Abstract
Live oral rotavirus (RV) vaccines used worldwide are most effective in reducing diarrheal hospitalizations from RV in high income countries and least effective in low income countries where RV remains a prime cause of death in children. Research has failed to fully explain the reason for this difference of efficacy for RV vaccines, an observation made with other live oral vaccines for polio, cholera and typhoid fever. Use of parenteral vaccines have been successful in overcoming this problem for both polio and typhoid and parenteral RV vaccines are now in development. This approach should be pursued for rotavirus vaccine as well because in low income countries where oral RV vaccines have been introduced and are only partially effective, RV remains the most common cause of diarrhea in children under 5 years. The ultimate control of RV diarrheal will likely require both oral and parenteral vaccines.
1. Introduction
Childhood mortality from diarrhea has declined remarkably from 4 million deaths annually in the late 1970s to fewer than 500,000 by 2015 [1] of which an estimated 150,000–200,000 are due to rotavirus (RV) and most occur in low income countries (LICs). In 2006, pivotal clinical trials conducted in high and middle-income countries in the Americas and Europe documented the 85–98% efficacy of two oral rotavirus vaccines (ORVs), RotaTeq (Merck) and Rotarix (GSK), against severe RV disease. Introduction of these vaccines in the immunization programs in many of these countries has dramatically reduced hospital admissions by 85–95% from RV [2]. In addition, Mexico documented a 40% decline in mortality in diarrheal deaths among children following vaccine introduction [3], raising the wonderful prospect that we might soon consider global control of RV disease with ORVs. In 2009, WHO recommended that all children be immunized against RV and GAVI began an ambitious program to provide vaccines at a substantially subsidized price in LICs to drastically reduce childhood deaths and disease from RV.
Recent data evaluating the impact of RV immunization programs in LICs is tempering this enthusiasm (Fig. 1). The median efficacy of both RotaTeq and Rotarix, as well as two nationally licensed vaccines in India, Rotavac (Bharat Biotech) and RotaSiil (Serum Institute of India) in LICs has been only 56% (range 18–79) against severe RV disease, significantly lower than the high efficacy (median ~90%) observed in high income settings. If we estimate that 150,000 deaths from RV occur in LICs, and if 75% of children receive a vaccine that has a median efficacy of 56%, we could expect to prevent only 42% (63,000) of these deaths. Consequently, while introduction of ORVs would substantially reduce the burden of RV in LICs by 58%, RV would remain a leading cause of severe or fatal childhood diarrhea even after vaccine introduction [4].
Fig. 1.
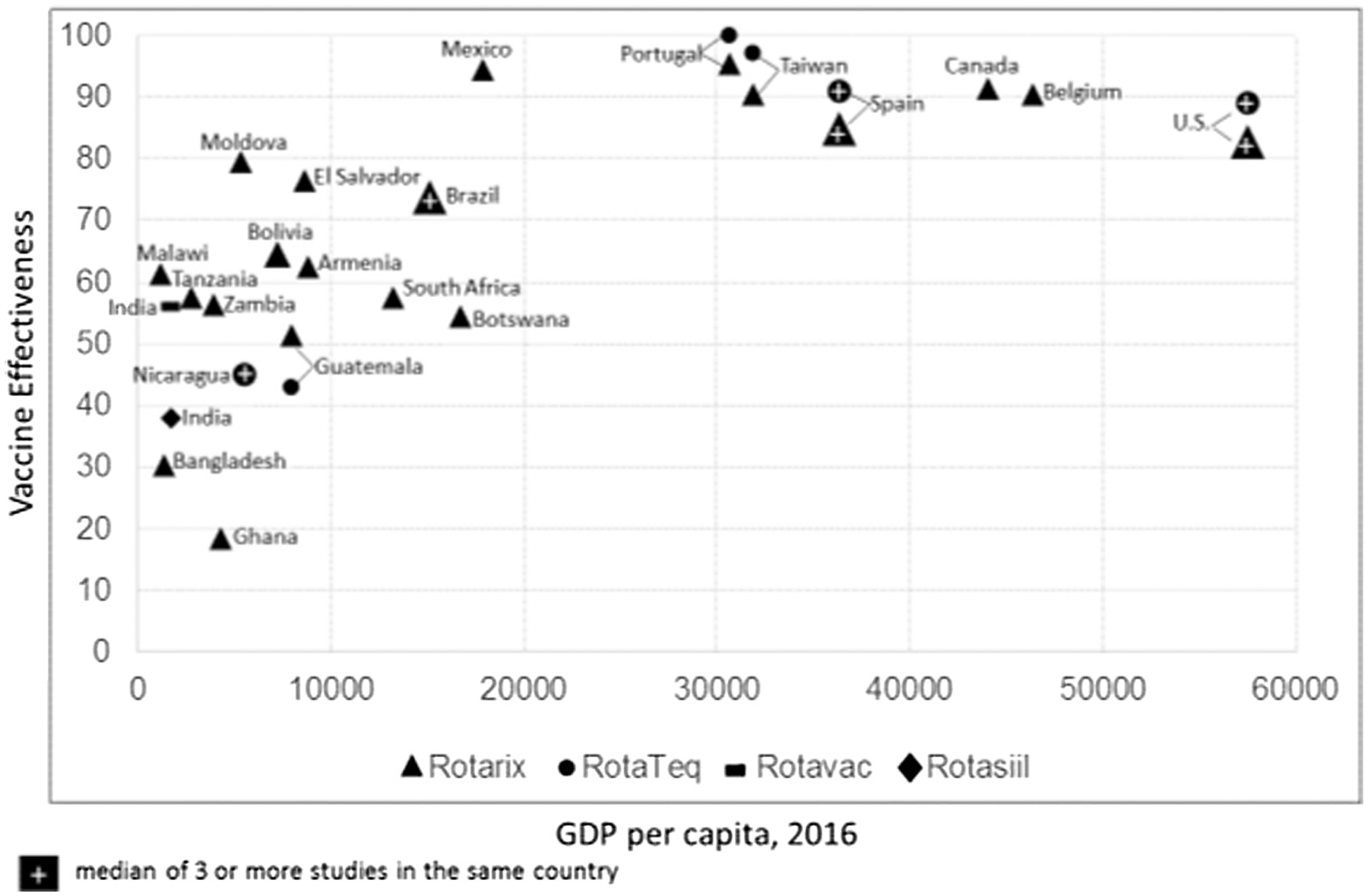
Effectiveness of oral rotavirus vaccines by a country’s per capita GDP.
The problem of lower performance of live oral vaccines in LICs is not unique to RV but was seen before for live oral polio (OPV), cholera and typhoid vaccines. For ORVs, hypotheses to explain their underperformance in LICs have focused on factors that might lower the titer of vaccine reaching target cells in the intestine – e.g., neutralization of the vaccine virus in the gut from high titers of transplacental or breast milk antibodies or stomach acid – or host factors that interfere with infant’s immune response – e.g., co-administration with OPV and other variations in the gut microbiome, or malnutrition [5]. Research to date has failed to demonstrate the reason for this lower efficacy or provided a clear strategy to improve the efficacy of ORVs. Trials to withhold breast milk or OPV at the very time of immunization, add a booster dose, change the vaccine schedule, or alter the microbiome with probiotics have shown only marginally improved outcomes [6]. In addition to these performance challenges, ORVs have been burdened with the rare risk of intussusception, prolonged shedding and severe disease in SCIDs children, and delivery issues around their relatively large volume in the cold chain.
2. Lessons from polio: IPV plays a role in polio endgame
The experience with OPV provides some cogent lessons to understand the current problems with ORVs. Early studies in India indicated that OPV performed less well than in other parts of the world and the reasons were never fully explained. The National Polio Eradication Program in India gave many children 10 or more doses of OPV and still found that some developed paralysis. Inactivated polio vaccine (IPV) played no role in the global eradication effort until the very end when research determined that a combination of OPV and IPV could significantly improve efficacy [7] even though it might not provide the herd protection seen with OPV. We may never know if more early research on both the cause of underperformance of OPV and the value of IPV as a booster in the eradication program might have accelerated the success and lowered the ultimate cost of this massive global effort.
3. Lessons from RV vaccine development: Don’t put all your eggs in one basket!
The history of RV vaccine development has provided some lessons on the importance of having multiple vaccines available. In 1998, when RotaShield was launched, the manufacturer, Wyeth-Lederle, never planned for a global market and other manufacturers were waiting to see how well a RV vaccine would be accepted. Nine months after its introduction in the United States, RotaShield was withdrawn for a rare adverse event, intussusception. Both Merck and GSK reassessed their strategies and accelerated development of their own ORVs each cautiously assessing the risk of intussusception from their own candidates.
Seven years and more than one million deaths later, these two companies launched their new ORVs. Over time, cost and programmatic challenges have favored Rotarix, which now commands 80% of ORVs supplied to GAVI for LICs. Again, despite having two products on the market, one was strongly preferred for use in LICs by virtue of its lower cost and two dose schedule, making the global program dependent primarily upon a single vaccine manufacturer. The lesson from this experience with both RotaShield and the current two vaccines is clear. As the search for next generation RV vaccines moves ahead, uncertainties around manufacturing, dosing, price, formulation, stability, and ability to have a combination product can all effect the ultimate acceptability of the next generation of vaccines. Consequently, we should consider developing multiple candidates and not depend upon a single product from a single manufacturer to supply the next generation RV vaccines for LICs for the future.
4. New approaches to RV vaccines
All the routine childhood vaccines recommended today, with the exception of oral polio and RV vaccines, are administered exclusively by injection and provide levels of protection that have made them universally acceptable for the global programs for childhood immunization. For polio, IPV is becoming an essential part of the endgame strategy for polio eradication with schedules ranging from administering a single booster dose of IPV to all children who were previously receiving only OPV, to providing three or more doses of IPV alone or combined with a parenteral vaccine. All currently licensed RV vaccines and many still in development are live oral vaccines. However, today to address the lower efficacy of the live oral RV vaccines in LICs, a number of parenteral RV vaccines (PRVs) are in active development (Table 1) [8]. The most advanced candidate is a non-replicating rotavirus vaccine (NRRV) derived from small VP8 fragments of the virus spikes of three strains of RV. This vaccine was immunogenic in infants against the target VP8 protein. Infants who received 3 doses of NRRV and placebo were subsequently administered a live attenuated vaccine, Rotarix. Shedding of Rotarix in the stool was reduced by 57% in the vaccinated group, indicating protection against a homologous virus [9]. Other approaches to parenteral vaccines have included intact rotaviruses inactivated with either heat or formaldehyde (IRVs) and selected RV proteins expressed in insect cells, E. coli, yeast or plants [10]. Finally, novel systems to deliver viral antigens with microneedles, skin patches or by intradermal injection have been considered [11]. IRVs could have other advantages if they were included in a childhood combination vaccine with either IPV or a traditional pentavalent product. Their use could not only improve the efficacy of the existing ORVs in low income countries, but would facilitate supply and delivery, decrease cold chain requirements, and remove the only oral vaccine from the immunization schedules of many countries. They are also less likely to be associated with adverse events related to replication of live virus in ORVs, such as intussusception and severe disease in babies with SCID. Challenges remain ahead to assess their efficacy, safety and cost and since a PRV will not be a standalone vaccine, its formulation with either IPV, pentavalent or hexavalent vaccine needs to be a priority.
Table 1.
Parenteral rotavirus vaccines in development.
| Vaccine | Characteristic | Developer | Status |
|---|---|---|---|
| Non-replicating RV vaccine (NRRV) | |||
| Monovalent | VP8 Fragment genotype [P8] | NIH – PATH | Phase 1: Safe in infants Phase 2: Immunogenic; protects against shedding of Rotarix |
| Trivalent | VP8: 3 Fragments [P8] [P4] [P6] | PATH – NIH | Phase 2: Ongoing |
| Inactivated RV vaccine (IRV) | |||
| By Heat | Strain CDC-9 (G1, P [8]) | CDC | Immunogenic: mice/monkeys/piglets Protective: piglets
|
| By Formaldehyde | Strain RV ZTR-68 (G1, P [8]) | Kunming Institute of Medical Biology, China | Immunogenic: mice Protective: mice |
| Expressed proteins | |||
| VP6 | VP6 combined with norovirus VLP† | Cincinnati Children’s Hospital; Univ. of Tampere, Finland | Immunogenic: mice Protective: mice |
| VLPa in insect cells | VLP containing VP2/6/7 or VP2/4/6/7 |
Baylor College of Medicine | Immunogenic: mice Protective: mice, rabbits |
| VLPa in plants | Medicago/Mitsubishi Tanabe Pharma, Japan | Immunogenic: mice Protective: mice |
|
This table is adapted from Kirkwood et al. [8].
Virus Like Particles (VLP).
Studies of natural and vaccine-acquired RV immunity provide several clues to the choice of antigens required in a PRV. While many serotypes of RV are in general circulation, children rarely get severe RV disease more than once so heterotypic immunity is clearly present [12]. Validating this observation, children immunized with a vaccine derived from a single serotype (e.g., Rotarix and Rotavac), are protected against severe disease from infections with different serotypes, again demonstrating that cross-protection is at play [13]. This cross-protection likely resides on multiple genes with different antigens and epitopes, such as those recently identified on the outer capsid protein VP7 and VP5 laying the blueprint for next generation IRVs [14]. We therefore believe that development of a PRV constructed from either the entire virus (similar to IPV) or a combination of many different antigens would provide the most direct approach to address the low efficacy of ORVs in LICs.
5. Conclusion
While ORVs in use today are having a major impact to reduce the burden of RV disease worldwide, the lower efficacy observed in LICs could markedly reduce their impact to decrease deaths and severe disease in these critical populations. Since research to date has failed to provide a novel approach to enhance the performance of ORVs, LICs that have adopted RV immunization programs will likely find that RV remains a leading cause of severe diarrhea in children. We believe that the optimal control of RV disease will therefore require a second-generation product, a PRV, composed of either an inactivated whole virus or a construct with multiple antigens derived from different gene segments. Experience with RV and other vaccines demonstrates that in early development, multiple candidate vaccines need to be competed to select the most promising candidates best suited for further development. If we want to ensure the ultimate control of severe and fatal RV disease in the most demanding settings in a timely fashion, research to develop these second-generation candidate PRVs must be accelerated today.
Footnotes
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. CDC holds the patents for CDC strains and inactivation methods developed by Drs. Jiang and Glass. No other conflicts to report.
References
- [1].GBD Diarrhoeal Diseases Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 2017;17:909–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J Infect Dis 2017;215(11):1666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gastañaduy PA, Sánchez-Uribe E, Esparza-Aguilar M, Desai R, Parashar UD, Patel M, et al. Effect of rotavirus vaccine on diarrhea mortality in different socioeconomic regions of Mexico. Pediatrics 2013;131:e1115–20. [DOI] [PubMed] [Google Scholar]
- [4].Operario DJ, Platts-Mills JA, Nadan S, et al. Etiology of severe acute watery diarrhea in children in the Global Rotavirus Surveillance Network using quantitative polymerase chain reaction. J Infect Dis 2017;216(2):220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Burnett E, Yen C, Tate JE, Parashar UD. Rotavirus vaccines: current global impact and future perspectives. Future Virol 2016;11:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Velasquez DE, Parashar U, Jiang B. Decreased performance of live attenuated, oral rotavirus vaccines in low-income settings: causes and contributing factors. Expert Rev Vaccines 2018;17:145–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].John TJ, Giri S, Karthikeyan AS, Lata D, Jeyapaul S, Rajan AK, et al. The duration of intestinal immunity after an inactivated poliovirus vaccine booster dose in children immunized with oral vaccine: a randomized controlled trial. J Infect Dis 2017;215(4):529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kirkwood CD, Ma LF, Carey ME, Steele AD. The rotavirus vaccine development pipeline. Vaccine 2017. 10.1016/j.vaccine.2017.03.076.S0264-410X(17)30410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Groome MJ, Koen A, Fix A, Page N, Jose L, Madhi SA, et al. Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet Inf Dis 2017;17:843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang Y, Azevedo M, Saif LJ, Gentsch JR, Glass RI, Jiang B. Inactivated rotavirus vaccine induces protective immunity in gnotobiotic piglets. Vaccine 2010;28:5432–6. [DOI] [PubMed] [Google Scholar]
- [11].Wang Y, Vlasova A, Velasquez DE, Saif LJ, Kandasamy S, Kochba E, et al. Skin vaccination against rotavirus using microneedles: proof of concept in gnotobiotic piglets. PLoS ONE 2016;11(11):e0166038. 10.1371/journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Velázquez FR, Matson DO, Calva JJ, Guerrero ML, Morrow AL, Carter-Campbell S, et al. Rotavirus infection in infants as protection against subsequent infections. N Engl J Med 1996;335:1022–8. [DOI] [PubMed] [Google Scholar]
- [13].Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006;354:11–22. [DOI] [PubMed] [Google Scholar]
- [14].Nair N, Feng N, Blum LK, Sanyal M, Ding S, Jiang B, Sen A, Morton JM, He X-S. Robinson WH, Greenberg HB. VP4- and VP7-specific antibodies mediate heterotypic immunity to rotavirus in humans. Sci Transl Med 2017;9(395). , http://stm.sciencemag.org/content/scitransmed/9/395/eaam5434.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]


