Abstract
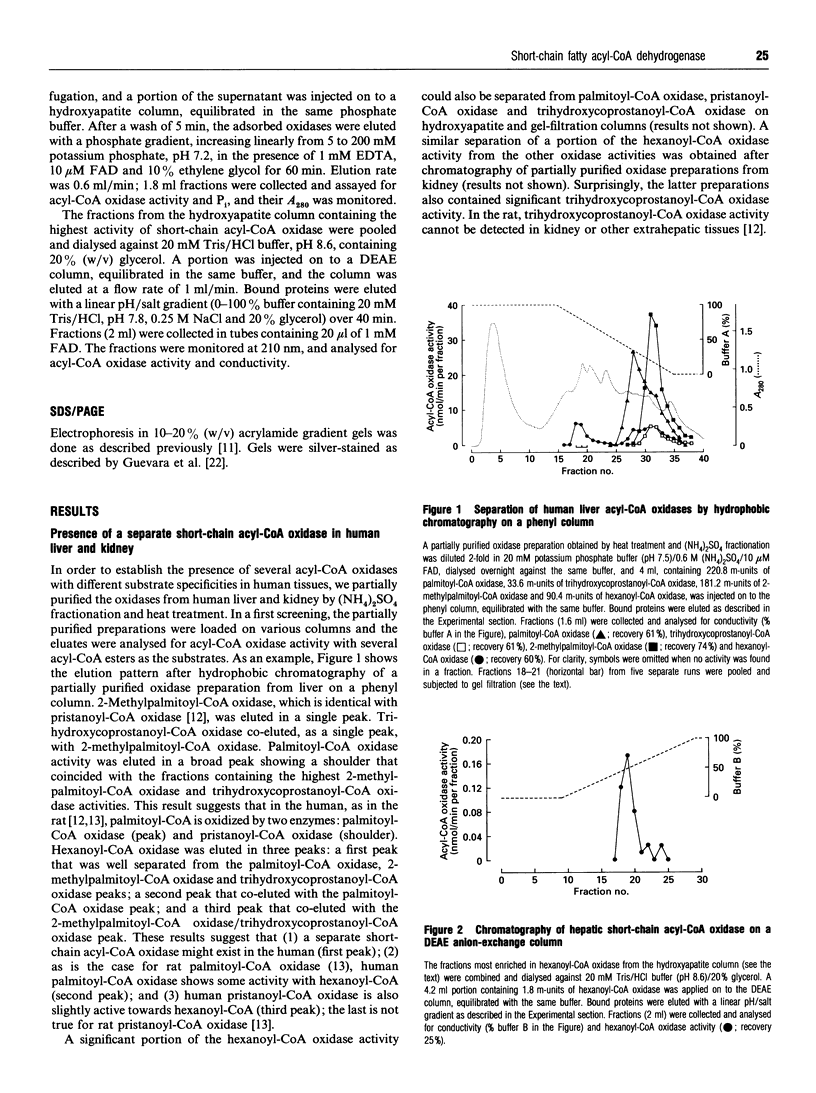
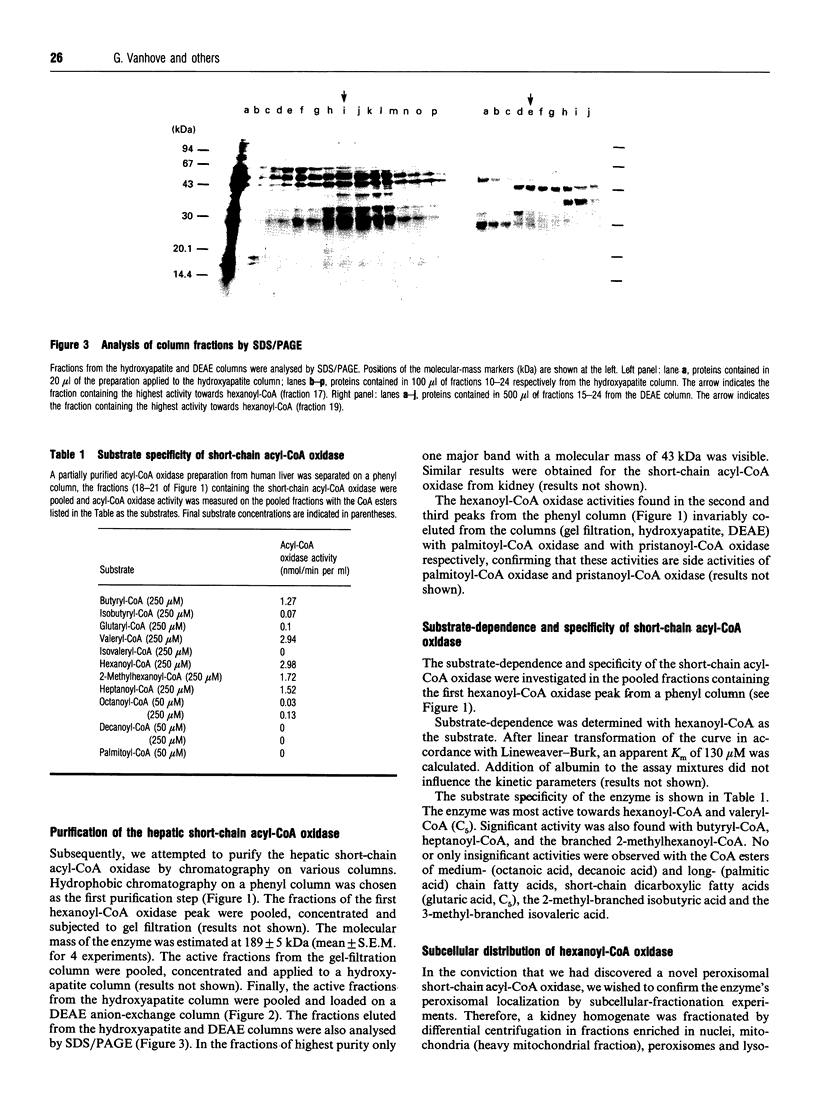
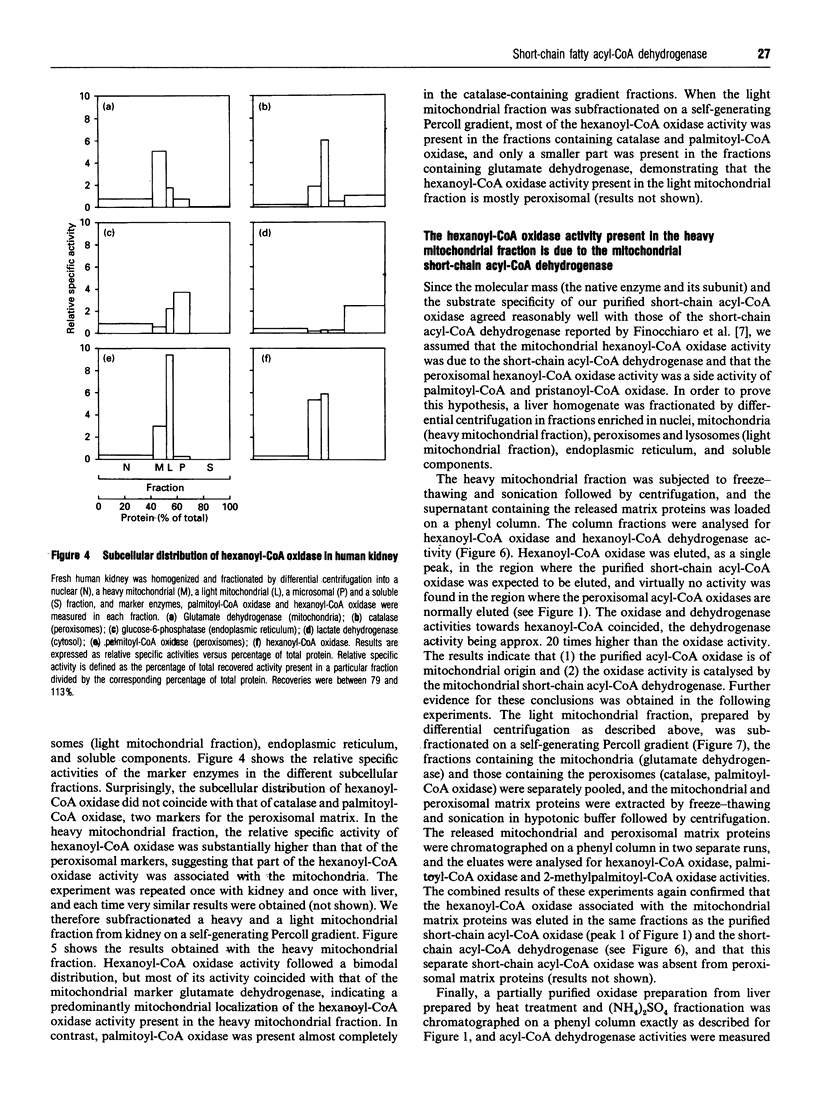
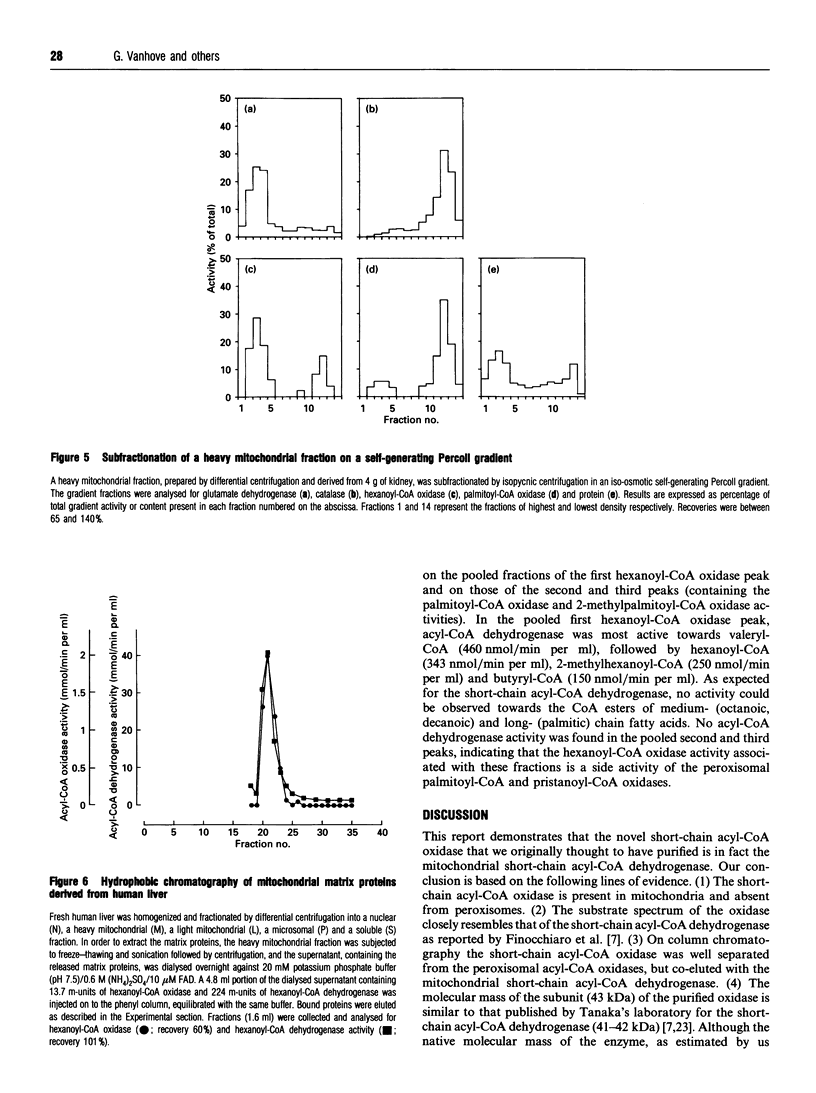
During an attempt to purify the peroxisomal acyl-CoA oxidases from human liver and kidney, we discovered a novel short-chain acyl-CoA oxidase, which was well separated from the known peroxisomal oxidases on various chromatographic columns. However, further experiments demonstrated that the novel oxidase is identical with the mitochondrial short-chain acyl-CoA dehydrogenase. (1) Subcellular fractionation revealed that the short-chain acyl-CoA oxidase is present in mitochondria and absent from peroxisomes. (2) The molecular mass (43 kDa) of the subunit of the purified oxidase was similar to that reported for the dehydrogenase. (3) The substrate spectrum of the oxidase was comparable with that described for the dehydrogenase. (4) On column chromatography, the oxidase and dehydrogenase activities co-eluted. Our results indicate that, in the absence of suitable electron acceptors, the short-chain acyl-CoA dehydrogenase is capable of transferring electrons directly to molecular oxygen, yielding potentially harmful H2O2. This raises the question as to whether the dehydrogenase might function as an oxidase in conditions in which the activity of the electron-transport chain is decreased, such as reperfusion after ischaemia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casteels M., Schepers L., Van Eldere J., Eyssen H. J., Mannaerts G. P. Inhibition of 3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-cholestanoic acid oxidation and of bile acid secretion in rat liver by fatty acids. J Biol Chem. 1988 Apr 5;263(10):4654–4661. [PubMed] [Google Scholar]
- Casteels M., Schepers L., Van Veldhoven P. P., Eyssen H. J., Mannaerts G. P. Separate peroxisomal oxidases for fatty acyl-CoAs and trihydroxycoprostanoyl-CoA in human liver. J Lipid Res. 1990 Oct;31(10):1865–1872. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq P. E., Haagsman H. P., Van Veldhoven P., Debeer L. J., Van Golde L. M., Mannaerts G. P. Rat liver dihydroxyacetone-phosphate acyltransferases and their contribution to glycerolipid synthesis. J Biol Chem. 1984 Jul 25;259(14):9064–9075. [PubMed] [Google Scholar]
- Finocchiaro G., Ito M., Tanaka K. Purification and properties of short chain acyl-CoA, medium chain acyl-CoA, and isovaleryl-CoA dehydrogenases from human liver. J Biol Chem. 1987 Jun 15;262(17):7982–7989. [PubMed] [Google Scholar]
- Henderson A. H., Most A. S., Parmley W. W., Gorlin R., Sonnenblick E. H. Depression of myocardial contractility in rats by free fatty acids during hypoxia. Circ Res. 1970 Apr;26(4):439–449. doi: 10.1161/01.res.26.4.439. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Dabrowski C., Tanaka K. Separation and properties of five distinct acyl-CoA dehydrogenases from rat liver mitochondria. Identification of a new 2-methyl branched chain acyl-CoA dehydrogenase. J Biol Chem. 1983 Jan 25;258(2):1066–1076. [PubMed] [Google Scholar]
- Ikeda Y., Okamura-Ikeda K., Tanaka K. Purification and characterization of short-chain, medium-chain, and long-chain acyl-CoA dehydrogenases from rat liver mitochondria. Isolation of the holo- and apoenzymes and conversion of the apoenzyme to the holoenzyme. J Biol Chem. 1985 Jan 25;260(2):1311–1325. [PubMed] [Google Scholar]
- Ikeda Y., Tanaka K. Purification and characterization of 2-methyl-branched chain acyl coenzyme A dehydrogenase, an enzyme involved in the isoleucine and valine metabolism, from rat liver mitochondria. J Biol Chem. 1983 Aug 10;258(15):9477–9487. [PubMed] [Google Scholar]
- Ikeda Y., Tanaka K. Purification and characterization of isovaleryl coenzyme A dehydrogenase from rat liver mitochondria. J Biol Chem. 1983 Jan 25;258(2):1077–1085. [PubMed] [Google Scholar]
- Izai K., Uchida Y., Orii T., Yamamoto S., Hashimoto T. Novel fatty acid beta-oxidation enzymes in rat liver mitochondria. I. Purification and properties of very-long-chain acyl-coenzyme A dehydrogenase. J Biol Chem. 1992 Jan 15;267(2):1027–1033. [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E., Ozasa H., Ikeda Y., Tanaka K. Molecular cloning and nucleotide sequence of complementary DNAs encoding human short chain acyl-coenzyme A dehydrogenase and the study of the molecular basis of human short chain acyl-coenzyme A dehydrogenase deficiency. J Clin Invest. 1989 May;83(5):1605–1613. doi: 10.1172/JCI114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E., Ozasa H., Ikeda Y., Tanaka K. Molecular cloning and nucleotide sequence of complementary DNAs encoding human short chain acyl-coenzyme A dehydrogenase and the study of the molecular basis of human short chain acyl-coenzyme A dehydrogenase deficiency. J Clin Invest. 1989 May;83(5):1605–1613. doi: 10.1172/JCI114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi T., Hashimoto T., Ui N. Purification and properties of acyl-CoA oxidase from rat liver. J Biochem. 1980 Jun;87(6):1735–1746. doi: 10.1093/oxfordjournals.jbchem.a132918. [DOI] [PubMed] [Google Scholar]
- Schepers L., Van Veldhoven P. P., Casteels M., Eyssen H. J., Mannaerts G. P. Presence of three acyl-CoA oxidases in rat liver peroxisomes. An inducible fatty acyl-CoA oxidase, a noninducible fatty acyl-CoA oxidase, and a noninducible trihydroxycoprostanoyl-CoA oxidase. J Biol Chem. 1990 Mar 25;265(9):5242–5246. [PubMed] [Google Scholar]
- Schulz H. Beta oxidation of fatty acids. Biochim Biophys Acta. 1991 Jan 28;1081(2):109–120. doi: 10.1016/0005-2760(91)90015-a. [DOI] [PubMed] [Google Scholar]
- Schulz H. Mitochondrial beta-oxidation. Prog Clin Biol Res. 1990;321:23–36. [PubMed] [Google Scholar]
- Van Veldhoven P. P., Brees C., Mannaerts G. P. D-aspartate oxidase, a peroxisomal enzyme in liver of rat and man. Biochim Biophys Acta. 1991 Jan 23;1073(1):203–208. doi: 10.1016/0304-4165(91)90203-s. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Vanhove G., Assselberghs S., Eyssen H. J., Mannaerts G. P. Substrate specificities of rat liver peroxisomal acyl-CoA oxidases: palmitoyl-CoA oxidase (inducible acyl-CoA oxidase), pristanoyl-CoA oxidase (non-inducible acyl-CoA oxidase), and trihydroxycoprostanoyl-CoA oxidase. J Biol Chem. 1992 Oct 5;267(28):20065–20074. [PubMed] [Google Scholar]
- Van Veldhoven P. P., Vanhove G., Vanhoutte F., Dacremont G., Parmentier G., Eyssen H. J., Mannaerts G. P. Identification and purification of a peroxisomal branched chain fatty acyl-CoA oxidase. J Biol Chem. 1991 Dec 25;266(36):24676–24683. [PubMed] [Google Scholar]
- Van Veldhoven P., Mannaerts G. P. Comparison of the activities of some peroxisomal and extraperoxisomal lipid-metabolizing enzymes in liver and extrahepatic tissues of the rat. Biochem J. 1985 May 1;227(3):737–741. doi: 10.1042/bj2270737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitch K., Hombroeckx A., Caucheteux D., Pouleur H., Hue L. Global ischaemia induces a biphasic response of the mitochondrial respiratory chain. Anoxic pre-perfusion protects against ischaemic damage. Biochem J. 1992 Feb 1;281(Pt 3):709–715. doi: 10.1042/bj2810709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders R. J., ten Brink H. J., van Roermund C. W., Schutgens R. B., Tager J. M., Jakobs C. Identification of pristanoyl-CoA oxidase activity in human liver and its deficiency in the Zellweger syndrome. Biochem Biophys Res Commun. 1990 Oct 30;172(2):490–495. doi: 10.1016/0006-291x(90)90699-n. [DOI] [PubMed] [Google Scholar]



