Abstract
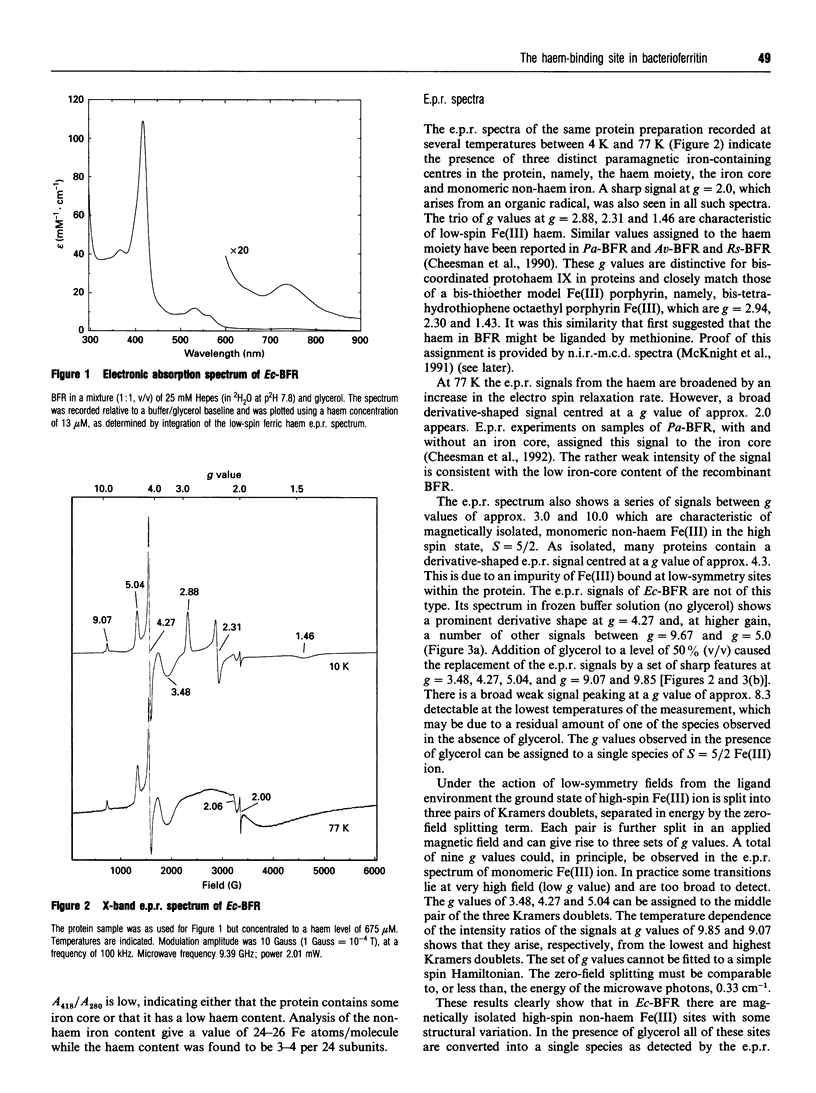
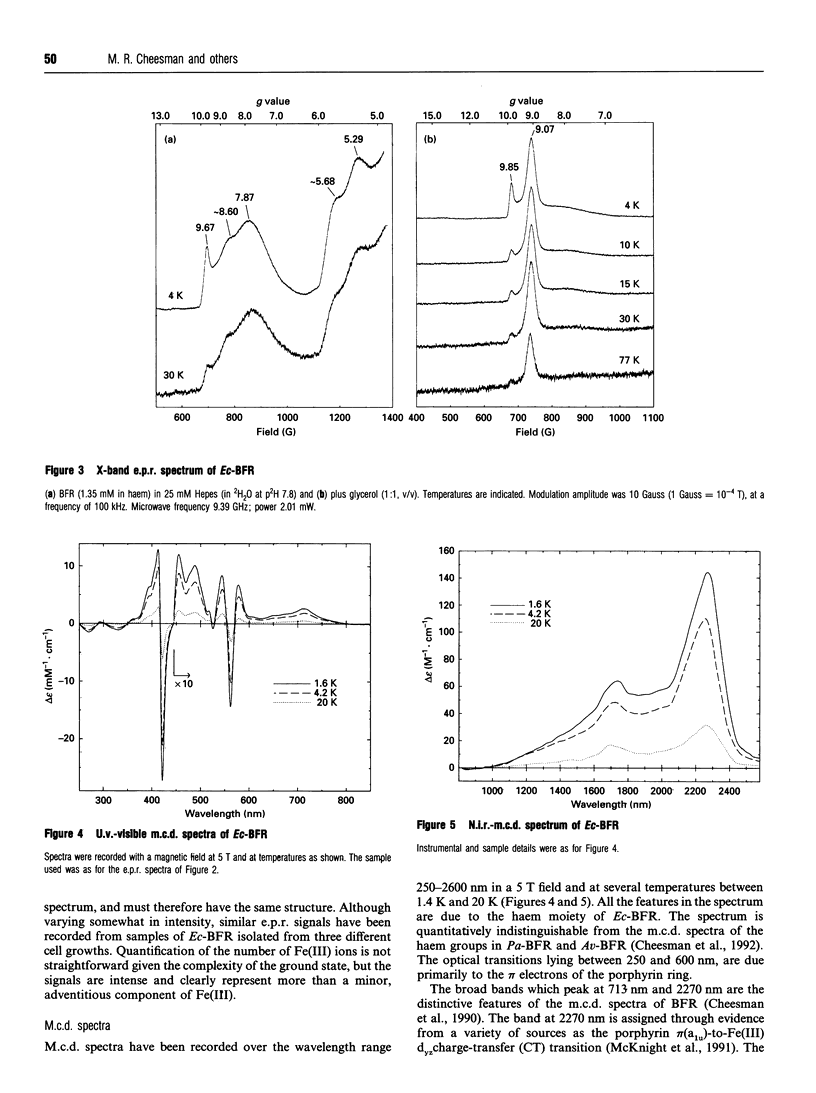
The bacterioferritin (BFR) of Escherichia coli is an iron-storage protein containing 24 identical subunits and between three and 11 protohaem IX groups per molecule. Titration with additional haem gave a maximum loading of 12-14 haems per molecule. The e.p.r. spectra and magnetic c.d. spectra of the protein-bound haem show it to be low-spin Fe(III), and coordinated by two methionine residues as previously reported for BFRs isolated from Pseudomonas aeruginosa and Azotobacter vinelandii [Cheesman, Thomson, Greenwood, Moore and Kadir, Nature (London) (1990) 346, 771-773]. A recent sequence alignment indicated that BFR may be structurally related to ferritin. The molecular model proposed for E. coli BFR has a four-alpha-helix-bundle subunit conformation and a quaternary structure similar to those of mammalian ferritins. In this model there are two types of hydrophobic pocket within which two methionine residues are correctly disposed to bind haem. The e.p.r. spectra also reveal a monomeric non-haem Fe(III) species with spin, S = 5/2. On the basis of sequence comparisons, a ferroxidase centre has recently been proposed to be present in BFR [Andrews, Smith, Yewdall, Guest and Harrison (1991) FEBS Lett. 293, 164-168] and the possibility that this Fe(III) ion may reside at or near the ferroxidase centre is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews S. C., Findlay J. B., Guest J. R., Harrison P. M., Keen J. N., Smith J. M. Physical, chemical and immunological properties of the bacterioferritins of Escherichia coli, Pseudomonas aeruginosa and Azotobacter vinelandii. Biochim Biophys Acta. 1991 May 30;1078(1):111–116. doi: 10.1016/0167-4838(91)90099-l. [DOI] [PubMed] [Google Scholar]
- Andrews S. C., Harrison P. M., Guest J. R. Cloning, sequencing, and mapping of the bacterioferritin gene (bfr) of Escherichia coli K-12. J Bacteriol. 1989 Jul;171(7):3940–3947. doi: 10.1128/jb.171.7.3940-3947.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. C., Smith J. M., Guest J. R., Harrison P. M. Amino acid sequence of the bacterioferritin (cytochrome b1) of Escherichia coli-K12. Biochem Biophys Res Commun. 1989 Jan 31;158(2):489–496. doi: 10.1016/s0006-291x(89)80075-0. [DOI] [PubMed] [Google Scholar]
- Andrews S. C., Smith J. M., Yewdall S. J., Guest J. R., Harrison P. M. Bacterioferritins and ferritins are distantly related in evolution. Conservation of ferroxidase-centre residues. FEBS Lett. 1991 Nov 18;293(1-2):164–168. doi: 10.1016/0014-5793(91)81177-a. [DOI] [PubMed] [Google Scholar]
- Bauminger E. R., Harrison P. M., Hechel D., Nowik I., Treffry A. Mössbauer spectroscopic investigation of structure-function relations in ferritins. Biochim Biophys Acta. 1991 Dec 11;1118(1):48–58. doi: 10.1016/0167-4838(91)90440-b. [DOI] [PubMed] [Google Scholar]
- Bauminger E. R., Harrison P. M., Nowik I., Treffry A. Mössbauer spectroscopic study of the initial stages of iron-core formation in horse spleen apoferritin: evidence for both isolated Fe(III) atoms and oxo-bridged Fe(III) dimers as early intermediates. Biochemistry. 1989 Jun 27;28(13):5486–5493. doi: 10.1021/bi00439a025. [DOI] [PubMed] [Google Scholar]
- Cheesman M. R., Kadir F. H., al-Basseet J., al-Massad F., Farrar J., Greenwood C., Thomson A. J., Moore G. R. E.p.r. and magnetic circular dichroism spectroscopic characterization of bacterioferritin from Pseudomonas aeruginosa and Azotobacter vinelandii. Biochem J. 1992 Sep 1;286(Pt 2):361–367. doi: 10.1042/bj2860361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheesman M. R., Thomson A. J., Greenwood C., Moore G. R., Kadir F. Bis-methionine axial ligation of haem in bacterioferritin from Pseudomonas aeruginosa. Nature. 1990 Aug 23;346(6286):771–773. doi: 10.1038/346771a0. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Harrison P. M., Rice D. W., Smith J. M., Treffry A., White J. L., Yariv J. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci. 1984 Feb 13;304(1121):551–565. doi: 10.1098/rstb.1984.0046. [DOI] [PubMed] [Google Scholar]
- Grossman M. J., Hinton S. M., Minak-Bernero V., Slaughter C., Stiefel E. I. Unification of the ferritin family of proteins. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2419–2423. doi: 10.1073/pnas.89.6.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuhara M., Takamune K., Takata R. Cloning and sequencing of an Escherichia coli K12 gene which encodes a polypeptide having similarity to the human ferritin H subunit. Mol Gen Genet. 1991 Mar;225(3):510–513. doi: 10.1007/BF00261694. [DOI] [PubMed] [Google Scholar]
- Kadir F. H., Moore G. R. Bacterial ferritin contains 24 haem groups. FEBS Lett. 1990 Oct 1;271(1-2):141–143. doi: 10.1016/0014-5793(90)80391-u. [DOI] [PubMed] [Google Scholar]
- Laulhère J. P., Labouré A. M., Van Wuytswinkel O., Gagnon J., Briat J. F. Purification, characterization and function of bacterioferritin from the cyanobacterium Synechocystis P.C.C. 6803. Biochem J. 1992 Feb 1;281(Pt 3):785–793. doi: 10.1042/bj2810785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D. M., Artymiuk P. J., Yewdall S. J., Smith J. M., Livingstone J. C., Treffry A., Luzzago A., Levi S., Arosio P., Cesareni G. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nature. 1991 Feb 7;349(6309):541–544. doi: 10.1038/349541a0. [DOI] [PubMed] [Google Scholar]
- Lawson D. M., Treffry A., Artymiuk P. J., Harrison P. M., Yewdall S. J., Luzzago A., Cesareni G., Levi S., Arosio P. Identification of the ferroxidase centre in ferritin. FEBS Lett. 1989 Aug 28;254(1-2):207–210. doi: 10.1016/0014-5793(89)81040-3. [DOI] [PubMed] [Google Scholar]
- Moore G. R., Mann S., Bannister J. V. Isolation and properties of the complex nonheme-iron-containing cytochrome b557 (bacterioferritin) from Pseudomonas aeruginosa. J Inorg Biochem. 1986 Oct-Nov;28(2-3):329–336. doi: 10.1016/0162-0134(86)80097-6. [DOI] [PubMed] [Google Scholar]
- Ramachandran G. N., Sasisekharan V. Conformation of polypeptides and proteins. Adv Protein Chem. 1968;23:283–438. doi: 10.1016/s0065-3233(08)60402-7. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Ford G. C., Harrison P. M., Yariv J., Kalb A. J. Molecular size and symmetry of the bacterioferritin of Escherichia coli. X-ray crystallographic characterization of four crystal forms. J Mol Biol. 1989 Jan 20;205(2):465–467. doi: 10.1016/0022-2836(89)90358-6. [DOI] [PubMed] [Google Scholar]
- Stiefel E. I., Watt G. D. Azotobacter cytochrome b557.5 is a bacterioferritin. Nature. 1979 May 3;279(5708):81–83. doi: 10.1038/279081a0. [DOI] [PubMed] [Google Scholar]
- Treffry A., Hirzmann J., Yewdall S. J., Harrison P. M. Mechanism of catalysis of Fe(II) oxidation by ferritin H chains. FEBS Lett. 1992 May 11;302(2):108–112. doi: 10.1016/0014-5793(92)80417-f. [DOI] [PubMed] [Google Scholar]
- Watt R. K., Frankel R. B., Watt G. D. Redox reactions of apo mammalian ferritin. Biochemistry. 1992 Oct 13;31(40):9673–9679. doi: 10.1021/bi00155a021. [DOI] [PubMed] [Google Scholar]
- Yariv J., Kalb A. J., Sperling R., Bauminger E. R., Cohen S. G., Ofer S. The composition and the structure of bacterioferritin of Escherichia coli. Biochem J. 1981 Jul 1;197(1):171–175. doi: 10.1042/bj1970171. [DOI] [PMC free article] [PubMed] [Google Scholar]