Abstract
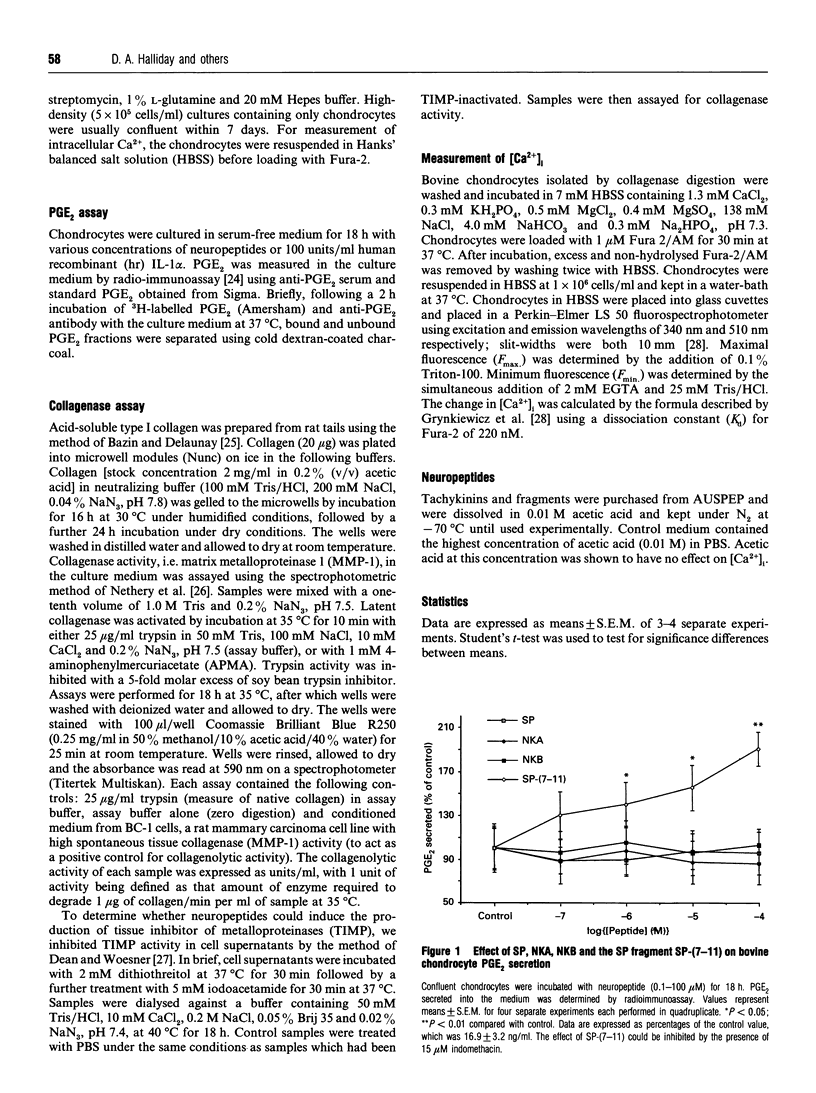
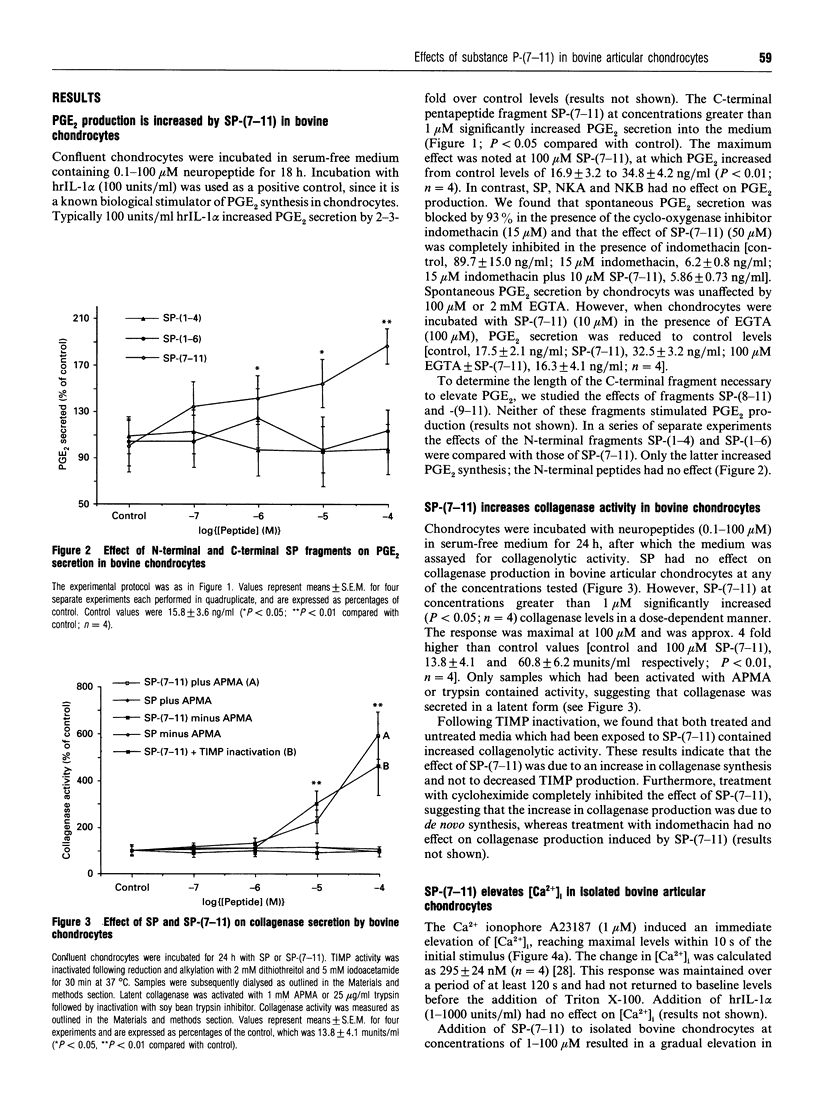
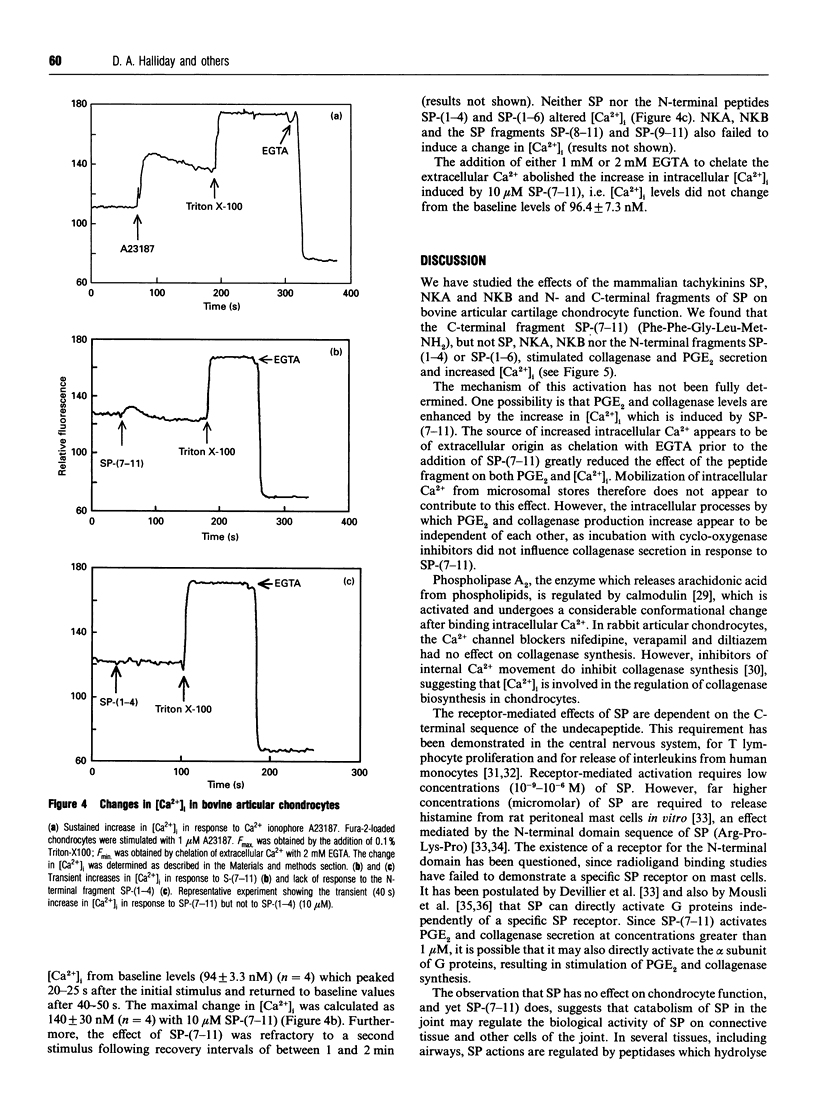
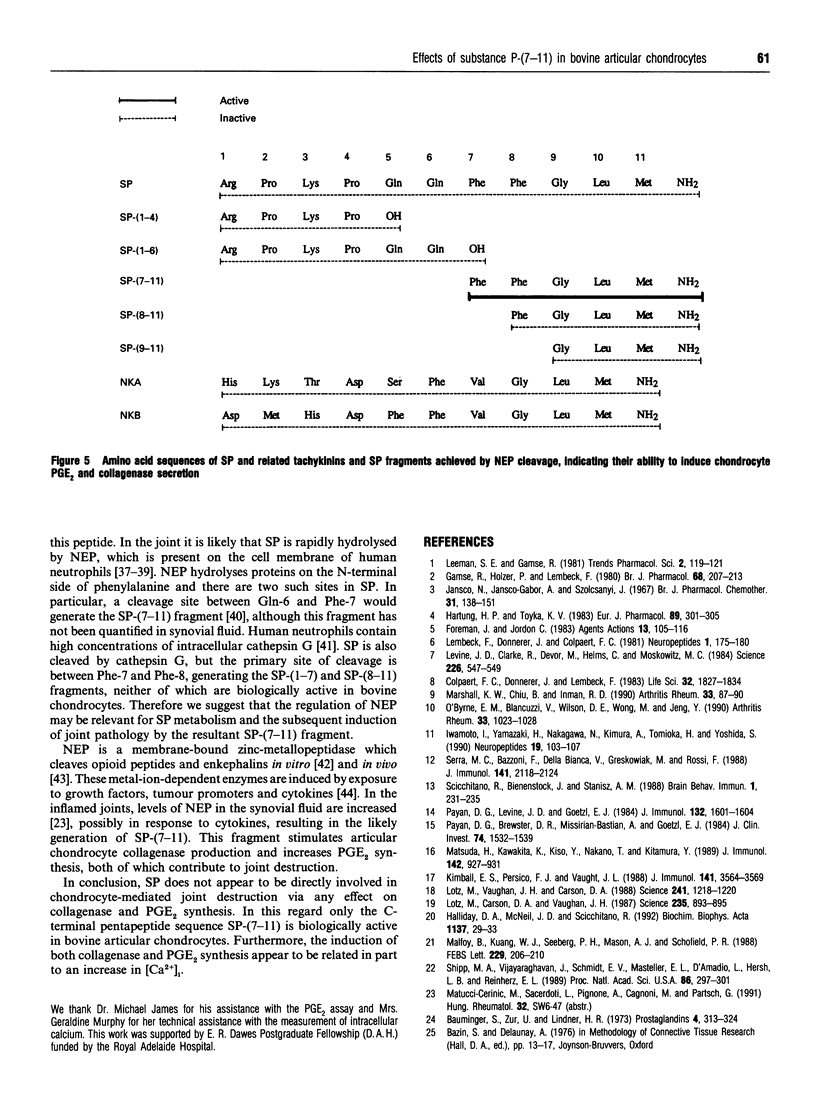
Substance P (SP) is found in increased concentrations in inflamed joints and is believed to play a role in joint pathology. Culture of bovine articular chondrocytes with SP or with the related mammalian tachykinins neurokinin A or B (NKA or NKB) produced no effect on prostaglandin E2 (PGE2) or collagenase production. However, the C-terminal fragment of SP, SP-(7-11), increased PGE2 and collagenase production at concentrations greater than 1 microM. The N-terminal fragments SP-(1-4) and SP-(1-6) had no effect on PGE2 or collagenase production. In addition, SP-(7-11), but not intact SP, SP-(1-4), SP-(1-6), SP-(8-11) or SP-(9-11), nor the tachykinins NKA and NKB, caused an increase in the intracellular calcium concentration as measured by the fluorescent dye Fura-2. The maximal change in intra-cellular calcium induced by 10 microM SP-(7-11) was 140 +/- 30 nM. We postulate that cleavage of SP by neutral endopeptidases which are present in the synovial fluid and which yield SP-(7-11) may be of biological importance in chondrocyte-mediated cartilage pathology.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauminger S., Zor U., Lindner H. R. Radioimmunological assay of prostaglandin synthetase activity. Prostaglandins. 1973 Sep;4(3):313–324. doi: 10.1016/0090-6980(73)90019-1. [DOI] [PubMed] [Google Scholar]
- Colpaert F. C., Donnerer J., Lembeck F. Effects of capsaicin on inflammation and on the substance P content of nervous tissues in rats with adjuvant arthritis. Life Sci. 1983 Apr 18;32(16):1827–1834. doi: 10.1016/0024-3205(83)90060-7. [DOI] [PubMed] [Google Scholar]
- Connelly J. C., Skidgel R. A., Schulz W. W., Johnson A. R., Erdös E. G. Neutral endopeptidase 24.11 in human neutrophils: cleavage of chemotactic peptide. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8737–8741. doi: 10.1073/pnas.82.24.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. D., Woessner J. F., Jr Extracts of human articular cartilage contain an inhibitor of tissue metalloproteinases. Biochem J. 1984 Feb 15;218(1):277–280. doi: 10.1042/bj2180277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillier P., Renoux M., Giroud J. P., Regoli D. Peptides and histamine release from rat peritoneal mast cells. Eur J Pharmacol. 1985 Oct 29;117(1):89–96. doi: 10.1016/0014-2999(85)90475-3. [DOI] [PubMed] [Google Scholar]
- Erdös E. G., Skidgel R. A. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989 Feb;3(2):145–151. [PubMed] [Google Scholar]
- Erdös E. G., Wagner B., Harbury C. B., Painter R. G., Skidgel R. A., Fa X. G. Down-regulation and inactivation of neutral endopeptidase 24.11 (enkephalinase) in human neutrophils. J Biol Chem. 1989 Aug 25;264(24):14519–14523. [PubMed] [Google Scholar]
- Foreman J., Jordan C. Histamine release and vascular changes induced by neuropeptides. Agents Actions. 1983 Apr;13(2-3):105–116. doi: 10.1007/BF01967311. [DOI] [PubMed] [Google Scholar]
- Gamse R., Holzer P., Lembeck F. Decrease of substance P in primary afferent neurones and impairment of neurogenic plasma extravasation by capsaicin. Br J Pharmacol. 1980 Feb;68(2):207–213. doi: 10.1111/j.1476-5381.1980.tb10409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Halliday D. A., McNeil J. D., Scicchitano R. Failure of tachykinins including substance P and its fragments to influence proteoglycan and protein synthesis in bovine chondrocytes in vitro. Biochim Biophys Acta. 1992 Oct 6;1137(1):29–33. doi: 10.1016/0167-4889(92)90095-s. [DOI] [PubMed] [Google Scholar]
- Hartung H. P., Toyka K. V. Activation of macrophages by substance P: induction of oxidative burst and thromboxane release. Eur J Pharmacol. 1983 May 6;89(3-4):301–305. doi: 10.1016/0014-2999(83)90511-3. [DOI] [PubMed] [Google Scholar]
- Iwamoto I., Yamazaki H., Nakagawa N., Kimura A., Tomioka H., Yoshida S. Differential effects of two C-terminal peptides of substance P on human neutrophils. Neuropeptides. 1990 Jun;16(2):103–107. doi: 10.1016/0143-4179(90)90119-j. [DOI] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967 Sep;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball E. S., Persico F. J., Vaught J. L. Substance P, neurokinin A, and neurokinin B induce generation of IL-1-like activity in P388D1 cells. Possible relevance to arthritic disease. J Immunol. 1988 Nov 15;141(10):3564–3569. [PubMed] [Google Scholar]
- Levine J. D., Clark R., Devor M., Helms C., Moskowitz M. A., Basbaum A. I. Intraneuronal substance P contributes to the severity of experimental arthritis. Science. 1984 Nov 2;226(4674):547–549. doi: 10.1126/science.6208609. [DOI] [PubMed] [Google Scholar]
- Lotz M., Carson D. A., Vaughan J. H. Substance P activation of rheumatoid synoviocytes: neural pathway in pathogenesis of arthritis. Science. 1987 Feb 20;235(4791):893–895. doi: 10.1126/science.2433770. [DOI] [PubMed] [Google Scholar]
- Lotz M., Vaughan J. H., Carson D. A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988 Sep 2;241(4870):1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Kuang W. J., Seeburg P. H., Mason A. J., Schofield P. R. Molecular cloning and amino acid sequence of human enkephalinase (neutral endopeptidase). FEBS Lett. 1988 Feb 29;229(1):206–210. doi: 10.1016/0014-5793(88)80828-7. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Swerts J. P., Guyon A., Roques B. P., Schwartz J. C. High-affinity enkephalin-degrading peptidase in brain is increased after morphine. Nature. 1978 Nov 30;276(5687):523–526. doi: 10.1038/276523a0. [DOI] [PubMed] [Google Scholar]
- Marshall K. W., Chiu B., Inman R. D. Substance P and arthritis: analysis of plasma and synovial fluid levels. Arthritis Rheum. 1990 Jan;33(1):87–90. doi: 10.1002/art.1780330111. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Kawakita K., Kiso Y., Nakano T., Kitamura Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989 Feb 1;142(3):927–931. [PubMed] [Google Scholar]
- Mazurek N., Pecht I., Teichberg V. I., Blumberg S. The role of the N-terminal tetrapeptide in the histamine releasing action of substance P. Neuropharmacology. 1981 Nov;20(11):1025–1027. doi: 10.1016/0028-3908(81)90091-5. [DOI] [PubMed] [Google Scholar]
- Moskowitz N., Shapiro L., Schook W., Puszkin S. Phospholipase A2 modulation by calmodulin, prostaglandins and cyclic nucleotides. Biochem Biophys Res Commun. 1983 Aug 30;115(1):94–99. doi: 10.1016/0006-291x(83)90973-7. [DOI] [PubMed] [Google Scholar]
- Mousli M., Bronner C., Bueb J. L., Tschirhart E., Gies J. P., Landry Y. Activation of rat peritoneal mast cells by substance P and mastoparan. J Pharmacol Exp Ther. 1989 Jul;250(1):329–335. [PubMed] [Google Scholar]
- Mousli M., Bronner C., Landry Y., Bockaert J., Rouot B. Direct activation of GTP-binding regulatory proteins (G-proteins) by substance P and compound 48/80. FEBS Lett. 1990 Jan 1;259(2):260–262. doi: 10.1016/0014-5793(90)80023-c. [DOI] [PubMed] [Google Scholar]
- Mousli M., Bueb J. L., Bronner C., Rouot B., Landry Y. G protein activation: a receptor-independent mode of action for cationic amphiphilic neuropeptides and venom peptides. Trends Pharmacol Sci. 1990 Sep;11(9):358–362. doi: 10.1016/0165-6147(90)90179-c. [DOI] [PubMed] [Google Scholar]
- Nethery A., Lyons J. G., O'Grady R. L. A spectrophotometric collagenase assay. Anal Biochem. 1986 Dec;159(2):390–395. doi: 10.1016/0003-2697(86)90358-1. [DOI] [PubMed] [Google Scholar]
- Nolan J. C., Gathright C. E., Wagner L. E. The effect of calcium channel blockers and calmodulin inhibitors on the macrophage factor-stimulated synthesis of collagenase by rabbit chondrocytes. Agents Actions. 1988 Aug;25(1-2):71–76. doi: 10.1007/BF01969097. [DOI] [PubMed] [Google Scholar]
- O'Byrne E. M., Blancuzzi V., Wilson D. E., Wong M., Jeng A. Y. Elevated substance P and accelerated cartilage degradation in rabbit knees injected with interleukin-1 and tumor necrosis factor. Arthritis Rheum. 1990 Jul;33(7):1023–1028. doi: 10.1002/art.1780330715. [DOI] [PubMed] [Google Scholar]
- Painter R. G., Dukes R., Sullivan J., Carter R., Erdös E. G., Johnson A. R. Function of neutral endopeptidase on the cell membrane of human neutrophils. J Biol Chem. 1988 Jul 5;263(19):9456–9461. [PubMed] [Google Scholar]
- Payan D. G., Brewster D. R., Missirian-Bastian A., Goetzl E. J. Substance P recognition by a subset of human T lymphocytes. J Clin Invest. 1984 Oct;74(4):1532–1539. doi: 10.1172/JCI111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payan D. G., Levine J. D., Goetzl E. J. Modulation of immunity and hypersensitivity by sensory neuropeptides. J Immunol. 1984 Apr;132(4):1601–1604. [PubMed] [Google Scholar]
- Regoli D., Drapeau G., Dion S., Couture R. New selective agonists for neurokinin receptors: pharmacological tools for receptor characterization. Trends Pharmacol Sci. 1988 Aug;9(8):290–295. doi: 10.1016/0165-6147(88)90013-2. [DOI] [PubMed] [Google Scholar]
- Schwartz J. C., Malfroy B., De La Baume S. Biological inactivation of enkephalins and the role of enkephalin-dipeptidyl-carboxypeptidase ("enkephalinase") as neuropeptidase. Life Sci. 1981 Oct 26;29(17):1715–1740. doi: 10.1016/0024-3205(81)90182-x. [DOI] [PubMed] [Google Scholar]
- Scicchitano R., Bienenstock J., Stanisz A. M. The differential effect with time of neuropeptides on the proliferative responses of murine Peyer's patch and splenic lymphocytes. Brain Behav Immun. 1987 Sep;1(3):231–237. doi: 10.1016/0889-1591(87)90025-0. [DOI] [PubMed] [Google Scholar]
- Serra M. C., Bazzoni F., Della Bianca V., Greskowiak M., Rossi F. Activation of human neutrophils by substance P. Effect on oxidative metabolism, exocytosis, cytosolic Ca2+ concentration and inositol phosphate formation. J Immunol. 1988 Sep 15;141(6):2118–2124. [PubMed] [Google Scholar]
- Shipp M. A., Vijayaraghavan J., Schmidt E. V., Masteller E. L., D'Adamio L., Hersh L. B., Reinherz E. L. Common acute lymphoblastic leukemia antigen (CALLA) is active neutral endopeptidase 24.11 ("enkephalinase"): direct evidence by cDNA transfection analysis. Proc Natl Acad Sci U S A. 1989 Jan;86(1):297–301. doi: 10.1073/pnas.86.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidgel R. A., Jackman H. L., Erdös E. G. Metabolism of substance P and bradykinin by human neutrophils. Biochem Pharmacol. 1991 May 1;41(9):1335–1344. doi: 10.1016/0006-2952(91)90106-f. [DOI] [PubMed] [Google Scholar]


