Abstract
Background
Neutral Lipid Storage Disease with Myopathy (NLSDM) is a rare lipid metabolism disorder caused by PNPLA2 gene mutations. Clinical manifestations are heterogeneous, and diagnosis is often delayed, usually gaining patients’ attention due to the increased risk of cardiomyopathy.
Case presentation
We herein report a 36-year-old Asian male presenting with progressive limb weakness, muscle atrophy of limbs and trunk, dysarthria, and heart failure. Electromyography indicated myogenic changes, and muscle biopsy results revealed characteristics of lipid storage myopathy. Genetic analysis of PNPLA2 revealed two heterozygous mutations: c.757 + 1G > T (chr11-823588, splice-5) on intron 6 and c.919delG (chr11-823854, p.A307Pfs*13) on exon 7. The patient improved limb strength, and dysarthria disappeared after the Medium Chain Fatty Acids diet.
Conclusions
In conclusion, we report for the first time that the two heterozygous mutations PNPLA2 c.919delG and c.757 + 1G > T together induced NLSDM, which was confirmed by muscle biopsy.
Keywords: Neutral lipid storage disease with myopathy, Patatin-like phospholipase domain-containing 2, Cardiomyopathy, Muscular atrophy, Medium-chain fatty acid diet
Introduction
Neutral lipid storage disease with myopathy (NLSDM) is a rare autosomal recessive disorder caused by mutations in the PNPLA2 gene [1]. These mutations lead to dysfunction of adipose triglyceride lipase, resulting in abnormal lipid metabolism and the subsequent manifestation of symptoms and signs. NLSDM is a disease with highly variable clinical manifestations, ranging from mild symptoms to more severe ones. Skeletal myopathy is present in more than 95% of patients, cardiomyopathy has been observed in about 40%, and other clinical presentations include hepatomegaly, diabetes, hearing loss, chronic pancreatitis, short stature, and intellectual disability [2].
We report a case of an Asian male diagnosed with NLSDM through muscle biopsy and PNPLA2 gene testing, and he exhibited asymmetric skeletal myopathy, dilated cardiomyopathy, and diabetes. The patient improved limb strength and dysarthria following targeted treatment with a medium-chain triglyceride diet.
Case presentation
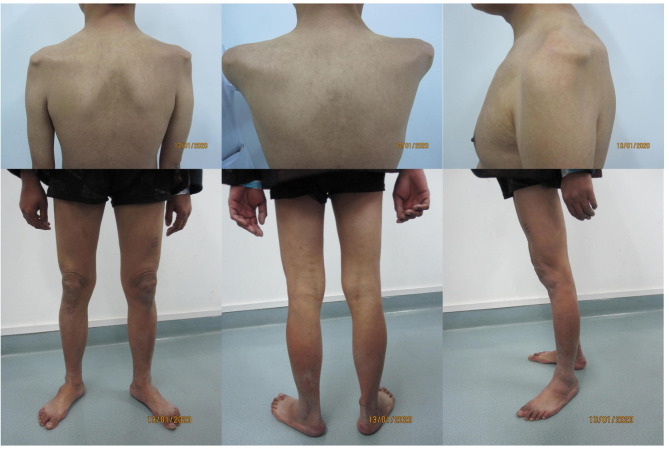
The patient is a 36-year-old Asian male admitted to our hospital primarily due to limb weakness, dysarthria, oliguria, and abdominal distension. The disease itself has a long duration and progresses slowly. The patient began to experience weakness in the right upper limb 6 years prior to admission, followed by muscle atrophy from the right shoulder to the limbs in the following year. Four years ago, the right upper limb and lower limbs gradually weakened, accompanied by muscle atrophy of the left shoulder, left upper limb, lower limbs, and paraspinal muscles. One year ago, symptoms of dysphonia, swelling in both lower limbs, oliguria, and abdominal distension were observed. Six months ago, numbness was experienced at the fingertips of the left hand upon waking up, which improved after exercise. The patient’s clinical image upon admission is shown in Fig. 1.
Fig. 1.
The clinical features of the patient
The patient’s intellectual development is normal. He reported no similar diseases within their family and denied the presence of other cardiovascular diseases and associated risk factors. He also denied any poisoning history. General Examination: The patient is alert and mentally stable, with dysarthria. There are no signs of entrapment neuropathy (negative Buried Eyelashes sign), and the patient does not exhibit air leakage when puffing cheeks. The patient is unable to stand on heels and demonstrates a duck-like gait. There is mild pitting edema in both lower extremities. Muscle Atrophy: There is noticeable muscle atrophy in the limbs, as well as in the scapular and paraspinal muscles. Tendon Reflexes: Tendon reflexes in the limbs are absent. Sensory System Examination: The sensitivity to pinprick pain in the limbs is normal, with no reduction.The results of muscle strength testing (MMT-8) are shown in Table 1.
Table 1.
Manual-muscle-testing 8 items
| Neck flexors | 5 |
| Shoulder abductors | 2 |
| Elbow flexors | 1 |
| Wrist extensors | 5- |
| Hip flexors | 5- |
| Ankle dorsiflexors | 5- |
| Hip abductors | 4+ |
| Hip extensors | 4+ |
| Total score (0 ~ 80) | 48 |
The blood routine examination indicates a decrease in platelet count (58 × 109/L, normal values: 94–268 × 109/L), and Wright’s stain shows changes in platelet size. The serum muscular and myocardial enzymes graph has significantly increased, including creatine kinase (1019.84 UL, normal values: 38–174 U/L), creatine kinase-MB (79.78 U/L, normal values: 1–25 U/L), lactate dehydrogenase (1691.0 UL, normal values: 313–618 U/L), and brain natriuretic peptide (1680.01 pg/mL, normal values: 0-100 pg/mL). There is a mild increase in aspartate aminotransferase (112.3 U/L, normal values: 15–40 U/L) and alanine aminotransferase (52.6 U/L, normal values: 9–50 U/L). The levels of triglycerides, total cholesterol, low-density lipoprotein cholesterol is normal. High-density lipoprotein cholesterol was lower (1.06 mmol/L, normal values: 1.16-1.42mmol/L). Glycosylated hemoglobin is higher than the normal limit (6.1%, normal values: 4-6%), and an oral glucose tolerance test indicates diabetes. Antinuclear antibodies are positive. Thyroid function tests are normal.
Electrocardiogram: occasional ventricular premature beats and T-wave changes are observed. The echocardiogram shows overall heart enlargement, left ventricular hypertrophy, reduced left ventricular systolic function (LVEF = 31%), and a small amount of pericardial effusion. Nerve conduction studies are normal. Electromyography shows myogenic changes in the left deltoid, right deltoid, biceps, gastrocnemius, and quadriceps femoris muscles, cranial MRI examination shows no abnormality.
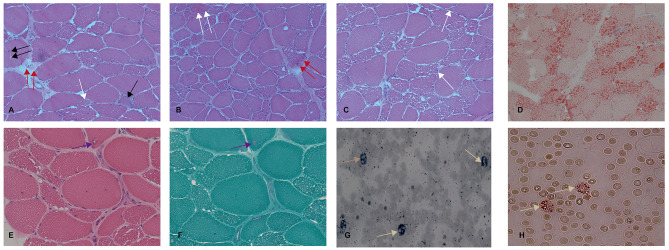
A muscle biopsy of the left quadriceps femoris in the patient revealed that the muscular tissue was partially substituted with fibro-adipose tissue. Hematoxylin and eosin (HE) staining revealed that the muscle fiber size and shape were significantly different, with many extremely type I atrophic muscle fibers, occasional muscle fibers splitting, occasional high contraction muscle fibers, a small amount of necrosis with phagocytosis and regeneration of muscle fibers. Moderate endomysial fibrous tissue hyperplasia was found in some areas. A pyknotic nuclear clump was also detected. Nucleus muscle fibers were not increased, and hypertrophic muscle fibers were not found. Importantly, many vacuoles were detected in the muscle fibers (Fig. 2A, B, C). Oil Red O (ORO) staining showed that the content of lipid droplets in muscle fibers increased significantly (Fig. 2D). HE staining and Modified Gomori Trichrome (MGT) staining revealed several vacuoles (Fig. 2E, F). Regarding peripheral blood smear tests, leucocytes vacuols that was positive at staining with ORO and Sudan (Jordans’s anomaly) was seen (Fig. 2G, H).
Fig. 2.
The pathology study of quadriceps biopsy and peripheral blood smear tests. A, B, and C: HE staining showed significant variation in muscle fiber size, massive vacuoles of various sizes within myocytes, and a moderate increase of endomysial fibrous tissue. Extremely atrophic muscle fibers (white arrow), muscle fibers splitting (double black arrow), high contraction muscle fibers (double white arrow), necrosis with phagocytosis (double red arrow), and regeneration of muscle fibers (black arrow) were also detected. Magnification: x200. D: Oil Red O staining revealed a large number of neutral lipid drops accumulated in myofibers. Magnification: x200. E and F: HE staining and modified MGT staining revealed RVs in myofibers. The arrows show the RVs. Magnification: x400. G and H: Red Oil O and Sudan IV staining of peripheral blood smear revealed cytoplasmic vacuolization in polymorphonuclear leukocytes (Jordan’s anomaly). Magnification: x400
The sequencing results revealed a PNPLA2 gene heterozygous mutation whose genomic location was chr11-823854 in exon 7 of c.919delG and chr11-823588 in intron 6 of c.757 + 1G > T (Fig. 3A, B). The results of his parents’ gene sequences showed that his father had a heterozygous mutation, with genomic location chr11-823854 in exon 7 of c.919delG (Fig. 3C, D), and his mother had a heterozygous mutation, with genomic location chr11-823588 in intron 6 of c.757 + 1G > T (Fig. 3E, F).
Fig. 3.
Sequence analysis of the PNPLA2 gene of the patient and his parents. A: The patient carried the heterozygous mutation of PNPLA2 c.919delG (A) and c.757 + 1G > T (B). The patient’s father showed heterozygous mutations of PNPLA2 c.919delG (C) but did not show the mutation of PNPLA2 c.757 + 1G > T (D). The patient’s mother carried the mutation of PNPLA2 c.757 + 1G > T (F) but did not carry the mutation of PNPLA2 c.919delG (E)
After diagnosis, the patient was given a medium-chain fatty acid (MCFA) diet. The main component of MCFAs is medium-chain triglycerides containing equal amounts of digestible energy (28.77 MJ/kg), carbohydrates (5%), protein (6%), and fat (117%). Follow-up was completed in the fourth month, and the patient’s limb weakness was mildly improved. We reevaluated the muscle strength as follows with MRC score: 4/5 in the extensor muscles of his neck, 4/5 in the left deltoid, 3/5 in the right deltoid, 2/5 in the left biceps, 4/5 in the right biceps, and 3/5 in the left flexor carpi radialis. The patient’s dysarthria also disappeared, and the blood lipid profiles returned to normal. Concurrently, the patient also took sacubitril valsartan, furosemide, and spironolactone to control chronic heart failure. Effective symptom control was achieved.
Discussion
Fisher et al. reported for the first time that mutations in the PNPLA2 gene caused NLSDM [3]. In 2007, the clinical phenotype was a combination of muscle and heart involvement without ichthyosis. PNPLA2 mutations are the cause of NLSDM onset, which may be helpful in antenatal diagnosis and genetic consulting [4].
The current case report describes a 36-year-old male in southwest China (Chongqing City) with progressive muscle weakness, skeletal muscle atrophy, and dysarthria. The subsequent muscle biopsy presented characteristic features of lipid storage myopathy. Genetic results further confirmed the diagnosis of NLSDM. We reported for the first time the simultaneous occurrence of heterozygous mutations of PNPLA2 c.757 + 1G > T and c.919del in an affected individual.
NLSDM is associated with PNPLA2 gene and is autosomal recessive disease. This means that pathogenic mutations must be present on two alleles (either as homozygous or compound heterozygous mutations) to cause NLSDM. The c.757 + 1G > T mutation has been proven to be pathogenic in previous studies [4], particularly in homozygous and compound heterozygous mutations [5, 6]. The c.919delG mutation results in a premature termination of protein translation, significantly affecting protein function. Based on ACMG guidelines, the c.919delG mutation is considered a likely pathogenic variant, though it has not been previously reported. From the perspective of the entire family, the heterozygous c.919delG mutation and the heterozygous c.757 + 1G > T mutation in the patient’s parents did not cause NLSDM, however, the presence of both c.757 + 1G > T and c.919delG mutation as compound heterozygous variants led to NLSDM in the patient. Our study confirmed that the c.919delG mutation is pathogenic in the context of compound heterozygous mutations.
Currently, there are no effective treatments or cures for NLSDM. Although some patients have been treated with bezafibrate [7] and beta-adrenergic drugs [8], more extensive clinical studies are needed to develop a standardized treatment protocol. Reports indicate that treatment with medium-chain triglycerides (MCTs) reduced body fat and improved lipid profiles in obese mice [9]. On the other hand, an MCT diet has already shown benefits in reducing liver size and improving liver function in CDS patients [4]. According to studies, in a mouse model of triglyceride deposit cardiomyovasculopathy, the triglyceride form of decanoic acid (a type of MCFA), Tricaprin, was able to rescue myocardial abnormalities [10]. Although the benefits of an MCT diet for NLSDM patients are still unclear, primary care physicians, after attempting communication with patients, decided to proceed with the dietary treatment. Surprisingly, by the fourth month of the diet, patients showed mild improvements in muscle weakness, articulation disorders disappeared, and lipid levels returned to normal after four months on the MCFA diet. Therefore, our case suggests that MCFAs can be used in the long-term diet of NLSDM patients and may offer benefits, necessitating further research.
Conclusion
In conclusion, we report that two heterozygous mutations of PNPLA2 c.919delG and c.757 + 1G > T together, induce NLSDM. We confirm that the c.919delG mutation is pathogenic in the context of compound heterozygous mutations. In addition to gene sequencing, detecting the vacuolization of granulocytes (Jordan’s anomaly) in peripheral blood can be used as a quick test method for NLSDM. Although there is no effective treatment or cure for NLSDM, an MCFA diet may have some clinical benefits.
Acknowledgements
This work is supported by the Key Clinical Specialty of Neurology of PLA to Daping Hospital.
Author contributions
Tong Yang and Jie Zhu wrote the manuscript. Yulai Kang and Chunhua Tang diagnosed, treated, and followed the patient. Lili Zhang and Lu Guo conceived the study and revised it critically for important intellectual content. All authors had read and approved the final version of the manuscript.
Funding
This work was supported by the Natural Science Foundation Project of Chongqing (CSTB2022NSCQ-MSX1585).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The ethics committee of the Army Medical Centre of PLA approved this study (ethical number: 2019 − 121).
Consent to participate
Written informed consent was obtained from patient and parents.
Consent to Publish
Written informed consent for publication was obtained from the patients and parents.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tong Yang and Jie Zhu equally contributed to this case report as co-first authors.
Contributor Information
Lili Zhang, Email: zhanglili@tmmu.edu.cn.
Lu Guo, Email: qiany0218@163.com.
References
- 1.Garcia MA, Rojas JA, Millán SP, Flórez AA. Neutral lipid storage disease with myopathy and dropped head syndrome. Report of a new variant susceptible of treatment with late diagnosis. J Clin Neurosci. 2018;58:207–9. 10.1016/j.jocn.2018.10.046 [DOI] [PubMed] [Google Scholar]
- 2.Missaglia S, Maggi L, Mora M, Gibertini S, Blasevich F, Agostoni P, et al. Late onset of neutral lipid storage disease due to novel PNPLA2 mutations causing total loss of lipase activity in a patient with myopathy and slight cardiac involvement. Neuromuscul Disord. 2017;27(5):481–6. 10.1016/j.nmd.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer J, Lefèvre C, Morava E, Mussini J-M, Laforêt P, Negre-Salvayre A, et al. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39(1):28–30. 10.1038/ng1951 [DOI] [PubMed] [Google Scholar]
- 4.Tan J, Yang H, Fan J, Fan Y, Xiao F. Patients with neutral lipid storage disease with myopathy (NLSDM) in Southwestern China. Clin Neurol Neurosurg. 2018;168:102–7. 10.1016/j.clineuro.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Wen B, Lu J, Zhao Y, Hong D, Zhao Z, et al. Neutral lipid storage disease with myopathy in China: a large multicentric cohort study. Orphanet J Rare Dis. 2019;14(1):234. 10.1186/s13023-019-1209-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latimer CS, Schleit J, Reynolds A, Marshall DA, Podemski B, Wang LH, et al. Neutral lipid storage disease with myopathy: further phenotypic characterization of a rare PNPLA2 variant. Neuromuscul Disord. 2018;28(7):606–9. 10.1016/j.nmd.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 7.van de Weijer T, Havekes B, Bilet L, Hoeks J, Sparks L, Bosma M, et al. Effects of bezafibrate treatment in a patient and a carrier with mutations in the PNPLA2 gene, causing neutral lipid storage disease with myopathy. Circ Res. 2013;112(5):e51–4. [DOI] [PubMed] [Google Scholar]
- 8.Reilich P, Horvath R, Krause S, Schramm N, Turnbull DM, Trenell M, et al. The phenotypic spectrum of neutral lipid storage myopathy due to mutations in the PNPLA2 gene. J Neurol. 2011;258(11):1987–97. 10.1007/s00415-011-6055-4 [DOI] [PubMed] [Google Scholar]
- 9.Liu Y-h, Zhang Y, Xu Q, Yu X-m, Zhang X-s, Wang J, et al. Increased norepinephrine by medium-chain triglyceride attributable to lipolysis in white and brown adipose tissue of C57BL/6J mice. Biosci Biotechnol Biochem. 2012;76(6):1213–8. 10.1271/bbb.120079 [DOI] [PubMed] [Google Scholar]
- 10.Suzuki A, Yamaguchi S, Li M, Hara Y, Miyauchi H, Ikeda Y, et al. Tricaprin rescues myocardial abnormality in a mouse model of triglyceride Deposit Cardiomyovasculopathy. J Oleo Sci. 2018;67(8):983–9. 10.5650/jos.ess18037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.