Abstract
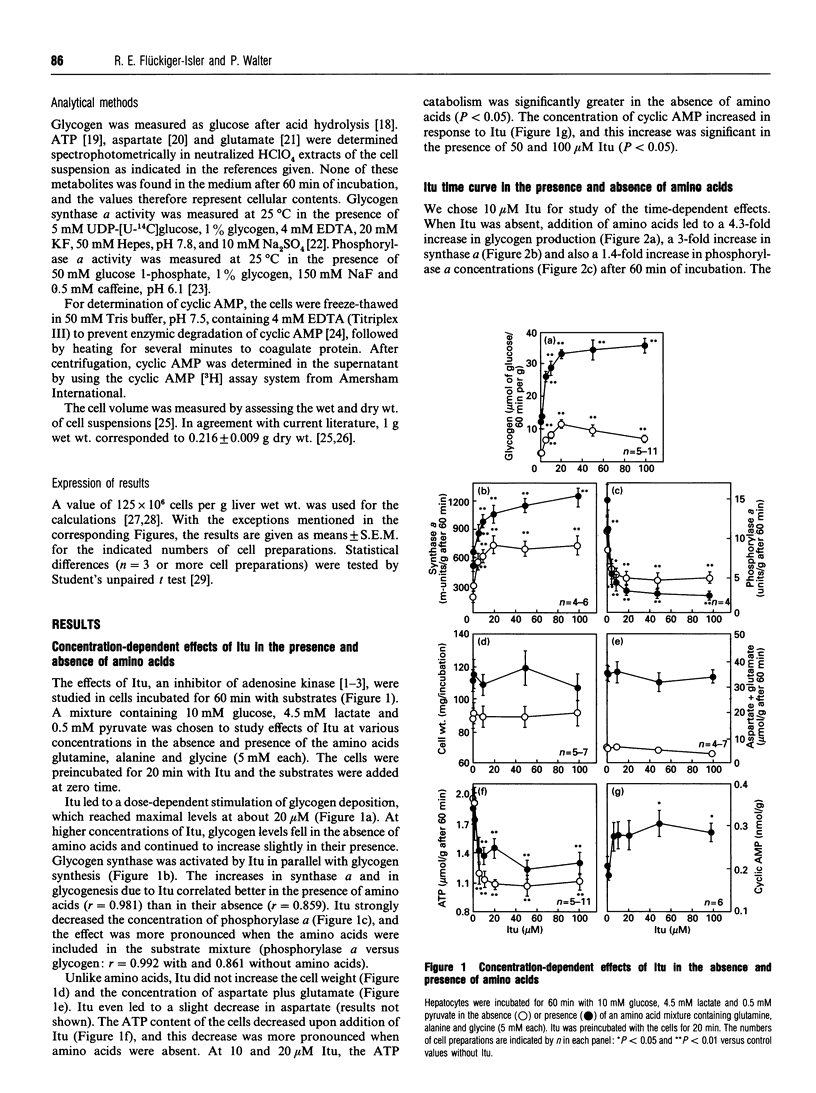
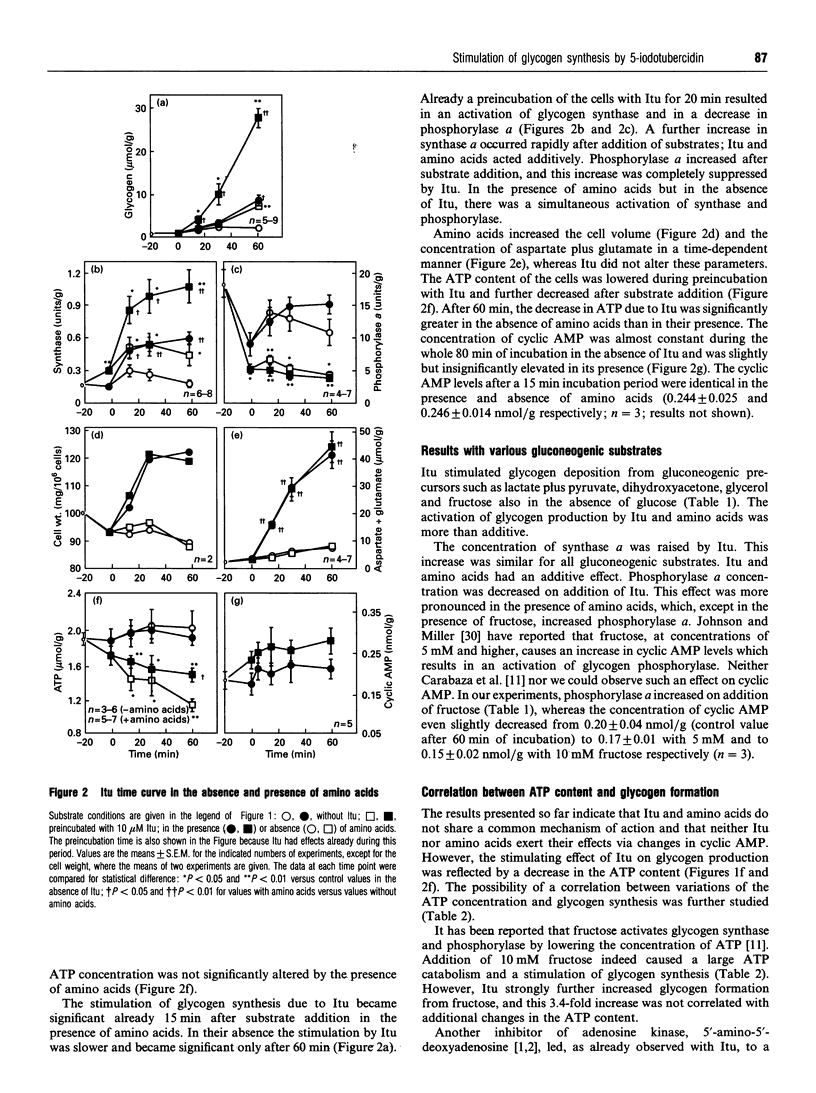
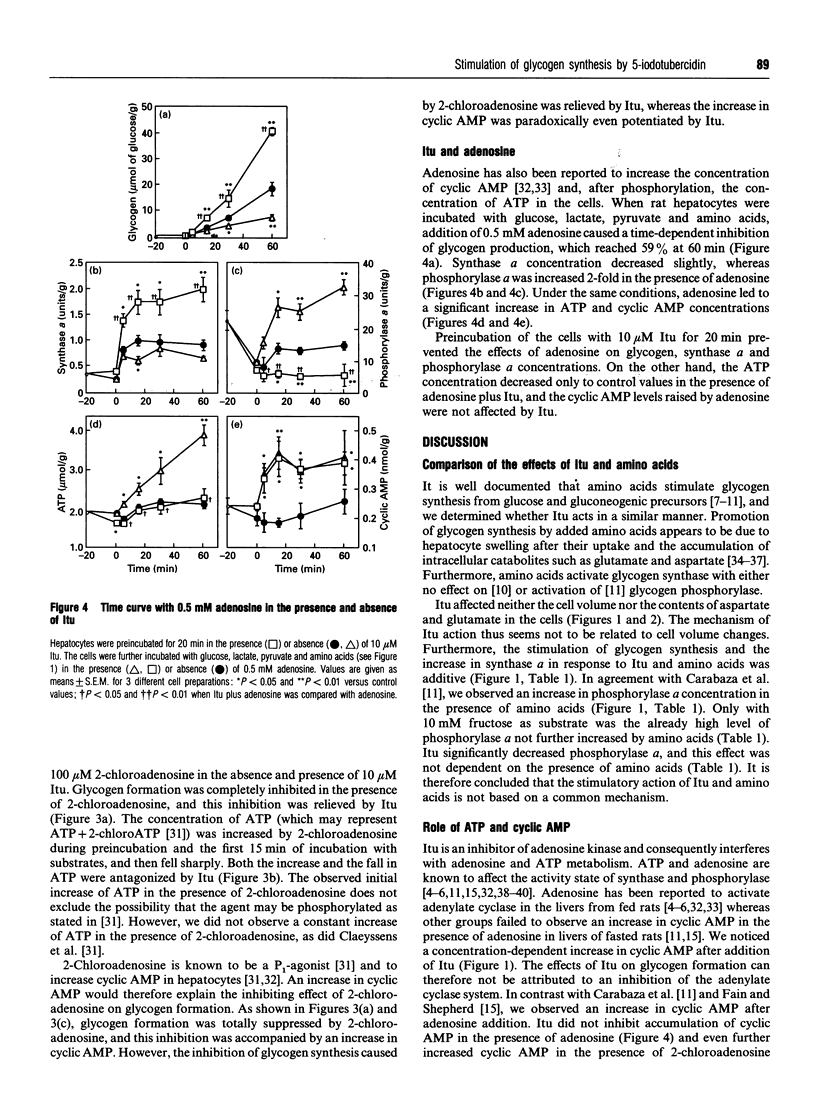
The adenosine kinase inhibitor 5-iodotubercidin (Itu) was found to have the following effects on glycogen metabolism in hepatocytes of fasted rats. (1) Itu strongly stimulated glycogen synthesis from different substrates (glucose, lactate plus pyruvate, dihydroxyacetone, glycerol and fructose). In cells incubated with these substrates, the well-known stimulating effect of amino acids and that of Itu was more than additive. (2) In parallel with the increase in glycogen deposition, there was an increase in synthase a and a decrease in phosphorylase a concentrations after administration of Itu. Synthase a was increased by Itu and amino acids in an additive manner, whereas the observed activation of phosphorylase after addition of amino acids was antagonized by Itu. (3) In contrast with amino acids, Itu increased neither the cell volume nor the aspartate and glutamate concentrations. (4) Itu enhanced the levels of cyclic AMP. The stimulation of glycogen deposition in the presence of Itu persisted when the cyclic AMP concentration was further increased by adenosine or 2-chloroadenosine. (5) Itu decreased the concentration of ATP, but its effects on glycogen synthesis, synthase a and phosphorylase a concentrations persisted when the ATP catabolism was prevented by adenosine. (6) The effect of Itu on glycogen synthesis was not the result of inhibition of adenosine kinase, since 5'-amino-5'-deoxyadenosine, another inhibitor of this enzyme, had no effect on glycogen deposition.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baquet A., Hue L., Meijer A. J., van Woerkom G. M., Plomp P. J. Swelling of rat hepatocytes stimulates glycogen synthesis. J Biol Chem. 1990 Jan 15;265(2):955–959. [PubMed] [Google Scholar]
- Baquet A., Hue L., Meijer A. J., van Woerkom G. M., Plomp P. J. Swelling of rat hepatocytes stimulates glycogen synthesis. J Biol Chem. 1990 Jan 15;265(2):955–959. [PubMed] [Google Scholar]
- Bartrons R., Van Schaftingen E., Hers H. G. The ability of adenosine to decrease the concentration of fructose 2,6-bisphosphate in isolated hepatocytes. A cyclic AMP-mediated effect. Biochem J. 1984 Feb 15;218(1):157–163. doi: 10.1042/bj2180157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. Evidence for a substrate cycle between AMP and adenosine in isolated hepatocytes. Proc Natl Acad Sci U S A. 1983 May;80(10):2829–2833. doi: 10.1073/pnas.80.10.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunengraber H., Boutry M., Lowenstein J. M. Fatty acid, 3-beta-hydroxysterol, and ketone synthesis in the perfused rat liver. Effects of (--)-hydroxycitrate and oleate. Eur J Biochem. 1978 Jan 16;82(2):373–384. doi: 10.1111/j.1432-1033.1978.tb12032.x. [DOI] [PubMed] [Google Scholar]
- Buxton D. B., Robertson S. M., Olson M. S. Stimulation of glycogenolysis by adenine nucleotides in the perfused rat liver. Biochem J. 1986 Aug 1;237(3):773–780. doi: 10.1042/bj2370773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabaza A., Ricart M. D., Mor A., Guinovart J. J., Ciudad C. J. Role of AMP on the activation of glycogen synthase and phosphorylase by adenosine, fructose, and glutamine in rat hepatocytes. J Biol Chem. 1990 Feb 15;265(5):2724–2732. [PubMed] [Google Scholar]
- Chagoya de Sánchez V., Brunner A., Sánchez M. E., López C., Piña E. Utilization of adenosine as a tool in studies on the regulation of liver glycogen biosynthesis. Arch Biochem Biophys. 1974 Jan;160(1):145–150. doi: 10.1016/s0003-9861(74)80019-6. [DOI] [PubMed] [Google Scholar]
- Chen K. S., Lardy H. A. Multiple requirements for glycogen synthesis by hepatocytes isolated from fasted rats. J Biol Chem. 1985 Nov 25;260(27):14683–14688. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Claeyssens S., Hamet M., Chedeville A., Basuyau J. P., Lavoinne A. Influence of 2-chloroadenosine on the nucleotide content of isolated rat hepatocytes. FEBS Lett. 1988 May 23;232(2):317–322. doi: 10.1016/0014-5793(88)80761-0. [DOI] [PubMed] [Google Scholar]
- Davies L. P., Baird-Lambert J., Marwood J. F. Studies on several pyrrolo[2,3-d]pyrimidine analogues of adenosine which lack significant agonist activity at A1 and A2 receptors but have potent pharmacological activity in vivo. Biochem Pharmacol. 1986 Sep 15;35(18):3021–3029. doi: 10.1016/0006-2952(86)90381-3. [DOI] [PubMed] [Google Scholar]
- Doperé F., Vanstapel F., Stalmans W. Glycogen-synthase phosphatase activity in rat liver. Two protein components and their requirement for the activation of different types of substrate. Eur J Biochem. 1980 Feb;104(1):137–146. doi: 10.1111/j.1432-1033.1980.tb04409.x. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Shepherd R. E. Adenosine, cyclic AMP metabolism, and glycogenolysis in rat liver cells. J Biol Chem. 1977 Nov 25;252(22):8066–8070. [PubMed] [Google Scholar]
- Gilboe D. P., Nuttall F. Q. Regulation of liver glycogen synthase phosphatase activity by ATP-Mg. Arch Biochem Biophys. 1986 Aug 15;249(1):34–45. doi: 10.1016/0003-9861(86)90557-6. [DOI] [PubMed] [Google Scholar]
- Gilboe D. P., Nuttall F. Q. The importance of nucleoside triphosphate inhibition of liver glycogen synthase phosphatase activity. Biochim Biophys Acta. 1989 May 31;991(2):340–346. doi: 10.1016/0304-4165(89)90126-8. [DOI] [PubMed] [Google Scholar]
- Gilboe D. P., Nuttall F. Q. The synergistic action of caffeine or adenosine on glucose stimulation of liver glycogen synthase phosphatase activity. FEBS Lett. 1984 May 21;170(2):365–369. doi: 10.1016/0014-5793(84)81345-9. [DOI] [PubMed] [Google Scholar]
- Guo Z. K., Wals P. A., Katz J. Stimulation of glycogen synthesis by proglycosyn (LY177507) by isolated hepatocytes of normal and streptozotocin diabetic rats. J Biol Chem. 1991 Nov 25;266(33):22323–22327. [PubMed] [Google Scholar]
- Harris R. A., Yamanouchi K., Roach P. J., Yen T. T., Dominianni S. J., Stephens T. W. Stabilization of glycogen stores and stimulation of glycogen synthesis in hepatocytes by phenacyl imidazolium compounds. J Biol Chem. 1989 Sep 5;264(25):14674–14680. [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. R., Miller T. B., Jr Adverse effects of fructose in perfused livers of diabetic rats. Metabolism. 1982 Feb;31(2):121–125. doi: 10.1016/0026-0495(82)90122-6. [DOI] [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Stimulation of hepatic glycogen synthesis by amino acids. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3433–3437. doi: 10.1073/pnas.73.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal R. L. Some properties of purified phosphoprotein phosphatases from rabbit liver. Biochim Biophys Acta. 1977 Dec 8;485(2):379–390. doi: 10.1016/0005-2744(77)90173-5. [DOI] [PubMed] [Google Scholar]
- Lavoinne A., Baquet A., Hue L. Stimulation of glycogen synthesis and lipogenesis by glutamine in isolated rat hepatocytes. Biochem J. 1987 Dec 1;248(2):429–437. doi: 10.1042/bj2480429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand J. C., Lavoinne A., Giroz M., Matray F. The influence of adenosine on intermediary metabolism of isolated hepatocytes. Biochimie. 1979;61(11-12):1273–1282. doi: 10.1016/s0300-9084(80)80286-0. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Baquet A., Gustafson L., van Woerkom G. M., Hue L. Mechanism of activation of liver glycogen synthase by swelling. J Biol Chem. 1992 Mar 25;267(9):5823–5828. [PubMed] [Google Scholar]
- Newby A. C., Holmquist C. A., Illingworth J., Pearson J. D. The control of adenosine concentration in polymorphonuclear leucocytes, cultured heart cells and isolated perfused heart from the rat. Biochem J. 1983 Aug 15;214(2):317–323. doi: 10.1042/bj2140317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyfeler F., Fasel P., Walter P. Short-term stimulation of net glycogen production by insulin in rat hepatocytes. Biochim Biophys Acta. 1981 Jun 11;675(1):17–23. doi: 10.1016/0304-4165(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Plomp P. J., Boon L., Caro L. H., van Woekom G. M., Meijer A. J. Stimulation of glycogen synthesis in hepatocytes by added amino acids is related to the total intracellular content of amino acids. Eur J Biochem. 1990 Jul 20;191(1):237–243. doi: 10.1111/j.1432-1033.1990.tb19115.x. [DOI] [PubMed] [Google Scholar]
- Vanstapel F., Waebens M., Van Hecke P., Decanniere C., Stalmans W. Modulation of maximal glycogenolysis in perfused rat liver by adenosine and ATP. Biochem J. 1991 Aug 1;277(Pt 3):597–602. doi: 10.1042/bj2770597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALAAS O., WALAAS E. Effect of epinephrine on rat diaphragm. J Biol Chem. 1950 Dec;187(2):769–776. [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley D. N. Binucleation in mammalian liver. Studies on the control of cytokinesis in vivo. Exp Cell Res. 1972 Oct;74(2):455–465. doi: 10.1016/0014-4827(72)90401-6. [DOI] [PubMed] [Google Scholar]
- Yamanouchi K., Stephens T. W., Chikada K., Dominianni S. J., Behforouz H., Scislowski P., DePaoli-Roach A., Allmann D. W., Harris R. A. Metabolic effects of proglycosyn. Arch Biochem Biophys. 1992 May 1;294(2):609–615. doi: 10.1016/0003-9861(92)90732-c. [DOI] [PubMed] [Google Scholar]


