Abstract
Arterial spin labeling (ASL) is a non-invasive magnetic resonance imaging (MRI) method for the assessment of cerebral blood flow (CBF). This review summarizes recent ASL-based investigations in adult and pediatric patients with migraine with aura, migraine without aura, and chronic migraine. A systematic search according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was conducted within PubMed and reference sections of articles identified from April 2014 to November 2022. Out of 236 initial articles, 20 remained after filtering, encompassing data from 1155 subjects in total. Cross-sectional studies in adults showed inconsistent results, while longitudinal studies demonstrated that cerebral perfusion changes over the migraine cycle can be tracked using ASL. The most consistent findings were observed in ictal states among pediatric migraine patients, where studies showed hypoperfusion matching aura symptoms during early imaging followed by hyperperfusion. Overall, ASL is a useful but currently underutilized modality for evaluating cerebral perfusion in patients with migraine. The generalizability of results is currently limited by heterogeneities regarding study design and documentation of clinical variables (e.g., relation of attacks to scanning timepoint, migraine subtypes). Future MRI studies should consider augmenting imaging protocols with ASL to further elucidate perfusion dynamics in migraine.
Keywords: Arterial spin labeling, cerebral blood flow, headache, magnetic resonance imaging, perfusion
Introduction
The current International Classification of Headache Disorders (ICHD) describes migraine as a highly prevalent primary headache disorder. 1 Current estimates indicate that more than one billion patients are suffering from migraine worldwide, rendering it one of the most prevalent diseases overall.2 –4 This makes migraine one of the top contributors to global disability by accounting for roughly 1.9% of disability-adjusted life years. 4 Despite the resulting need to better understand the mechanisms underlying migraine, migraine pathophysiology remains insufficiently understood.
A variety of neuroimaging modalities have been employed in the investigation of migraine.5,6 A preeminent role in this field has been occupied by magnetic resonance imaging (MRI) due to its non-invasive and multi-parametric imaging capabilities.5,6 In this context, both structural imaging investigating white matter (WM) lesions, 7 glymphatic system function, 8 WM streamlines, 9 or muscular pathologies, 10 as well as functional imaging based on the blood oxygen level-dependent (BOLD) effect11,12 have been utilized in migraine patients.
However, the results have only partially contributed to an improved understanding of the disease. Reasons for this include the heterogeneity of pathophysiology, symptoms, and imaging characteristics regarding migraine (e.g., different subtypes including migraine with aura [MwA], migraine without aura [MwoA], chronic migraine [CM], or different lateralization patterns, combined with oftentimes inconsistent imaging intervals in relation to the attack cycle). Additional complications arise from a lack of reproduction by studies investigating representative cohort sizes with homogeneous migraine characteristics.11,12
Convergent evidence implicates trigeminovascular mechanisms in migraine pathophysiology. 13 Neurogenic inflammation with vasodilation of meningeal vessels can cause perfusion abnormalities and contributes to the characteristic migraine-related pain.13 –17 Additionally, both the involvement of central pain pathways as well as phenomena such as cortical spreading depolarization (CSD) likely result in changes of brain perfusion patterns in migraine patients.13,18 Migraine patients have also been shown to be subject to an increased risk for cerebrovascular diseases such as ischemic19,20 or hemorrhagic stroke, 20 as well as subclinical WM hyperintensities (WMH) as potential correlates of micro-vascular pathology.7,21 While cerebral blood flow (CBF) can also be influenced by other factors such as blood pressure, blood oxygenation, or carbon dioxide levels, 22 the abovementioned findings motivate the use of perfusion imaging in investigations in migraine. Recent advantages have enabled the progressive adoption of perfusion MRI using arterial spin labeling (ASL) in scientific and clinical neuroimaging.23,24 Specifically, ASL is a non-invasive method for assessing CBF without the need for injection of contrast media, radioactive tracers, or ionizing radiation. 24 Considering its range of applicability and increasing use in migraine, the present review aimed to summarize the current literature landscape of ASL applications to yield an overview of current strengths and weaknesses of the method. We introduce technical aspects of ASL, highlight relevant applications in investigations of migraine, and put a special focus on findings that have been replicated across studies. Herein, we section the reviewed studies primarily according to whether scans were conducted in adult or pediatric cohorts, and, secondarily, whether scans were conducted longitudinally or cross-sectionally in adult cohorts.
Methods
Technical overview
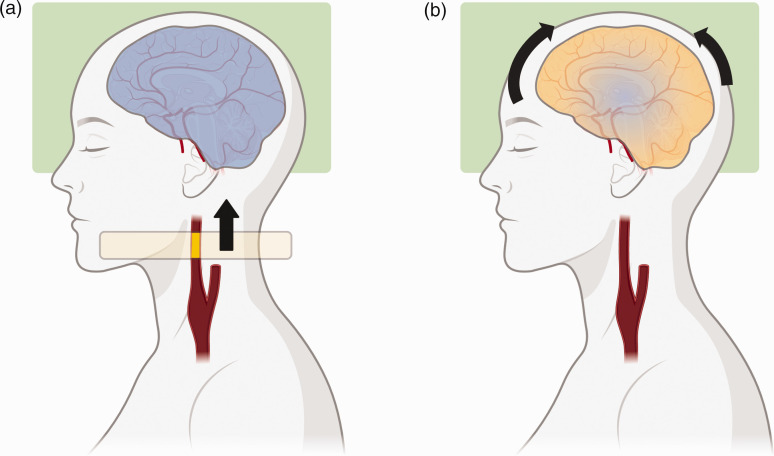
Perfusion imaging by ASL uses water within blood as an endogenous contrast tracer. The inflowing blood is magnetically labeled within the so-called labeling plane or labeling slab, usually placed onto a straight segment of the brain-feeding arteries to ensure maximum labeling efficiency. The labeled water molecules travel along the vessels to the intracranial space, where they change the equilibrium magnetization of brain tissue during perfusion (Figure 1). To account for the arterial transit time (ATT), i.e. the time blood takes to pass from the labeling to the imaging volume, the post-label delay (PLD) is introduced prior to image acquisition. Additionally, a control image is usually acquired, where no effective labeling is performed. By subtracting both images, perfusion data can be calculated and CBF quantified (in ml/100 g/min). Recommendations for setting up ASL sequences have been published recently.25,26
Figure 1.
Concept of pseudo-continuous arterial spin labeling (pCASL). (a) Inflowing blood is magnetically labeled (indicated by the yellow section of the internal carotid artery [ICA]) when passing the labeling plane (light yellow) using a train of short radiofrequency pulses. The cerebral volume of interest (green box) is imaged, and the tissue signal is not influenced by any labeled blood (blue overlay). (b) As the now labeled blood continues to travel towards the brain to perfuse the tissue, the tissue magnetization is altered (blue/yellow overlay). The transit time is considered by introducing a post-label delay (PLD) prior to imaging. By subtracting from the prior image with no effective labeling (a), perfusion can by quantified in terms of cerebral blood flow (CBF). Figure created with BioRender.com.
Historically, there have been mainly two approaches for labeling: continuous ASL (CASL 27 ) and pulsed ASL (PASL 28 ). However, the state-of-the-art method is pseudo-continuous ASL (pCASL), proposed as a hybrid of both methods.29,30 This technique was introduced to achieve both general availability on most clinical MRI systems and sufficient signal intensities.
Search strategy
Our literature search was conducted according to a protocol previously registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42021238822, Supplementary Material 1), in compliance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards. 31 At conception, we intended to cover both BOLD functional MRI (fMRI) as well as ASL literature in migraine. Due to the high volume of BOLD fMRI studies compared to ASL, the review was split and the fMRI literature was summarized separately. 12
We searched the PubMed database (www.pubmed.ncbi.nlm.nih.gov), as well as reference sections of articles that passed our inclusion criteria. Initially, we covered articles published between April 2014 and January 2021. This was motivated by the initial fMRI part of the review, following up on a previous fMRI review that covered articles before April 2014. 32 In order not to miss relevant studies that have been published since January 2021, we further extended our literature search until November 2022. Additionally, we decided to also include pediatric studies after an initial screening of studies performed in adults only, and lowered the participant threshold for adult cohorts to 10 investigated participants during the review process as a modification to the initially registered protocol (CRD42021238822, Supplementary Material 1). Afterwards, the reference sections of the included articles were screened for additional studies that may have eluded our initial PubMed search. An overview of the literature selection is presented in Figure 2.
Figure 2.
Literature selection. This figure shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart for our literature search and demonstrates the number of studies excluded at different stages of the literature search procedure. Examples for exclusion reasons: article types outside the inclusion criteria (e.g., literature reviews, case reports); false topic, referring to articles not dealing with ASL investigations of migraine; too few participants. Literature search was conducted according to a previously registered protocol (International Prospective Register of Systematic Reviews [PROSPERO] database, CRD42021238822, Supplementary Material 1), with the following adaptions after the initial search: extension of surveyed timeframe from January 2021 to November 2022; inclusion of studies in pediatric cohorts; longitudinal studies with a threshold of a minimum of 10 instead of 15 investigated participants.
Data extraction
Data extraction was performed by SS, supervised by NS, and included the following characteristics: studied population and control groups (including sex distribution and potential dropouts), description of imaging-related task (if applicable), region of interest (ROI) selection, details regarding statistical processing, statistical tests underlying results, documentation of quality control measures, scan timing in relation to ictality, documentation of headache medication (whenever applicable), and main findings.
Additionally, we extracted potential sources of bias or heterogeneity, specifically whether patients were recruited consecutively or randomly, whether more than 90% of recruited patients were included in the analyses, whether basic MRI parameters were documented (i.e., static field strength, labeling duration, PLD, and readout method), and whether age, sex, hematocrit, volumetry, blood pressure, and CO2 were controlled or corrected for in each study (Table 1).
Table 1.
Documentation of selected quality criteria.
| First author | Consecutive or random patient sample? | 90% or more of enrolled patients analyzed? | Correction/Control: Sex | Correction/Control: Age | Correction/Control: Volumetry | Correction/Control: Hematocrit | Correction/Control: Blood Pressure | Correction/Control: CO2 | Essential MRI parameters a given? |
|---|---|---|---|---|---|---|---|---|---|
| Bai X 38 | N/S | Y | N | N | N | N | N | N | Y |
| Cadiot D 56 | Y | Y | N | N | N/S | N | N | N | Y |
| Chen Z 33 | N/S | Y | Y | Y | N | N | N | N | Y |
| Fu T 44 | N/S | Y | Y | Y | N | N | N | N | Y |
| Gil-Gouveia R 51 | N/S | Y | Y; self-control | Y; self-control | Y; self-control | N | N | N | Y |
| Hodkinson DJ 34 | N/S | Y | Y | Y | N | N | N | N | Y |
| Li X 41 | N/S | Y | Y | N | N | N | N | N | Y |
| Liu M 37 | N/S | Y | N | N | N | N | N | N | Y |
| Meylakh N 48 | N/S | Y | N | N | N | N | N | N | Y |
| Michels L 36 | N/S | N/S | Y | Y | N | N | N | N | Y |
| Nahman-Averbuch H 53 | N/S | N | Y | Y | N | N | N | N | N |
| Park S 45 | N/S | N | Y | Y | N | N | N | N | Y |
| Stankewitz A 49 | N/S | N | Y; self-control | Y; self-control | Y; self-control | N | N | N | Y |
| Uetani H 52 | Y | Y | N/A | N/A | N/A | N | N | N | Y |
| Xu Z-G 47 | N | Y | Y | Y | Y | N | N | N | Y |
| Younis S 50 | N | Y | Y; self-control | Y; self-control | Y; self-control | N | N b | Y | Y |
| Youssef AM 54 | N | N | Y | Y | Y | N | N | N | N |
| Zhang C 43 | N/S | Y | N/S | N/S | N | N | N | N | Y |
| Zhang D 35 | N/S | N | Y | Y | N | N | N | N | N |
| Zhang Q 42 | N/S | N | Y | Y | Y | N | N | N | Y |
Table 1 yields an overview of certain quality criteria and their documentation within the reviewed studies. N: no; N/A: not applicable; N/S: not specified; Y: yes.
Essential magnetic resonance imaging (MRI) parameters were defined as static field strength, labeling duration, post-label delay (PLD), and readout method (if applicable to the specific arterial spin labeling [ASL] method).
Monitored during scanning, but not used during analysis of ASL data.
Evidence synthesis
After data extraction, the evidence was synthesized and summarized in batches primarily sorted according to cohort age (adult versus pediatric cohorts), and secondarily according to study design (cross-sectional, longitudinal, combined with other cerebrovascular imaging, and other designs).
Results
After filtering 236 studies, 20 studies remained for the evidence synthesis. Studies included on average 58 ± 49 participants, with a median of 40 participants for a total of 1155 participants across all studies. Studied populations included MwoA in 13 studies, MwA in 8 studies, migraine without further specification in 1 study, CM in 5 studies, and menstrual-related migraine in 1 study.
In 12 studies, at least one ASL acquisition was conducted within the interictal interval, with that interval being subject to different definitions between studies (e.g., 72 hours prior to the scan – 24 hours after the scan versus 72 hours prior to the scan – no interval after the scan). In 4 studies, ASL was collected at least once during the migraine attack. Furthermore, 5 studies did not document the timing of their scans in relation to the migraine cycle. Medication intake was documented and controlled to different degrees. Specifically, 7 studies did not document migraine-related medication in any way.
All studies except one were conducted using a 3-Tesla MRI scanner. The remaining study was conducted on a 1.5-Tesla scanner. Furthermore, 4 studies employed PASL, while 16 studies employed pCASL.
ASL in adult cohorts
Perfusion in cross-sectional studies with scans at one time point within the migraine cycle
Cross-sectional designs have been used in a variety of migraine populations and study designs to investigate cerebral perfusion. The most commonly studied subgroup of migraine patients was MwoA.33 –36 An overview of cross-sectional studies with scans at one time point within the migraine cycle is given by Table 2.
Table 2.
Cross-sectional studies in adults.
| First Author | Year | Group 1 (F/M) | Group 2 (F/M) | Group 3 (F/M) | Group 4 (F/M) | Scan timing | Medication | Field strength & sequence details | Main findings |
|---|---|---|---|---|---|---|---|---|---|
| Chen Z 33 | 2018 | MwoA (11/4) | HC (11/4) | Interictal (3 days pre) | No preventative medication 3 months pre | 3 T pCASL; LD 1.5 s/PLD 1.5 s; 3D SR | - Heightened CBF in the left BA38 (lSTG) in MwoA compared to HC. - Correlated with HAMD scores in MwoA. - No other significant clusters, no correction for multiple comparisons. | ||
| Hodkinson DJ 34 | 2015 | MwoA (12/5) | HC (12/5) | Interictal (72 h pre, 24 h post) | No daily medication | 3 T pCASL; LD 1.5 s/PLD 1.3 s; 2 D EPI | - Higher CBF in bS1 and lM1 in MwoA compared to HC. - Correlated with AF. | ||
| Michels L 36 | 2019 | MwoA (4/1) | MwA (9/3) | HC (11/8) | Interictal (48 h pre, 48 h post) | Information provided, not controlled | 3 T pCASL; LD 1.65 s/PLD 1.53 s; 2 D EPI | - Heightened CBF in MwoA and MwA compared to HC within rV5. - Heigthened CBF in MwA within the rSTG compared to HC. | |
| Zhang D 35 | 2021 | MwoA (30/10) | HC (27/15) | Interictal (48 h pre, 48 h post) | No vasoactive medication 1 week pre | 3 T pCASL; LD 1.650 s/PLD 1.6 s; Readout n/s | - Heightened CBF of MwoA CBF in rOFC and rMFG compared to HC. - Lowered CBF in the CrbVerm compared to HC. - Reduced CBF FC of rOFC to rPut, lSFG, rCau as well as rAG in MwoA compared to HC. - Heightened CBF FC to lCalC in MwoA compared to HC. | ||
| Liu M 37 | 2022 | CM (13 total) | HC (15) | Interictal (otherwise unspecified) | No alcohol, nicotine, caffeine or “other substances” 12 h pre | 3 T pCASL; LD 1.5 s/PLD 1.5 s; 3D SR | - Reduced CBF of the lNucA in CM compared to HC. - Correlated with PI. | ||
| Bai X 38 | 2022 | CM (11/7) | HC (8/7) | NDPH (7/8) | No information | No information | 3 T pCASL; multi-LD/multi-PLD; 3D SR | - Heightened CBF in bvlTh in CM compared to HC. - Heightened aCBV within the lvlTh in CM compared to HC.- Heightened CBF and aCBV in multiple other brain regions of CM compared to NDPH. | |
| Li X 41 | 2021 | Menstrual-related migraine (14/0) | HC (15/0) | Interictal (3 days pre) | No preventative or acute medication | 3 T PASL; 3D TGSE | - Heightened CBF in the lIns and rSMA of Menstruation-related migraine patients compared to HC. - CBF in the rSMA correlated with DD. | ||
| Zhang Q 42 | 2017 | MwoA (45/15) | MwA (48/8) | HC(39/15) | No information | No information | 3 T pCASL; LD 1.125 s/PLD 1.2 s; 2D EPI | - Reduced global CBF in MwA patients with high WMH load compared to no or low WMH load. - No CBF differences between groups with varying WMH load in MwoA or HC. | |
| Zhang C 43 | 2018 | MwoA (43/15) | MwA (45/7) | HC (39/13) | No information | No information | 3 T pCASL; LD 1.125 s/PLD 1.2 s; 2D EPI | - Posterior cerebral artery CBF based on territory masks did not differ between HC, MwA and MwoA. | |
| Park S 45 | 2022 | MwA (8/3) | MwoA (43/4) | Interictal (otherwise unspecified) | No information | 3 T pCASL; LD 1.65 s/PLD 1.8 s; 3 D GRASE | - Lowered assortative coefficients in an ASL based FC graph analysis for MwA compared to MwoA. | ||
| Fu T 44 | 2022 | MwoA (45/11) | MwA (25/7) | Additional patients in testing sample (23/7) | HC (44 total) | Conflicting information (unspecified interictal per abstract, unspecified ictal per limitations) | No information | 3 T pCASL; LD 1.65 s/PLD 1.6 s; 3D readout | - CBF differences in 6 regions between MwA, MwoA and HC (SFG, PoCG, Crb, MFG, Th, mvOcCor). - MwoA/MwA SVM-classifier based on the CBF of the above regions achieved an AUC of 0.86 in an independent testing set. |
| Xu Z-G 47 | 2021 | Tin with CM or MwoA (15/5) | Tin without migraine (15/10) | Non-Tin (30/20) | Not during attack, otherwise unspecified | No information | 3 T pCASL; LD 1.65 s/PLD 2.0 s; 2 D EPI | - Decreased CBF within rSTG, bMFG, lSFG in Tin patients compared to non-Tin. - CBF in rSFG and rMFG was further lowered when Tin patients also had Migraine. |
Table 2 yields an overview over key parameters and findings of the reviewed cross-sectional studies in adults. Abbreviations: Arterial cerebral blood volume (aCBV); attack frequency (AF); angular gyrus (AG); arterial spin labeling (ASL); area under the curve (AUC); bilateral (b); Brodmann Area (BA); calcarine cortex (CalC); caudate nucleus (Cau); cerebral blood flow (CBF); chronic migraine (CM); cerebellar vermis (CrbVerm); disease duration (DD); dorsolateral (dl); echo planar imaging (EPI); functional connectivity (FC); gradient and spin echo (GRASE); Hamilton Rating Scale for Depression (HAMD); healthy controls (HC); hypothalamus (HyTh); insula (Ins); left (l); labeling duration (LD); primary motor cortex (M1); middle frontal gyrus (MFG); medioventral (mv); migraine with aura (MwA); migraine without aura (MwoA); new daily persistent headache (NDPH); nucleus accumbens (NucA); occipital cortex (OcCor); orbitofrontal cortex (OFC); pulsed arterial spin labeling (PASL); pseudo-continuous arterial spin labeling (pCASL); prefrontal cortex (PFC); pain intensity (PI); post-label delay (PLD); postcentral gyrus (PoCG); precentral gyrus (PrCG); putamen (Put); right (r); primary somatosensory cortex (S1); superior frontal gyrus (SFG); supplementary motor area (SMA); spiral readout (SR); superior temporal gyrus (STG); support vector machine (SVM); turbo-gradient spin echo (TGSE); thalamus (Th); tinnitus (Tin); visual motion area (V5); ventrolateral (vl); white-matter hyperintensities (WMH).
In one study, the authors reported increased CBF in the left superior temporal gyrus in MwoA patients during the interictal period compared to healthy controls (HC). 33 Furthermore, CBF within the left superior temporal gyrus was positively correlated with Hamilton Rating Scale for Depression (HAMD) scores. 33 However, the voxel-wise analysis between groups has not been corrected for multiple comparisons. 33 In another study of interictal MwoA, the authors did not replicate this finding but observed CBF increases in the bilateral primary somatosensory cortices and left primary motor cortex, which correlated positively with attack frequency. 34
In another approach, interictal MwoA compared to HC demonstrated heightened CBF in the right orbitofrontal gyrus and right middle frontal gyrus, lowered CBF in the cerebellar vermis, and significant correlations between right orbitofrontal cortex CBF and attack frequency as well as results from the Visual Light Sensitivity Questionnaire (VSLQ-8). 35 Additionally, CBF connectivity analyses were conducted by computing the correlation coefficient for CBF values of regions that differed in the between-group analysis and all other brain voxels. 35 High correlation coefficients were interpreted as demonstrating CBF connectivity. 35 The authors observed that in MwoA compared to HC, the right orbitofrontal gyrus demonstrated reduced CBF connectivity to the right putamen, left superior frontal gyrus, right caudate nucleus, as well as the right angular gyrus, contrasted by heightened CBF connectivity to the left calcarine cortex. 35 Another publication investigated perfusion differences between MwoA, MwA, and HC. 36 Interictally, a group with MwoA plus MwA and patients with only MwA demonstrated heightened CBF within the right visual motion area (V5) compared to HC. 36 Additionally, MwA compared to HC demonstrated hyperperfusion within the right superior temporal gyrus. 36
Another subgroup prevalent in the identified literature was CM.37,38 In one comparison between interictal CM and HC, patients with CM demonstrated reduced CBF of the left nucleus accumbens that correlated with pain intensity (PI). 37 In another study, CM compared to HC demonstrated heightened CBF in the bilateral ventral lateral thalamus. 38 Arterial cerebral blood volume (aCBV, the volume of labeled arterial blood measured within a specific voxel), calculated from CBF maps and ATT maps based on methodology previously employed in HC, 39 was higher in the left ventral lateral thalamus. 38 Additionally, CM patients were compared to a cohort of new daily persistent headache (NDPH), a subtype of primary headache disorders distinct from migraine but sharing some of its characteristics. 40 In this context, CM patients demonstrated heightened CBF and aCBV compared to NDPH in multiple brain regions (e.g., bilateral thalami, right orbitofrontal cortex, right amygdala, or right anterior cingulate cortex). 38 Most perfusion differences were right-hemispheric (15 right-hemispheric areas/19 total areas), which the authors speculate as being connected to hemispheric dominance. 38
One study investigated menstrual-related migraine patients. 41 Compared to HC, patients with interictal menstruation-related migraine demonstrated heightened CBF in the left insula and right supplementary motor area. 41 Disease duration correlated with CBF in the right supplementary motor area. 41
In one study, the authors investigated WMH load in migraine patients and cerebral perfusion. 42 The WMH burden was identified on T2-weighted fluid-attenuated inversion recovery (FLAIR) images as well as T1-weighted images. 42 For the assessment of WMH, the authors counted the number of lesions and sorted them in three categories by size, then multiplying the number of lesions with a factor dependent on the WMH size group. 42 The authors found a significant difference in global CBF between MwA patients with no or low WMH load and MwA patients with high WMH load, with the latter showing reduced CBF. 42 No significant differences were found in MwoA or HC. 42 Interestingly, WMH load differences between MwoA, MwA, and HC did not reach statistical significance, 42 thus contrasting previous literature that has identified migraine (especially MwA) as a potential risk factor for WMH. 7
Additionally, in a retrospective analysis, MwA patients demonstrated a significantly higher curvature of the basilar artery compared to HC as measured via time-of-flight magnetic resonance angiography (TOF-MRA), which correlated with attack frequency. 43 The authors discussed potential causal connections to migraine symptoms, such as enhanced endothelial shear stress, compression of surrounding structures, or the curvature resulting from migraine attacks or related medication (e.g., triptan intake). 43 However, CBF of the posterior cerebral artery territory did not significantly differ between groups. 43
Advanced statistical modeling has been applied to ASL data to create classifier models. 44 In a corresponding study, the authors first demonstrated differences in CBF in 6 regions between MwA, MwoA, and HC (superior frontal gyrus, postcentral gyrus, cerebellum, middle frontal gyrus, thalamus, and medioventral occipital cortex). 44 In a comparison of different MwoA/MwA classifiers based on the CBF of the abovementioned regions, a support vector machine trained on the above data achieved the maximum area under the curve (AUC) of 0.86 in an independent testing set for discriminating patients with MwoA and MwA. 44 Furthermore, graph theoretical connectivity analyses have been applied to ASL datasets. 45 In the corresponding study, the authors constructed brain connectivity matrices based on an automated anatomical labeling (AAL) template, defining connectivity via the correlation of CBF between different AAL regions. 45 From the resulting matrix, the authors extracted measures such as global efficiency, transitivity, or mean clustering coefficient for further analyses. 45 Herein, patients with MwA demonstrated significantly lowered assortative coefficients compared to MwoA. 45 The assortative coefficient measures represented the tendency of nodes within a given network to preferentially connect to similar nodes (e.g., high assortativity is present when nodes with many connections preferentially connect to other nodes with many connections). 46 Information flow within low-assortativity networks tended to be more easily disrupted. 45 Thus, the authors interpreted their results as hyperresponsiveness that is inherent in cerebral networks of MwA patients. 45
Lastly, one study investigated the relationship between migraine and tinnitus. 47 The authors demonstrated that tinnitus patients compared to patients without tinnitus showed decreased CBF within the right superior temporal gyrus, bilateral middle frontal gyrus, and left superior frontal gyrus. 47 Herein, CBF in the right superior frontal gyrus and right middle frontal gyrus was further lowered when the tinnitus patients also had migraine. 47 Additionally, the study reported associations between Headache Impact Test (HIT-6) scores and CBF in the right superior temporal gyrus, as well as for PI and CBF in the right middle frontal gyrus. 47
Perfusion in longitudinal studies with several scans throughout the migraine cycle
Some studies investigated cerebral perfusion dynamics at multiple time points within the migraine cycle.48 –51 An overview of longitudinal studies with more than one scan acquired throughout the migraine cycle is provided by Table 3.
Table 3.
Longitudinal studies in adults.
| First Author | Year | Group 1 (F/M) | Group 2 (F/M) | Group 3 (F/M) | Group 4 (F/M) | Scan timing | Medication | Field strength & sequence details | Main findings |
|---|---|---|---|---|---|---|---|---|---|
| Meylakh N 48 | 2020 | MwoA (16/7) | MwA (8/1) | CM (0/2) | HC (22/4) | Pre- (24 h before attack), post (72 h after attack) and interictal (>24 h before-, >72 h after attack) | Not controlled, detailed information given | 3 T pCASL; LD 1.65 s/PLD 1.6 s; 2D EPI | - Decreased CBF in rHyTh, RSC and lVisC of migraine patients compared to HC only pre-ictally. |
| Younis S 50 | 2021 | MwoA (24/2) | Interictal (48 h pre) | No daily medication | 3 T pCASL; LD 1.65 s/multi-PLD; 2D EPI | - Increased regional CBF in the ipsilateral dlPons (respective to the most painful side) during pharmaceutically triggered attacks in MwoA compared to Baseline. - No change in CBF for patients that did not develop migraine attacks after trigger application. | |||
| Gil-Gouveia R 51 | 2017 | MwoA (13/0) | Ictal (otherwise unspecified) and interictal (no attack 48 h pre) | No acute medication 12 h pre, no prolonged medication outside oral contraceptives | 3 T PASL; 2D EPI | - No difference in CBF in MwoA during spontaneous attacks versus interictal (48 h pre). | |||
| Stankewitz A 49 | 2021 | EM (11/1) | Scanned longitudinally over whole cycle | No preventative medication 6 months pre, no acute medication before ictal scan | 3 T pCASL; LD 1.65 s/PLD 1.7 s; 2D EPI | - Increasing perfusion in rNucA, rIns and rPrCG leading up to the migraine attack. |
Table 3 yields an overview over key parameters and findings of the reviewed longitudinal studies in adults. Abbreviations: arterial spin labeling (ASL); calcarine cortex (CalC); cerebral blood flow (CBF); chronic migraine (CM); dorsolateral (dl); echo planar imaging (EPI); gradient and spin echo (GRASE); healthy controls (HC); hypothalamus (HyTh); insula (Ins); left (l); labeling duration (LD); migraine with aura (MwA); migraine without aura (MwoA); nucleus accumbens (NucA); pulsed arterial spin labeling (PASL); pseudo-continuous arterial spin labeling (pCASL); post-label delay (PLD); precentral gyrus (PrCG); putamen (Put); right (r); restrosplenial cortex (RSC); visual cortex (VisC); ventrolateral (vl).
In one study recruiting mixed migraine subtypes (MwA, MwoA, and CM), patients were scanned at varying time points within their respective migraine cycle, resulting in 22 interictal scans, 7 scans immediately preceding headache attacks (<24 h before headache onset), and 13 scans shortly following a headache attack (<72 h after headache end). 48 Migraine patients’ CBF in the right hypothalamus, right retrosplenial cortex, and left visual areas was decreased compared to HC, but only <24 h before a migraine attack. 48
Scanning 12 migraine subjects on an ictal day and periodically acquiring images until the next attack, another study demonstrated progressive hyperperfusion in the right nucleus accumbens, right insula, and right precentral gyrus prior to the migraine attack. 49 This was interpreted as a sign of increasing sensitivity of the patient’s brain to sensory input. 49
Another study employed pharmaceutical migraine triggers (i.e., calcitonin gene-related peptide and sildenafil) to study provoked attacks in MwoA. 50 During these attacks, regional CBF increased in the ipsilateral dorsolateral pons (respective to the most painful side) compared to baseline. 50 However, CBF did not change in patients that did not develop migraine attacks after trigger application. 50
Contrasting those previous studies, one study including 13 patients with MwoA who were scanned during spontaneous attacks (4 patients scanned <5 h after headache onset; 9 patients scanned >5 h after headache onset; average time from onset to scanning: 16.2 ± 19.7 h) and interictally demonstrated no difference in CBF between the different states. 51
Perfusion studies in pediatric cohorts
Overall, studies in pediatric populations were rarer than studies in adults. An overview of the respective studies in pediatric cohorts is given by Table 4.52 –54
Table 4.
Pediatric studies.
| First Author | Year | Group 1 (F/M) | Group 2 (F/M) | Scan timing | Medication | Field strength & sequence details | Main findings |
|---|---|---|---|---|---|---|---|
| Uetani H 52 | 2018 | Migraine, not further specified (27/22) | Varied (<24 h after onset - > 7 days after onset) | Not controlled, some documentation is presented | 3 T PASL;3D GRASE | - Hypoperfusion in mostly occipital and parietal areas in 11 out of 49 patients.- Hypoperfusion in all patients scanned within 24 h of symptom onset. | |
| Cadiot D 56 | 2018 | MwA (8/9) | Varied (7 min – 5 h 32 min after onset) | No information | 1.5 T PASL; 2 D EPI | - Hypoperfusion in 94% of pediatric MwA patients scanned during the attack.- In 75% of patients with perfusion abnormalities, TOF-MRA revealed matching vasospasm.- In 93 % of patients, the hypoperfusion pattern matched aura lateralization. | |
| Nahman-Averbuch H 53 | 2020 | MwoA, MwA, CM (14 total) | Implied not ictal, otherwise unspecified. | No preventative medication 5 half-lives pre, no acute medication more than 3 times a week (not migraine specific) or 6 times a month (migraine specific) | 3 T pCASL;LD 1.65 s/PLD 1.8 s;readout n/s | - Higher perfusion in rOFC, dlPFC and vlPFC in pediatric migraine patients after CBT compared to before.- Lower perfusion in bCrb, which the authors note could be due to susceptibility artifacts.- Headache reduction following CBT correlated with perfusion of bilateral occipital areas. | |
| Youssef AM 54 | 2017 | Migraine (12/10) | HC (12/10) | Interictal (72 h pre, 24 h post) | No daily preventative medication, no other medication 4 h pre | 3 T pCASL;LD 1.48 s/PLD 1.3 s; readout n/s | - Heightened CBF within bS1 in migraine patients compared to HC.- rS1 CBF correlated with AF and cutaneous allodynia. |
Table 4 yields an overview over key parameters and findings of the reviewed studies in pediatric patients. Abbreviations: attack frequency (AF); arterial spin labeling (ASL); cerebral blood flow (CBF); cognitive behavioral therapy (CBT); chronic migraine (CM); cerebellum (Crb); dorsolateral (dl); echo planar imaging (EPI); gradient and spin echo (GRASE); healthy controls (HC); left (l); labeling duration (LD); middle frontal gyrus (MFG); medioventral (mv); migraine with aura (MwA); migraine without aura (MwoA); orbitofrontal cortex (OFC); pulsed arterial spin labeling (PASL); pseudo-continuous arterial spin labeling (pCASL); prefrontal cortex (PFC); post-label delay (PLD); right (r); primary somatosensory cortex (S1); superior frontal gyrus (SFG); time-of-flight magnetic resonance angiography (TOF-MRA); ventrolateral (vl).
In one study, ASL has been used to investigate effects of cognitive behavioral therapy (CBT). 53 The authors demonstrated higher brain perfusion in the right orbitofrontal cortex, dorsolateral prefrontal cortex, and ventrolateral prefrontal cortex in pediatric migraine patients (MwoA, MwA, and CM) after CBT (8 sessions about 45 minutes each, once per week) when compared to the condition before CBT (scanning one week before and after the CBT block with 8 sessions). 53 The authors also demonstrated lowered perfusion in the bilateral cerebellum, which they noted could be artificial due to susceptibility artifacts. 53 Additionally, an association between reduction in attack frequency following CBT and perfusion of bilateral occipital areas was observed. 53
Another study in pediatric migraine patients (subtype unspecified) replicated a finding previously observed in adult migraine patients. 54 Compared to HC, patients demonstrated heightened CBF in bilateral primary somatosensory cortices during the interictal period. 54 For the right primary somatosensory cortex, CBF was associated with attack frequency and cutaneous allodynia (assessed by a questionnaire derived from the Allodynia Symptom Questionnaire 55 ). 54
A more consistent pattern of ASL alterations was observed in ictal imaging of pediatric MwA.52,56 In one study, the authors reported hypoperfusion of mostly occipital and parietal areas in 11 out of 49 analyzed patients. 52 Notably, hypoperfusion was observed in all 9 patients that were scanned within 24 hours of symptom onset. 52 Additionally, visually assessed diffusion-weighted imaging (DWI) did not show any hyperintense signal alterations, which would be suggestive of cytotoxic edema (e.g., as a result of cerebral ischemia). 52
Adding further context to and replicating parts of this finding, another study in pediatric MwA patients retrospectively analyzed ASL imaging during migraine attacks. 56 The authors reported hypoperfusion in 16 out of 17 scans, with the hypoperfusion pattern visually matching the expected brain area based on aura neurological symptoms (e.g., right-sided motor deficit – alterations within the left hemisphere) in 15 of these cases, which was confirmed by CBF measurements. 56 In 75% of the patients with perfusion abnormalities, visually assessed TOF-MRA revealed vasospasms matching the areas of hypoperfusion (e.g., left-hemispheric hypoperfusion – vasospasm of the left middle cerebral artery). 56 Again, DWI did not show any alterations suggestive of diffusion restrictions within the brain. 56
Discussion
We conducted a review of 20 ASL studies in migraine (16 in adult populations, 4 in pediatric populations) published between April 2014 and November 2022. Overall, ASL has been used in a variety of study designs and migraine subgroups to investigate disease-associated phenomena of cerebral perfusion.
State of the literature
Overall, ASL has found application in a variety of settings and study designs. In cross-sectional designs, studies demonstrated a variety of altered perfusion patterns between different migraine subtypes and HC.33 –38,41 There was little overlap regarding the results of the individual studies, as well as little overlap with other known areas (e.g., parietal association areas, retrosplenial cortex, ectorhinal cortex) implicated in the perception of migraine pain (Figure 3). 57
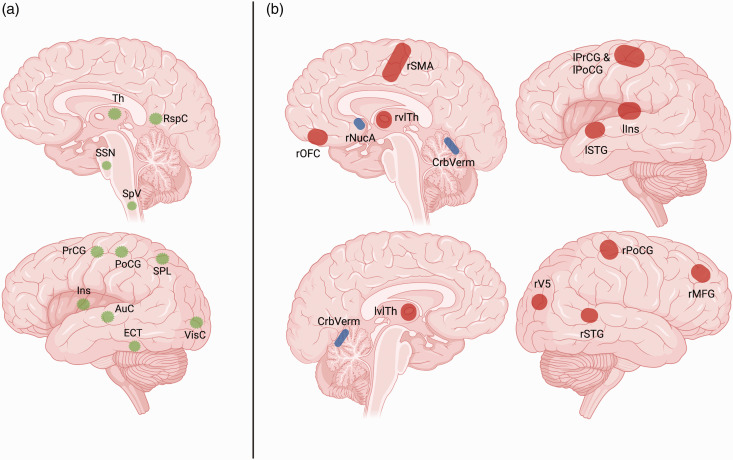
Figure 3.
Migraine pain matrix and cross-sectional perfusion changes in migraine patients compared to healthy controls (HC). This figure depicts areas involved in the perception of migraine pain as reported by Ashina 57 (a, green), vice versa the perfusion changes of migraine patients compared to HC are shown as reported in the reviewed literature (b, red & blue). Red markers indicate hyperperfusion, and blue markers indicate hypoperfusion in patients compared to HC. Data visualized in b is taken from the cross-sectional studies reviewed across all subtypes of migraine. Notably, while some overlap appears to exist (thalamus [Th], superior temporal gyrus [STG], insula [Ins], visual cortex [VisC], precentral gyrus [PrCG], and postcentral gyrus [PoCG]), the observed perfusion changes were mostly identified in different studies with no replication between different publications.
AuC: auditory cortex; CrbVerm: cerebellar vermis; ECT: ectorhinal cortex; MFG: middle frontal gyrus; NucA: nucleus accumbens; OFC: orbitofrontal cortex; RspC: retrosplenial cortex; r: right; SpV: spinal trigeminal nucleus; SPL: superior parietal lobule; SSN: superior salivatory nucleus; SMA: supplementary motor area; vl: ventrolateral; V5: visual motion area. Areas within the left hemisphere are labeled with initial letter “l”, areas within the right hemisphere are labeled with initial letter “r”. Figure created with BioRender.com.
One notable replicated finding was reported in two studies conducted by the same group, demonstrating heightened interictal CBF of bilateral primary somatosensory cortices in migraine patients compared to HC, one study recruiting adults and the other investigating pediatric and adolescent patients.34,54 Both studies also demonstrated associations between CBF of primary somatosensory cortices and attack frequency.34,54 Hyperresponsiveness of the somatosensory cortex in migraine patients has long been considered a common phenomenon in migraine, resulting from pathological habituation processes. 58 In this context, CBF increases could be considered an imaging-based correlate due to increased metabolic needs arising from hyperresponsiveness. However, more recent reports aiming to reduce study biases (e.g., by blinding assessing personnel) were unable to reproduce electrophysiological correlates of cortical hyperresponsiveness and thus called this hypothesis into question. 59 Interestingly, in stimulation paradigms, BOLD signal changes of somatosensory cortices were found reduced in response to painful stimuli in migraine and medication-overuse headache compared to HC, which was interpreted as a correlate of reduced analgesic activity.60 –62 It should however be reminded that stimulation-induced BOLD signal changes are fundamentally different from group differences in ASL signals, especially when no stimulation paradigm was employed.
The concept of hyperresponsiveness has also been used to explain the observation of migraine patients having an increased risk of developing WMH. 63 One recent study employed CO2 targeting to investigate the relationship between cerebrovascular reactivity and the presence of WMH. 63 The authors found that on a voxel-to-voxel basis, reduced cerebrovascular reactivity (CRV) correlated with an increased likelihood of WMH being present within the respective voxels. 63 This was explained as the result of a hyperresponsivity-induced higher baseline metabolic demand, leading to higher vulnerability during periods of metabolic stress such as related to CSD. 63 Higher resting CBF velocity of migraine patients in both anterior and posterior circulation was proposed in a recent systematic review. 18 However, the ASL results reviewed apparently conflict with this hypothesis, indicating that patients with higher WMH load exhibit reduced CBF. 42 One potential way to reconcile these findings would be to consider whether the observed hypoperfusion is in fact a consequence of decreased global metabolic demand due to progressive neuronal death and replacement via WMH, as the authors discussed in their work. 42
Other studies conducted measurements across multiple timepoints within the migraine cycle in longitudinal designs.48 –51 Longitudinal studies may allow for investigation and better control of potentially confounding variables inherent in a cyclical disease, specifically scan timing in reference to the migraine cycle. The observed results (e.g., failure to replicate certain CBF alterations48,49,51) appear partially conflicting, but reasons for these differences could be found within the underlying designs (e.g., continuous scanning of individuals versus acquisition of scans within different individuals at varying timepoints within the migraine cycle).46,47,49 Thus, while longitudinal designs hold promise in migraine specifically, future studies testing for reproducibility of the abovementioned findings are much needed.
In this context, one should also consider the possibility that inter-individual differences between patients in migraine-related perfusion alterations could be so particular so that they would not be necessarily observable on the group comparison level, but only longitudinally. This should be even more of a concern when conducting studies in cohorts of mixed subtypes of migraine (e.g., MwA, MwoA, or vestibular migraine), and again speaks to the importance of longitudinal measurements and their potential value over cross-sectional approaches. Naturally, findings on a group level would likely miss any processes with high spatial variance between individuals. These would likely only become visible in longitudinal imaging studies. Furthermore, this line of thought highlights the key importance of any generalizable, replicable and robust cross-sectional findings as pointing towards shared mechanisms in migraine.
More consistently replicated findings were found in pediatric migraine imaging.52,56,64 –66 Specifically, ASL has repeatedly demonstrated early-phase hypoperfusion matching the hemisphere of aura,52,56,64 –66 with unaltered DWI, 56 vasospasm-like phenomena in TOF-MRA, 56 and subsequent hyperperfusion.52,56 This replicates analogous findings from case series demonstrating similar perfusion alterations in smaller cohorts.64 –66 This time-dependent hypo-/hyperperfusion pattern could resemble the luxury perfusion seen in ischemic stroke, where tissue subject to transitory hypoperfusion is subsequently hyperperfused.67 –69 These findings might be especially interesting for clinicians, since collectively they describe a plausible mechanistic timeline for perfusion abnormalities during the migraine attack that may also relate to findings such as heightened risks for WMH. Taken together, the reviewed studies may imply cerebral hypoperfusion as one consistent component of pediatric migraine aura, fitting the concept of CSD as an important pathophysiological surrogate of aura (Figure 4).70,71
Figure 4.
Cortical spreading depolarization (CSD) and perfusion. This figure depicts a schematic representation of the presumed connection between CSD and perfusion. (a) CSD (right hemisphere, green) begins in occipital areas and spreads in rostral direction. (b) Following initial depolarization, hyperpolarization (yellow) sets in, accompanied by initial hypoperfusion (left hemisphere, blue). (c) Following initial hypoperfusion, a relative hyperperfusion analogous to postischemic luxury perfusion sets in (left hemisphere, gradient red). (d) In the subacute phase, hyperperfusion has supplanted the initial hypoperfusion (left hemisphere, red). Figure created with BioRender.com.
Extending our discussion to the adjacent modality of fMRI, some of the areas of interest observed in our ASL review (e.g., insula, brainstem nuclei, or central region) have been reported as showing various BOLD signal alterations compared to HC or other migraine subgroups. 12 For example, the insular cortex has demonstrated altered BOLD functional connectivity with a variety of other brain areas in fMRI, 12 which could plausibly be related to the hyperperfusion observed in some of the present ASL studies.41,49 Analogous conclusions could be drawn for observed ASL signal changes in the hypothalamus or thalamus.38,48 However, as recent reviews have pointed out, fMRI literature of migraine is generally subject to many limitations and partially contradictory findings, mostly due to methodological inconsistencies (e.g., group sizing, data preprocessing, statistical analyses) and resulting lack of replications.11,12 Therefore, it stands to reason whether the literature base of fMRI is at this time truly robust enough to directly relate any specific fMRI results to the ASL findings presented in this review.
Perspectives for future research
Overall, ASL may have a valuable role in investigating vascular mechanisms and pathologies in migraine related to alterations of meningeal vessels and phenomena of cerebral perfusion.13 –17 Current recommendations by the Perfusion Study Group of the International Society for Magnetic Resonance in Medicine (ISMRM) and the European Cooperation in Science and Technology (COST) action for ASL in Dementia may aid to harmonize ASL acquisition schemes, especially of pCASL-based imaging. 25 Technical advances may reveal additional information, which can be derived from innovations such as multi-PLD, vessel-selective ASL, velocity-selective ASL, or recent blood-brain barrier modeling approaches.26,69,72 –79 Such more advanced ASL-based techniques could aid the investigation of perfusion- and vessel-related phenomena in migraine by providing non-invasive access to data that previously may have required invasive procedures or may have not been delivered by any other imaging technique.
To generate generalizable findings, larger datasets are appealing, especially considering the rising prevalence of machine learning methodologies for functional data. 80 Data must however be available, adequately structured, preferably labeled, as well as comparable to other datasets. Tackling these challenges requires a multi-faceted approach, including data sharing,81 –83 data harmonization,84,85 data storage (e.g., using the Brain Imaging Data Structure [BIDS]),86,87 and data analyses (e.g., ExploreASL, ASLPrep, or Bayesian Inference for ASL MRI [BASIL]).88 –90
Regarding data labeling, migraine poses unique challenges compared to many other disorders. Specifically, migraine encompasses a variety of subtypes with different clinical phenotypes that likely correspond to different alterations demonstrable in neuroimaging.5,6,12 This necessitates precise documentation standards regarding cohort recruitment. Additionally, some of the reviewed studies indicated that cerebral perfusion is subject to considerable changes over the course of the migraine cycle.48,49 Currently, most cross-sectional studies referred to their scan timing (if documentation was present) as either “ictal” or “interictal”, with interval definitions varying between studies.34,36 While consistent nomenclature (e.g., by referring to prodrome and postdrome intervals as defined by the ICHD 1 ) would be one contributor to higher inter-study comparability, a more elegant solution could be found in a stronger focus on longitudinal study designs. Some of the reviewed studies modeled perfusion changes over the migraine cycle by scanning subjects at different time points within the migraine cycle.48,49 While these types of studies tend to require more resources than cross-sectional designs, they could potentially account more easily for inter-individual differences confounding cross-sectional designs.
Limitations
While we have searched both the Pubmed database and reference sections from the included publications, we have not searched other databases (e.g., Web of Science, Scopus or similar) and therefore cannot exclude the possibility of having missed isolated publications in our search process. Additionally, we have not attempted a meta-analysis of the underlying image data, both due to the technical issues inherent in attempts at synthesizing heterogeneous MRI data, as well as the underlying differences in study designs referred to above, both of which make data between different studies difficult to compare.
Conclusion
Perfusion imaging by ASL has seen both numerous validation studies and technical innovations contributing to its progressive adoption in research and clinical frameworks. Despite this, ASL applications in migraine studies appear sparse. In this context, it needs to be emphasized that this technique does not require intravenous contrast media, radioactive tracers, or ionizing radiation, making it especially appealing for longitudinal setups and pediatric cohorts. Newer developments such as vessel-selective perfusion mapping or time-resolved 4 D angiography have not yet found application within migraine imaging. However, primarily on the basis of pCASL in ictal imaging of pediatric MwA, evidence converges on a common pattern of perfusion alterations characterized by initial hypoperfusion shortly after symptom onset followed by subsequent hyperperfusion, which seems in line with the pathophysiological concept of CSD.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X241237733 for Perfusion imaging by arterial spin labeling in migraine: A literature review by Severin Schramm, Corinna Börner, Miriam Reichert, Gabriel Hoffmann, Stephan Kaczmarz, Michael Griessmair, Kirsten Jung, Maria T Berndt, Claus Zimmer, Thomas Baum, Florian Heinen, Michaela V Bonfert and Nico Sollmann in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work of NS is supported by the Dr.-Ing. Leonhard Lorenz Foundation, the German Migraine and Headache Society (DMKG), and the Joachim Herz Foundation. The work of MB is supported by the Bavarian Gender Equality Grant of the Free State of Bavaria, the German Migraine and Headache Society (DMKG), and the ZNS-Hannelore Kohl Stiftung. GH’s work is supported by a personal grant of the Ev. Studienwerk Villigst. FH received a grant “Innovationsfonds” of the joint federal committee of health insurance companies (GBA) for a nation wide study on an early multimodal intervention program for children with migraine. The Division of Pediatric Neurology and Developmental Medicine, Dr. von Hauner Children’s Hospital, LMU Hospital, Munich is provided by an emFieldPro magnetic stimulator by Zimmer MedizinSysteme GmbH (Neu-Ulm, Germany).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material: Supplemental material for this article is available online.
ORCID iDs: Gabriel Hoffmann https://orcid.org/0000-0002-5233-7532
Stephan Kaczmarz https://orcid.org/0000-0001-7694-7012
Maria T Berndt https://orcid.org/0000-0001-9124-231X
Nico Sollmann https://orcid.org/0000-0002-8120-2223
References
- 1.Olesen J. International classification of headache disorders. Lancet Neurol 2018; 17: 396–397. [DOI] [PubMed] [Google Scholar]
- 2.Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 2018; 17: 954–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020; 396: 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 2019; 18: 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans RW, Burch RC, Frishberg BM, et al. Neuroimaging for migraine: the American headache society systematic review and evidence‐based guideline. Headache 2020; 60: 318–336. [DOI] [PubMed] [Google Scholar]
- 6.Ellingson BM, Hesterman C, Johnston M, et al. Advanced imaging in the evaluation of migraine headaches. Neuroimaging Clin N Am 2019; 29: 301–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eikermann-Haerter K, Huang SY. White matter lesions in migraine. Am J Pathol 2021; 191: 1955–1962. [DOI] [PubMed] [Google Scholar]
- 8.Lee DA, Lee HJ, Park KM. Normal glymphatic system function in patients with migraine: a pilot study. Headache 2022; 62: 718–725. [DOI] [PubMed] [Google Scholar]
- 9.DeSouza DD, Woldeamanuel YW, Sanjanwala BM, et al. Altered structural brain network topology in chronic migraine. Brain Struct Funct 2020; 225: 161–172. [DOI] [PubMed] [Google Scholar]
- 10.Sollmann N, Mathonia N, Weidlich D, et al. Quantitative magnetic resonance imaging of the upper trapezius muscles – assessment of myofascial trigger points in patients with migraine. J Headache Pain 2019; 20: 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirill S, Degan D, Anastasia P, et al. Functional connectivity studies in migraine: what have we learned? J Headache Pain 2019; 20: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schramm S, Börner C, Reichert M, et al. Functional magnetic resonance imaging in migraine: a systematic review. Cephalalgia 2023; 43: 3331024221128278. [DOI] [PubMed] [Google Scholar]
- 13.Ashina M, Hansen JM, Do TP, et al. Migraine and the trigeminovascular system – 40 years and counting. Lancet Neurol 2019; 18: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason BN, Russo AF. Vascular contributions to migraine: time to revisit? Front Cell Neurosci 2018; 12: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol 2018; 17: 174–182. [DOI] [PubMed] [Google Scholar]
- 16.Goadsby PJ, Holland PR. An update: pathophysiology of migraine. Neurol Clin 2019; 37: 651–671. [DOI] [PubMed] [Google Scholar]
- 17.Bushnell MC, Čeko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013; 14: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzator JS, Howe PR, Wong RH. Profiling cerebrovascular function in migraine: a systematic review and meta-analysis. J Cereb Blood Flow Metab 2021; 41: 919–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Øie LR, Kurth T, Gulati S, et al. Migraine and risk of stroke. J Neurol Neurosurg Psychiatry 2020; 91: 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmoud AN, Mentias A, Elgendy AY, et al. Migraine and the risk of cardiovascular and cerebrovascular events: a meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open 2018; 8: e020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruit MC, van Buchem MA, Hofman PA, et al. Migraine as a risk factor for subclinical brain lesions. Jama 2004; 291: 427–434. [DOI] [PubMed] [Google Scholar]
- 22.Claassen JA, Thijssen DH, Panerai RB, et al. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev 2021; 101: 1487–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Telischak NA, Detre JA, Zaharchuk G. Arterial spin labeling MRI: clinical applications in the brain. J Magn Reson Imaging 2015; 41: 1165–1180. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Garcia L, Lahiri A, Schollenberger J. Recent progress in ASL. Neuroimage 2019; 187: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European Consortium for ASL in dementia. Magn Reson Med 2015; 73: 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindner T, Bolar DS, Achten E, et al. Current state and guidance on arterial spin labeling perfusion MRI in clinical neuroimaging. Magn Reson Med 2023; 89: 2024–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Detre JA, Leigh JS, Williams DS, et al. Perfusion imaging. Magn Reson Med 1992; 23: 37–45. [DOI] [PubMed] [Google Scholar]
- 28.Kim SG. Quantification of relative cerebral blood flow change by flow‐sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med 1995; 34: 293–301. [DOI] [PubMed] [Google Scholar]
- 29.Garcia D, Bazelaire C, Alsop D. Pseudo-continuous flow driven adiabatic inversion for arterial spin labeling. Proc Int Soc Magn Reson Med 2005; 13: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai W, Garcia D, De Bazelaire C, et al. Continuous flow‐driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 2008; 60: 1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021; 88: 105906. [DOI] [PubMed] [Google Scholar]
- 32.Schwedt TJ, Chiang C-C, Chong CD, et al. Functional MRI of migraine. Lancet Neurol 2015; 14: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z, Chen X, Liu M, et al. Evaluation of gray matter perfusion in episodic migraine using voxel-wise comparison of 3D pseudo-continuous arterial spin labeling. J Headache Pain 2018; 19: 36–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodkinson DJ, Veggeberg R, Wilcox SL, et al. Primary somatosensory cortices contain altered patterns of regional cerebral blood flow in the interictal phase of migraine. PLoS One 2015; 10: e0137971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang D, Huang X, Mao C, et al. Assessment of normalized cerebral blood flow and its connectivity with migraines without aura during interictal periods by arterial spin labeling. J Headache Pain 2021; 22: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michels L, Villanueva J, O'Gorman R, et al. Interictal hyperperfusion in the higher visual cortex in patients with episodic migraine. Headache 2019; 59: 1808–1820. [DOI] [PubMed] [Google Scholar]
- 37.Liu M, Sun Y, Li X, et al. Hypoperfusion in nucleus accumbens in chronic migraine using 3D pseudo-continuous arterial spin labeling imaging MRI. J Headache Pain 2022; 23: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai X, Wang W, Zhang X, et al. Cerebral perfusion variance in new daily persistent headache and chronic migraine: an arterial spin-labeled MR imaging study. J Headache Pain 2022; 23: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai W, Robson PM, Shankaranarayanan A, et al. Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn Reson Med 2012; 67: 1252–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamani N, Olesen J. New daily persistent headache: a systematic review on an enigmatic disorder. J Headache Pain 2019; 20: 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Khan A, Li Y, et al. Hyperconnection and hyperperfusion of overlapping brain regions in patients with menstrual-related migraine: a multimodal neuroimaging study. Neuroradiology 2021; 63: 741–749. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q, Datta R, Detre JA, et al. White matter lesion burden in migraine with aura may be associated with reduced cerebral blood flow. Cephalalgia 2017; 37: 517–524. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C, Detre JA, Kasner SE, et al. Basilar artery lateral displacement may be associated with migraine with aura. Front Neurol 2018; 9: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu T, Liu L, Huang X, et al. Cerebral blood flow alterations in migraine patients with and without aura: an arterial spin labeling study. J Headache Pain 2022; 23: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park S, Lee DA, Lee HJ, et al. Brain networks in migraine with and without aura: an exploratory arterial spin labeling MRI study. Acta Neurol Scand 2022; 145: 208–214. [DOI] [PubMed] [Google Scholar]
- 46.Newman ME. Assortative mixing in networks. Phys Rev Lett 2002; 89: 208701. [DOI] [PubMed] [Google Scholar]
- 47.Xu Z-G, Xu J-J, Chen Y-C, et al. Aberrant cerebral blood flow in tinnitus patients with migraine: a perfusion functional MRI study. J Headache Pain 2021; 22: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meylakh N, Marciszewski KK, Di Pietro F, et al. Altered regional cerebral blood flow and hypothalamic connectivity immediately prior to a migraine headache. Cephalalgia 2020; 40: 448–460. [DOI] [PubMed] [Google Scholar]
- 49.Stankewitz A, Keidel L, Rehm M, et al. Migraine attacks as a result of hypothalamic loss of control. Neuroimage Clin 2021; 32: 102784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Younis S, Christensen CE, Vestergaard MB, et al. Glutamate levels and perfusion in pons during migraine attacks: a 3T MRI study using proton spectroscopy and arterial spin labeling. J Cereb Blood Flow Metab 2021; 41: 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gil-Gouveia R, Pinto J, Figueiredo P, et al. An arterial spin labeling MRI perfusion study of migraine without aura attacks. Front Neurol 2017; 8: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uetani H, Kitajima M, Sugahara T, et al. Perfusion abnormality on three-dimensional arterial spin labeling with a 3T MR system in pediatric and adolescent patients with migraine. J Neurol Sci 2018; 395: 41–46. [DOI] [PubMed] [Google Scholar]
- 53.Nahman‐Averbuch H, Schneider VJ, Chamberlin LA, et al. Alterations in brain function after cognitive behavioral therapy for migraine in children and adolescents. Headache 2020; 60: 1165–1182. [DOI] [PubMed] [Google Scholar]
- 54.Youssef AM, Ludwick A, Wilcox SL, et al. In child and adult migraineurs the somatosensory cortex stands out… again: an arterial spin labeling investigation. Hum Brain Mapp 2017; 38: 4078–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol 2008; 63: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cadiot D, Longuet R, Bruneau B, et al. Magnetic resonance imaging in children presenting migraine with aura: association of hypoperfusion detected by arterial spin labelling and vasospasm on MR angiography findings. Cephalalgia 2018; 38: 949–958. [DOI] [PubMed] [Google Scholar]
- 57.Ashina M. Migraine. N Engl J Med 2020; 383: 1866–1876. [DOI] [PubMed] [Google Scholar]
- 58.Coppola G, Pierelli F, Schoenen J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia 2007; 27: 1427–1439. [DOI] [PubMed] [Google Scholar]
- 59.Omland PM, Uglem M, Hagen K, et al. Visual evoked potentials in migraine: is the “neurophysiological hallmark” concept still valid? Clin Neurophysiol 2016; 127: 810–816. [DOI] [PubMed] [Google Scholar]
- 60.Russo A, Tessitore A, Esposito F, et al. Pain processing in patients with migraine: an event-related fMRI study during trigeminal nociceptive stimulation. J Neurol 2012; 259: 1903–1912. [DOI] [PubMed] [Google Scholar]
- 61.Ferraro S, Grazzi L, Mandelli ML, et al. Pain processing in medication overuse headache: a functional magnetic resonance imaging (fMRI) study. Pain Med 2012; 13: 255–262. [DOI] [PubMed] [Google Scholar]
- 62.Tedeschi G, Russo A, Conte F, et al. Increased interictal visual network connectivity in patients with migraine with aura. Cephalalgia 2016; 36: 139–147. [DOI] [PubMed] [Google Scholar]
- 63.Lee MJ, Park B-y, Cho S, et al. Cerebrovascular reactivity and deep white matter hyperintensities in migraine: a prospective CO2 targeting study. J Cereb Blood Flow Metab 2022; 42: 1879–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cobb-Pitstick K, Munjal N, Safier R, et al. Time course of cerebral perfusion changes in children with migraine with aura mimicking stroke. Am J Neuroradiol 2018; 39: 1751–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boulouis G, Shotar E, Dangouloff‐Ros V, et al. Magnetic resonance imaging arterial‐spin‐labelling perfusion alterations in childhood migraine with atypical aura: a case–control study. Dev Med Child Neurol 2016; 58: 965–969. [DOI] [PubMed] [Google Scholar]
- 66.Kim S, Kang M, Choi S. A case report of sporadic hemiplegic migraine associated cerebral hypoperfusion: comparison of arterial spin labeling and dynamic susceptibility contrast perfusion MR imaging. Eur J Pediatr 2016; 175: 295–298. [DOI] [PubMed] [Google Scholar]
- 67.Shimonaga K, Matsushige T, Hosogai M, et al. Hyperperfusion after endovascular reperfusion therapy for acute ischemic stroke. J Stroke Cerebrovasc Dis 2019; 28: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 68.Yu S, Liebeskind DS, Dua S, et al. Postischemic hyperperfusion on arterial spin labeled perfusion MRI is linked to hemorrhagic transformation in stroke. J Cereb Blood Flow Metab 2015; 35: 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Togao O, Obara M, Helle M, et al. Vessel-selective 4D-MR angiography using super-selective pseudo-continuous arterial spin labeling may be a useful tool for assessing brain AVM hemodynamics. Eur Radiol 2020; 30: 6452–6463. [DOI] [PubMed] [Google Scholar]
- 70.Carneiro-Nascimento S, Levy D. Cortical spreading depression and meningeal nociception. Neurobiol Pain 2022; 11: 100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olesen J, Burstein R, Ashina M, et al. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol 2009; 8: 679–690. [DOI] [PubMed] [Google Scholar]
- 72.Obara M, Togao O, Beck GM, et al. Non‐contrast enhanced 4D intracranial MR angiography based on pseudo‐continuous arterial spin labeling with the keyhole and view‐sharing technique. Magn Reson Med 2018; 80: 719–725. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki Y, Fujima N, van Osch MJ. Intracranial 3D and 4D MR angiography using arterial spin labeling: technical considerations. Magn Reson Med Sci 2020; 19: 294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sollmann N, Hoffmann G, Schramm S, et al. Arterial spin labeling (ASL) in neuroradiological diagnostics – methodological overview and use cases. In: RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren 2024; 196: 36–51. [DOI] [PubMed]
- 75.Hernandez Petzsche MR, Reichert M, Hoffmann G, et al. Non-invasive perfusion territory quantification and time-resolved angiography by arterial spin labeling in a patient with a large right-hemispheric AVM: case report. J Neurol 2022; 269: 4539–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaczmarz S, Reichert M, Petzsche MRH, et al. Clinical application of ASL-based non-invasive perfusion territory mapping and time-resolved angiography in cerebrovascular diseases. Ismrm & smrt. Online Conference, 2021. https://archive.ismrm.org/2021/0871.html (accessed 5 March 2024).
- 77.Qin Q, Alsop DC, Bolar DS, et al. Velocity‐selective arterial spin labeling perfusion MRI: a review of the state of the art and recommendations for clinical implementation. Magn Reson Med 2022; 88: 1528–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahroo A, Buck MA, Huber J, et al. Robust Multi-TE ASL-based blood–brain barrier integrity measurements. Front Neurosci 2021; 15: 719676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahn H-S, Park J, Sohn CH, et al. Quantification of blood-brain barrier water permeability and arterial blood volume with multi-slice multi-delay diffusion-weighted ASL. ISMRM SMRT Annu Meet Exhib 2021; 470. [Google Scholar]
- 80.Khosla M, Jamison K, Ngo GH, et al. Machine learning in resting-state fMRI analysis. Magn Reson Imaging 2019; 64: 101–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poldrack RA, Baker CI, Durnez J, et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 2017; 18: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Etkin A. A reckoning and research agenda for neuroimaging in psychiatry. Am J Psychiatry 2019; 176: 507–511. [DOI] [PubMed] [Google Scholar]
- 83.White T, Blok E, Calhoun VD. Data sharing and privacy issues in neuroimaging research: opportunities, obstacles, challenges, and monsters under the bed. Hum Brain Mapp 2022; 43: 278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karayumak SC, Bouix S, Ning L, et al. Retrospective harmonization of multi-site diffusion MRI data acquired with different acquisition parameters. Neuroimage 2019; 184: 180–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mirzaalian H, Ning L, Savadjiev P, et al. Multi-site harmonization of diffusion MRI data in a registration framework. Brain Imaging Behav 2018; 12: 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clement P, Petr J, Dijsselhof MB, et al. A beginner's guide to arterial spin labeling (asl) image processing. Front Radiol 2022; 2: 929533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gorgolewski KJ, Auer T, Calhoun VD, et al. The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci Data 2016; 3: 160044–160049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mutsaerts HJ, Petr J, Groot P, et al. ExploreASL: an image processing pipeline for multi-center ASL perfusion MRI studies. Neuroimage 2020; 219: 117031. [DOI] [PubMed] [Google Scholar]
- 89.Adebimpe A, Bertolero M, Dolui S, et al. ASLPrep: a platform for processing of arterial spin labeled MRI and quantification of regional brain perfusion. Nat Methods 2022; 19: 683–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chappell MA, Groves AR, Whitcher B, et al. Variational Bayesian inference for a nonlinear forward model. IEEE Trans Signal Process 2009; 57: 223–236. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X241237733 for Perfusion imaging by arterial spin labeling in migraine: A literature review by Severin Schramm, Corinna Börner, Miriam Reichert, Gabriel Hoffmann, Stephan Kaczmarz, Michael Griessmair, Kirsten Jung, Maria T Berndt, Claus Zimmer, Thomas Baum, Florian Heinen, Michaela V Bonfert and Nico Sollmann in Journal of Cerebral Blood Flow & Metabolism