Abstract
The human brain undergoes rapid development during the first years of life. Beginning in utero, a wide array of biological, social, and environmental factors can have lasting impacts on brain structure and function. To understand how prenatal and early life experiences alter neurodevelopmental trajectories and shape health outcomes, several NIH Institutes, Centers, and Offices collaborated to support and launch the HEALthy Brain and Child Development (HBCD) Study. The HBCD Study is a multi-site prospective longitudinal cohort study, that will examine human brain, cognitive, behavioral, social, and emotional development beginning prenatally and planned through early childhood. Influenced by the success of the ongoing Adolescent Brain Cognitive DevelopmentSM Study (ABCD Study®) and in partnership with the NIH Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, the HBCD Study aims to establish a diverse cohort of over 7000 pregnant participants to understand how early life experiences, including prenatal exposure to addictive substances and adverse social environments as well as their interactions with an individual’s genes, can affect neurodevelopmental trajectories and outcomes. Knowledge gained from the HBCD Study will help identify targets for early interventions and inform policies that promote resilience and mitigate the neurodevelopmental effects of adverse childhood experiences and environments.
Keywords: HBCD, Brain development, Neuroimaging, Longitudinal, Prenatal substance use, Social determinants of health
1. Introduction
The brain undergoes rapid development from early gestation through childhood, and that development continues through adolescence and into young adulthood, supporting cognitive and emotional maturation. This early rapid growth represents a highly vulnerable period when a variety of biological, social, and environmental factors influence physical growth and brain development and exert a large and potentially enduring impact on developmental trajectories and future health outcomes. Factors affecting development can include substance exposures, emotional and physical neglect, pollutants and toxins, neighborhood disadvantage, structural racism, education quality, access to healthcare, among many others, and often these factors are interrelated.
Thus far, it has been difficult to disentangle the effects of different early exposures and influences, as well as their interactions with each other or with the genome. Our understanding has been limited by the paucity of research on normative brain development from birth through adolescence across diverse social and environmental contexts. A large prospective study of pregnant participants and their offspring followed through early childhood could dramatically advance the science and have immediate and substantial implications for healthcare practice and policy.
The HEALthy Brain and Child Development (HBCD) Study was conceived to address this need through a nationwide multi-site, longitudinal, prospective study that aims to enroll over 7000 pregnant participants and follow them, along with their children, for up to ten years. The study is designed to address two main objectives. First, to provide a comprehensive characterization of early life brain development starting in the first month of life using neuroimaging and EEG, along with genetic, biological, behavioral, social, and environmental contextual assessments. Second, to identify and understand the developmental impacts of various risk and resilience factors, including substance or polysubstance exposure during pregnancy. The HBCD Study will recruit participants from diverse populations from across the country to determine variability in child development and identify factors that may be protective or disruptive.
This report elaborates on the scientific and policy rationale for this study, explains why the time is right for such a landmark collaborative project, and recounts the process of developing the scientific framework needed to guide its implementation, including tackling the study’s unique methodological and ethical challenges.
2. Scientific and policy rationale
Families, healthcare providers, policymakers, and researchers increasingly want to understand the implications of prenatal exposures for subsequent brain development and growth trajectories. We know that in utero, neonatal, and early childhood experiences, such as exposure to substances, have the potential to negatively affect child development in a variety of ways (Nunez et al., 2011, Peterson et al., 2020, Ross et al., 2015, Thompson et al., 2009).
It is equally or perhaps more important to identify prenatal and early life resilience factors, given the neuroplasticity during the first decade of life, to help guide and implement evidence-based policies and interventions that could have long-lasting and cumulative benefits. For example, environments rich with parental social support (Lähdepuro et al., 2023), good nutrition (Prado and Dewey, 2014), access to greenspace (McCormick, 2017), and participation in social welfare benefits and services (i.e., WIC, Venkataramani et al., 2022) lead to greater well-being during child, adolescent, and young adult development. Parenting interventions, such as prenatal and early-life home visits, have been shown to provide positive impacts on child cognitive outcomes, including school readiness, and brain development in populations residing in different regions of the United States (Bick et al., 2019, Brody et al., 2017, Ho et al., 2020, Michalopoulos et al., 2017). Characterization of a broad array of early-life experiences and exposures and childhood outcomes, mapped to neurodevelopmental trajectories, can help identify which resilience factors may be the most amenable to intervention to promote and advance healthy child development.
To effectively leverage knowledge gained about risk and resilience, we need to understand typical neurodevelopmental trajectories and how much they vary from person to person from birth through childhood. Detailed characterization of a diverse cohort and inclusion of participant populations that are commonly excluded from research is critical to this process. People of color are systematically underrepresented in research, and changes in recruitment and retention strategies as well as data analytic methods are needed to address exclusionary practices (Hansen et al., 2020, Ricard et al., 2023). Similarly, individuals with a history of substance use during pregnancy face myriad barriers to participating in research, including stigma and fear of legal repercussions (Beasley et al., 2020, Davis et al., 2019). Fully understanding the scope of variation in neurodevelopmental trajectories across diverse populations is needed to understand how prenatal and early life experiences can alter these trajectories to shape health outcomes.
The HBCD Study will include deep phenotyping of the parent-child dyad using multi-modal data collection (Fig. 1) across the first decade of life to provide the duration and scope necessary to detect potential impacts that may manifest as the child develops (See Nelson et al., this issue, for an overview of the HBCD Study protocol). Whereas other studies may have focused on specific child outcome domains, or investigated risk and resilience factors separately, the deep phenotyping of the HBCD Study will allow for researchers and policy makers to identify the impact of risk factors, including social and environmental determinants of health, in combination with mitigating factors, on a wide array of child outcomes, including neurodevelopmental trajectories and physical, behavioral, cognitive, and mental health. The longitudinal design of the HBCD Study, including brain imaging, will also enable investigators to study for the first time how risk and resilience factors influence an individual’s brain neurodevelopmental trajectory. Knowledge gained from these data may help identify targets for personalized early interventions and inform policies that promote resilience and mitigate detrimental effects of adverse childhood experiences and environments.
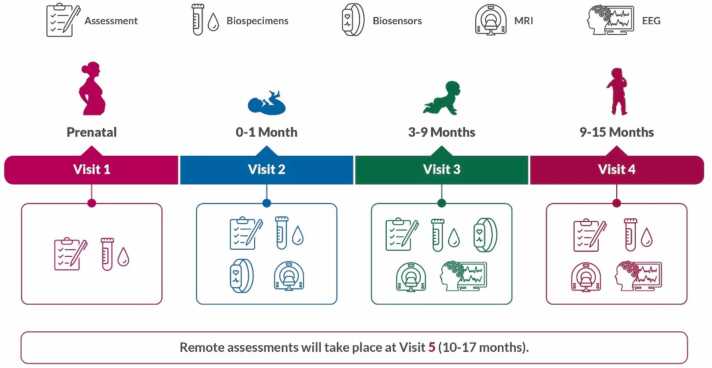
Fig. 1.
Overview of the HBCD Study protocol starting from the prenatal visit (Visit 1) to 17 months of age (Visit 5). The study protocol contains assessments across a wide range of domains (Nelson et al., this issue) including pregnancy exposures (e.g., substance use, mental health; Gurka et al., this issue), social and environmental determinants of health (Cioffredi, Yerby, et al., this issue), physical health (Cioffredi, Garner, et al., this issue), child behavior and child-caregiver relationships (Edwards et al., this issue), and neurocognition and language development (Kable et al., this issue). Biospecimens are collected from both the birth parent and child to identify biomarkers and classify exposures (Sullivan et al., this issue) and wearable biosensors calculate infant movement and heartbeat (Pini et al., this issue). Measures of brain structure and function begin with collection of MRIs starting in the first month of life (Dean et al., this issue) and EEGs starting at 3 months of age (Fox et al., this issue). Protocol development is ongoing for additional visits through 10 years of age.
Advances in neuroimaging, bioinformatics, and genomic sequencing (among others) will enable data collection and ongoing data sharing with the wider research community for hypothesis testing and exploratory data analyses. Similar to the success of the ABCD Study®, with over 1000 peer-reviewed publications to date, open access to HBCD Study data in annual data releases can greatly expand the range of questions and utility of the data in ways that we may not even be aware of at this time. Data generated from this study will be a long-term resource for studying neurodevelopment and a wide variety of positive and negative health outcomes.
3. Why now?
Ultimately, four key factors contributed to the decision to embark on the HBCD Study at this time. First, the increase of perinatal opioid use in the United States as well as the increasingly widespread use of alcohol and other substances, especially cannabis, during pregnancy have created an urgent need to understand the short- and long-term health outcomes of early exposures. Second, there is growing recognition of economic and social determinants of health and their influences on multiple developmental outcomes. Geocoded data and direct assessment now give us the ability to acquire detailed information about an individual’s dynamic environment, enabling us to identify modifiable social and economic factors for promoting healthy child development. Third, the successes of other nationwide, multi-site, multi-modal, longitudinal cohort studies that combine genetics, genomics, neuroimaging, and big data analytics, such as the ongoing Adolescent Brain Cognitive DevelopmentSM Study (ABCD Study®), have paved the way for large-scale data collection and harmonization with an open-science model of data release on an ongoing basis for timely analysis. Fourth, the confluence of support and interest among several NIH Institutes, Centers, and Offices (ICOs), including The Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, has facilitated the launch of such an ambitious study.
3.1. Substance use during pregnancy
The opioid crisis has created an urgent need to better understand the short- and longer-term impacts of perinatal opioid exposure. The number of individuals presenting with opioid use disorder (OUD) at labor and delivery increased fourfold between 1999 and 2014 (Haight et al., 2018), and the number of infants born with neonatal opioid withdrawal syndrome (NOWS) increased fivefold between 2004 and 2014 (Winkelman et al., 2018). Recent estimates indicate that approximately 8.8/1000 in-hospital births are affected by NOWS or neonatal abstinence syndrome (NAS; Leech et al., 2020).
The rising rates of other substance exposures during pregnancy are also worrisome. Reports of cannabis use during pregnancy nearly doubled between 2002 and 2017 (Volkow et al., 2019). This may be driven in part by perceptions that cannabis is a ‘natural’ remedy for nausea during pregnancy and that prenatal exposure does not negatively affect child health outcomes (Raifman et al., 2024). Stimulant use during pregnancy has also been rising over the last two decades (Smid et al., 2019, Young-Wolff et al., 2022), as has alcohol misuse. The prevalence of binge drinking during pregnancy increased by 8.9 % per year and heavy alcohol consumption increased by 11.6 % per year between 2011 and 2022 (Howard et al., 2022). According to 2022 data from the National Survey on Drug Use and Health (NSDUH), 11.0 % of pregnant 15- to 44-year olds in the United States used alcohol in the past month (Center for Behavioral Health Statistics, 2023), while other studies suggest that nearly 14 % (or 1 in 7) of pregnant 18- to 49-year olds report current drinking in the past 30 days (Gosdin et al., 2022). While recent reports from the Centers for Disease Control and Prevention (CDC) indicate that cigarette smoking during pregnancy declined by approximately one-third in the United States from 2016 to 2021 (Martin et al., 2023), use of e-cigarettes and other Electronic Nicotine Delivery Devices (sometimes used for smoking cessation) is increasingly common (Wen et al., 2023, Wen et al., 2023).
Many studies have documented the negative impact of alcohol on development in the context of fetal alcohol spectrum disorder, and other studies have documented the negative impacts of prenatal drug and environmental exposures on child health outcomes. New findings also suggest that tobacco use during pregnancy is associated with alterations in brain development in preadolescents (Zou et al., 2022). However, the relatively small sample sizes in most studies limit the ability to disentangle the many factors (including polysubstance use, mental illness, and environmental adversity) that may cause or contribute to the measured outcomes (Boggess and Risher, 2022, Metz et al., 2023, Nygaard et al., 2020, Yeoh et al., 2019). Large longitudinal studies are needed to answer the many open questions about the impacts of perinatal substance exposures.
The HBCD Study plans to enroll 25 % of its cohort, pregnant participants whose babies were exposed pre- or perinatally to substances (prescription and/or illicit opioids, cannabis, stimulants, alcohol, and tobacco/nicotine) and another 25 % will be those with comparable characteristics, who did not use substances during pregnancy (Si et al., this issue). The remaining 50 % will be from the general population, matched as closely as possible to the racial, ethnic, and socioeconomic demographics of the American Community Survey (ACS) for individuals of reproductive age, to allow for the careful assessment and measurement of the heterogeneity in child development. The cohort will be enriched for participants with substance exposures during pregnancy compared to the general population, which can range on average between 8 % and 11 % (Center for Behavioral Health Statistics, 2023). The social and environmental characteristics of participants who use substances during pregnancy are also expected to vary from that of the general population, thus the need for a “matched” sample to better understand how demographic factors and other determinants of health may impact associations between prenatal exposures and child health outcomes.
3.2. Economic and social determinants of health
A growing body of evidence suggests that economic disadvantage and racial inequalities, including discrimination and structural racism, childhood adversity, environmental toxins, occupational exposures can have dramatic effects on birth outcomes (Chantarat et al., 2022), and child health (Shonkoff et al., 2021, Trent et al., 2019), including brain development (Dumornay et al., 2023, Tooley et al., 2021). Determining how high-risk environments and experiences can influence child health and brain development is critical for identifying strategies that could mitigate adverse impacts. For example, adverse childhood experiences are a known risk factor for later psychopathology, acting on a variety of neurodevelopmental mechanisms, including threat-related social information processing, emotional reactivity and regulation, and reward processing, that could be targeted for novel interventions that optimize youth development (McLaughlin et al., 2019).
Environmental pollutants and toxins are often more prevalent in communities with greater socioeconomic disadvantage and in communities of color (Mikati et al., 2018); their impact can include reduced motor, cognitive, and language function and increased behavioral problems (Ni et al., 2022) among other outcomes. Similarly, racial and ethnically minoritized communities and groups that have been economically/socially marginalized are more likely to experience adverse occupational exposures (Asfaw, 2022, Landsbergis, 2010, Schnake-Mahl et al., 2021).
Underlying racial and socioeconomic inequities can also exacerbate the burden of substance use during pregnancy on already marginalized populations due to limited access to care and treatment, fear of criminal punishment or penalties, and other social barriers such as stigma (Volkow, 2023). The intersectionality, or the cumulative overlap, of these factors highlights the importance of directly investigating the associations of adverse environments, structural racism, and socioeconomic disadvantage with the development of both brain and behavior throughout early childhood. Recruitment sites for the HBCD Study are expected to engage diverse populations including participants from urban and rural communities, from varying racial, ethnic, and socioeconomic backgrounds to enable us to better understand such associations and to ensure that the findings from the study will be relevant to everyone.
3.3. Advances in technology and large-scale data including genetics and genomics
Until recently, we have lacked the technical ability to conduct large, prospective, longitudinal neuroimaging studies; to integrate neuroimaging, electrophysiological data (EEGs), genomics, and behavioral data; or to analyze the massive quantities of multi-modal data that would be necessary to understand normative development and the impacts of adverse exposures. Over the past decade, technological improvements have greatly increased our ability to conduct large-scale studies and harmonize data collected across multiple platforms.
The ABCD Study® was launched in September 2015 as the largest long-term study of brain development and child and adolescent health in the United States (Volkow et al., 2018). ABCD has successfully demonstrated the feasibility of conducting large multi-site neuroimaging studies starting at 9 or 10 years of age, including maintaining high retention rates (>90 % at 7 years) and leveraging the cohort to collect data related to unexpected events (e.g., COVID). Additionally, advances in genetics and genomics provide the basis for studying how genetic backgrounds influence brain development trajectories as a function of diverse settings as was demonstrated by the Pediatric Imaging, Neurocognition, and Genetics study (PING, Jernigan et al., 2016). Banked biospecimens collected from HBCD participants (birth parents and children) will allow researchers to assess the contribution of genetics, environmental exposures and other factors on neurodevelopment and child health. Similarly, advances in non-invasive neuroimaging, most notably in functional magnetic resonance imaging (fMRI) have facilitated safe and effective studies of the developing brain. Notably, the Baby Connectome Project (BCP, Howell et al., 2019) and the NIH MRI Study of Normal Brain Development (Evans, 2006), have shown that it is possible to address the limitations and difficulties inherent in scanning very young populations.
The field has advanced in terms of MRI protocols, processing pipelines, and the creation of standardized tools and atlases (Pollatou et al., 2022). Real-time in-scanner motion detection is possible using software such as FIRMM (framewise integrated real-time MRI monitoring), which allows for more efficient data acquisition and reduction of costs associated with repeat scanning sessions (Dosenbach et al., 2017). Equally importantly, advances have been made in our understanding of best practices that aid in high-quality MRI data acquisition in infants and young children, including extensive pre-visit planning with families, creating environments conducive to sleep during MRI scans, and the use of a mock scanner training environment to acclimate children before awake MRI scans (Howell et al., 2019, Raschle et al., 2012).
Similarly, other large-scale projects such as the Human Connectome Project (HCP, Van Essen et al., 2012); the UK Biobank (Sudlow et al., 2015); IMAGEN (Mascarell Maričić et al., 2020); and Environmental Impacts of Child Health Outcomes (ECHO, Knapp et al., 2023) have demonstrated the utility and importance of collecting (and releasing) multi-modal data. Advances in bioinformatics data platforms have resulted in increased capabilities of secure data storage, harmonization, and sharing that enable effective communication of detailed research findings, tools for data access and analysis, supporting documentation, and necessary training materials (Elam et al., 2021). Furthermore, these platforms can house data from various sources (i.e., MRI, EEG, wearable sensors, surveys, genetics, etc.), harmonize with existing large-scale neurodevelopmental research efforts, and interface with high-performance computing infrastructure, enabling researchers to easily access and analyze anonymized data with state-of-the-art analytical tools and pipelines.
3.4. Broad institutional interest and support
Even as the ABCD Study® was being planned in 2014/2015, it was clear that an impactful developmental window, beginning prenatally and lasting through birth and early childhood, was still being missed, and discussions had started among several NIH Institutes on the importance of such a study. When funds were allocated to the NIH HEAL Initiative®, the opportunity emerged to initiate a new cohort study beginning during pregnancy that would clarify the impact of opioid use during pregnancy on infant and child outcomes. Together the ABCD Study® and the HBCD Study will provide a rich and comprehensive set of complementary data on brain development from birth through young adulthood that is unparalleled in scope and depth.
The NIH HEAL Initiative aims to speed up the development and implementation of cross-cutting scientific solutions to the national opioid public health crisis, which is increasing mortality in all age groups, including individuals who are pregnant (Han et al., 2023). One of its strategic goals was to improve postpartum outcomes in individuals with OUD during the postpartum period, enhance treatments for infants with NAS/NOWS, and find the best approaches to address the medical and social needs of children living with a family member with OUD. An understanding of brain and cognitive development in infants exposed to opioids (and/or to other substances) is critical and will rely on a careful characterization of typical neurodevelopmental trajectories in diverse populations to enhance our understanding of how and when interventions may be necessary to yield optimal long-term health outcomes. Thus, the initial planning and establishment of the HBCD Study involved the partnership of several NIH Institutes, Centers, and Offices (ICs), which have invested in the HBCD Study, and are providing key contributions to the study’s development, design, and goals (see Table 1).
Table 1.
Broad HBCD Study Interest and Contributions from Multiple NIH Institutes.
| NIH Institute | Contributions and Interest in the HBCD Study | |
|---|---|---|
| National Institute on Drug Abuse | (NIDA) |
|
| National Institute of Mental Health | (NIMH) |
|
| National Cancer Institute | (NCI) |
|
| National Eye Institute | (NEI) |
|
| National Institute on Alcohol Abuse and Alcoholism | (NIAAA) |
|
| National Institute of Biomedical Imaging and Bioengineering | (NIBIB) |
|
| Eunice Kennedy Shriver Institute of Child Health and Human Development | (NICHD) |
|
| National Institute of Environmental Health Sciences | (NIEHS) |
|
| National Institute on Minority Health and Health Disparities | (NIMHD) |
|
| National Institute of Neurological Disorders and Stroke | (NINDS) |
|
| Office of Behavioral and Social Sciences Research | (OBSSR) |
|
| Office of Research on Women’s Health | (ORWH) |
|
4. Laying the groundwork for the HBCD Study
Early on, it was recognized that the HBCD Study would be a highly complex project, even more so than projects with other developmental cohorts such as the ABCD Study®. Since development occurs at a very rapid rate in the first few years of life, frequent assessments are required to fully characterize brain and behavioral growth. However, assessment frequency must be balanced with participant burden to ensure appropriate retention and data quality across the sample. Individuals with a history of substance use during pregnancy may face significant barriers to research that may be exacerbated by the demands of high-frequency time-intensive longitudinal assessments. Those at high risk for substance use during pregnancy may be living in particularly stressful or unstable home situations. They may be at risk of losing custody of their baby and/or subject to other punitive measures, depending on the policies and practices of the states where they live. Further, as an observational study, there are unique ethical concerns that must be addressed given that researchers will not be offering interventions as part of the study. At a minimum, pregnant participants must be given information about available treatments or services, if they desire assistance. Moreover, the importance of including an adequate control group of pregnant participants living in similar circumstances to those using substances is critical in order not to repeat the stigmatizing impacts of past research, such as when a nationwide fear of babies exposed to crack cocaine was greatly exaggerated and highly detrimental to those struggling with substance use during pregnancy and their children (Thompson et al., 2009).
Being cognizant of all of these factors, two expert panels were convened, focused on the ethics and legal considerations that were necessary to “do no harm” to study participants (NIDA, 2018a) https://archives.nida.nih.gov/meetings/2018/10/expert-panel-meeting-to-discuss-study-design-longitudinal-study-impact-prenatal-opioid-other, and the feasibility of the study, sampling considerations, and retention strategies (NIDA, 2018b) https://archives.nida.nih.gov/meetings/2018/09/research-methodologies-to-understand-long-term-consequences-prenatal-opioid-other-substance-exposure. Discussions from these panels identified a need to focus efforts on creating a study design with thorough considerations for the well-being of the participant dyads and special protections to help mitigate risks to participants. The complexity of the solutions needed to address these issues contributed to NIH’s decision to support a planning phase for HBCD, which was initiated in the Fall of 2018.
The planning phase involved the funding of 29 sites across the U.S., which sought to establish the feasibility of conducting such a study. As part of these efforts, investigators, staff, and colleagues formed five nationwide working groups with more than 18 subgroups in key domains, including neuroimaging and neurophysiology; study design; ethical and legal considerations; biospecimen collection; and maternal, neurodevelopmental, and contextual assessments. These workgroups met weekly or biweekly to discuss progress, identify challenges and brainstorm solutions, and address key questions. These national working groups revealed important considerations and nuances that informed the design of the full-scale HBCD Study, which are documented in a special issue of Adversity and Resilience Science, “Informing Longitudinal Studies on the Effects of Maternal Stress and Substance Use on Child Development: Planning for the HEALthy Brain and Child Development (HBCD) Study” (Jordan et al., 2020). Briefly, the special issue discussed the importance of establishing a study design (e.g., sampling methods, and appropriate controls) that minimizes the potential for stigmatizing conclusions that harm participants and their families (Singer et al., 2020), set guiding principles for the selection of neurodevelopmental assessments of risk and resilience (Morris et al., 2020), and identified best practices for engaging and retaining pregnant participants that are considered high-risk for substance use during pregnancy (Beasley et al., 2020). Two reports also outlined the framework for biospecimen collection, including special considerations for American Indian/ Alaskan Native populations (Bakhireva et al., 2020, Croff et al., 2020). Additional summary documents from the planning phase workgroups and meeting reports are available on the NIH HEAL website (https://heal.nih.gov/research/infants-and-children/healthy-brain).
The planning phase also prompted a workshop led by NIH staff and HBCD Study Investigators on “Engaging Child Welfare Systems in Research on Young Children” (https://heal.nih.gov/news/events/engaging-child-welfare-systems) to better understand how researchers can engage with local child welfare systems to promote study participant retention and wellbeing. Discussions surrounding these challenges are ongoing and will likely continue to evolve throughout the duration of the study.
5. Conclusion
The HBCD Study holds the potential to provide a profound and nuanced understanding of the many factors that affect a child’s health, brain, and behavioral development. The ongoing release of data to the wider research community, expected annually, will magnify its impact, advancing scientific methods and technologies, fostering the development of the scientific workforce, democratizing access to information, and enabling researchers to explore questions that were unimaginable during the study conception. The accumulated knowledge gained is expected to inform prevention science by identifying targets for early intervention, promoting resilience, and determining optimal neurodevelopmental windows for intervention implementation. Ultimately, we hope the results of this study will inform child-rearing practices, community support structures, and healthcare policies that nurture personal, interpersonal, and structural resilience and mitigate the developmental effects of adverse environments (Fordyce et al., 2017) for generations to come.
The launch of this study required extensive planning and effort, and its success hinges not only on the dedication and commitment of the NIH and extramural research staff but also on the contributions of numerous individuals, including research assistants, site monitors, study navigators, other recruitment and site staff, and community advisory boards. These individuals play crucial roles in maintaining participant engagement and motivation throughout the comprehensive and complex study protocol. We will depend heavily upon the community advisory boards to be our local ambassadors and to keep us focused on our participants’ needs and values. Above all, the study participants are of utmost importance as they allow the HBCD Study researchers to gain firsthand knowledge of their lives to develop the evidence base needed to inform policies and practices that we hope will promote health and well-being for all families and children.
CRediT authorship contribution statement
Bruce J. Tromberg: Writing – review & editing, Funding acquisition, Conceptualization. Christopher S. Sarampote: Writing – review & editing. Richard P. Woychik: Writing – review & editing, Funding acquisition, Conceptualization. Jane M. Simoni: Writing – review & editing, Funding acquisition, Conceptualization. Michelle P. Freund: Writing – review & editing, Project administration. Katherine M. Cole: Writing – review & editing, Writing – original draft, Project administration. Gayathri J. Dowling: Writing – review & editing, Project administration. Benjamin Xu: Writing – review & editing, Project administration. Julia L. Zehr: Writing – review & editing, Project administration. Rebecca Hommer: Writing – review & editing, Project administration. Erica L. Spotts: Writing – review & editing, Project administration. Traci M. Murray: Writing – review & editing, Project administration. Vani Pariyadath: Writing – review & editing, Project administration. Nora D. Volkow: Writing – review & editing, Funding acquisition, Conceptualization. Katia D. Howlett: Writing – review & editing, Writing – original draft, Project administration, Conceptualization. Chloe J. Jordan: Writing – review & editing, Writing – original draft, Project administration. Walter J. Koroshetz: Writing – review & editing, Funding acquisition, Conceptualization. Eliseo J. Pérez-Stable: Writing – review & editing, Funding acquisition, Conceptualization. William M. Klein: Writing – review & editing, Funding acquisition, Conceptualization. George F. Koob: Writing – review & editing, Funding acquisition, Conceptualization. Michael F. Chiang: Writing – review & editing, Funding acquisition, Conceptualization. Susan R.B. Weiss: Writing – review & editing, Writing – original draft, Project administration, Conceptualization. Janine A. Clayton: Writing – review & editing, Funding acquisition, Conceptualization. Janani Prabhakar: Writing – review & editing, Project administration. Joshua A. Gordon: Writing – review & editing, Funding acquisition, Conceptualization. Michele L. Rankin: Writing – review & editing, Project administration. Diana W. Bianchi: Writing – review & editing, Funding acquisition, Conceptualization.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. Julia L. Zehr reports a relationship with Jazz Pharmaceuticals Inc that includes: equity or stocks. Co-author’s spouse is employed by Jazz Pharmaceuticals, Inc - JLZ If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgement
The views and opinions expressed in this manuscript are those of the authors only and do not necessarily represent the views, official policy or position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies. The authors thank Dr. Eric Wargo for his valuable contributions to the writing and preparation of the manuscript.
References
- Asfaw A. Racial disparity in potential occupational exposure to Covid-19. J. Racial Ethn. Health Disparities. 2022;9(5):1726–1739. doi: 10.1007/s40615-021-01110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhireva L.N., Nebeker C., Ossorio P., Angal J., Thomason M.E., Croff J.M. Inclusion of American Indians and Alaskan Natives in large national studies: ethical considerations and implications for biospecimen collection in the HEALthy Brain and Child Development Study. Advers. Resil. Sci. 2020;1(4):285–294. doi: 10.1007/s42844-020-00020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley L.O., Ciciolla L., Jespersen J.E., Chiaf A.L., Schmidt M., Shreffler K.M., Breslin F.J., Bakhireva L.N., Sanjuan P.M., Stephen J.M., Coles C.D., Chambers C.D., Kable J.A., Leeman L., Singer L.T., Zellner J., Morris A.S., Croff J.M. Best practices for engaging pregnant and postpartum women at risk of substance use in longitudinal research studies: a qualitative examination of participant preferences. Advers. Resil. Sci. 2020;1(4):235–246. doi: 10.1007/s42844-020-00019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J., Palmwood E.N., Zajac L., Simons R., Dozier M. Early parenting intervention and adverse family environments affect neural function in middle childhood. Biol. Psychiatry. 2019;85(4):326–335. doi: 10.1016/j.biopsych.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggess T., Risher W.C. Clinical and basic research investigations into the long-term effects of prenatal opioid exposure on brain development. J. Neurosci. Res. 2022;100(1):396–409. doi: 10.1002/jnr.24642. [DOI] [PubMed] [Google Scholar]
- Brody G.H., Gray J.C., Yu T., Barton A.W., Beach S.R., Galván A., MacKillop J., Windle M., Chen E., Miller G.E., Sweet L.H. Protective prevention effects on the association of poverty with brain development. JAMA Pedia. 2017;171(1):46–52. doi: 10.1001/jamapediatrics.2016.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics. (2023). Results from the 2022 National Survey on Drug Use and Health: Detailed tables. https://www.samhsa.gov/data/report/2022-nsduh-detailed-tables. Retrieved from: 〈https://www.samhsa.gov/data/report/2022-nsduh-detailed-tables〉.
- Chantarat T., Van Riper D.C., Hardeman R.R. Multidimensional structural racism predicts birth outcomes for black and white Minnesotans. Health Serv. Res. 2022;57(3):448–457. doi: 10.1111/1475-6773.13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffredi, L., Garner, B., Maxwell, J.R., Merhar, S., Peralta-Carcelen, M., Scott, L.S., Sisodia, M., DeMauro, S.B., & HBCD Physical Health Working Group. (this issue). Infant and early childhood physical health assessments in the HEALthy Brain and Child Development (HBCD) Study. Developmental Cognitive Neuroscience. [DOI] [PMC free article] [PubMed]
- Cioffredi, L., Yerby, L.G., Burris, H.H., Cole, K.M., Engel, S.M., Murray, T.M., Slopen, N., Volk, H.E., Acheson, A., & HBCD Social and Environmental Determinants Working Group. (this issue). Assessing prenatal and early childhood social and environmental determinants of health in the HEALthy Brain and Child Development Study. Developmental Cognitive Neuroscience.
- Croff J.M., Bogdan R., Johnson S.B., Bakhireva L.N. Early environmental exposures and contaminants: a design framework for biospecimen collection and analysis for a prospective national birth cohort. Advers. Resil. Sci. 2020;1(4):269–283. doi: 10.1007/s42844-020-00024-4. [DOI] [Google Scholar]
- Davis J.M., Yao L., Bierer B.E. Protecting pregnant women with substance use disorders and their neonates participating in research. JAMA. 2019;322(7):609–610. doi: 10.1001/jama.2019.9002. [DOI] [PubMed] [Google Scholar]
- Dean, D.C., Tisdall, M.D., Wisnowski, J.L., Fezko, E., Gagoski, B., Alexandar, A.L., Edden, R.A.E., Gao, W., Hendrickson, T.J., Howell, B.R., Huang, H., Humphreys, K.L., Riggins, T., Sylvester, C.M., Weldon, K.B., Yacoub, E., Ahtam, B., Beck, N., Banerjee, S., … Elison, J.T. (this issue). Quantifying brain development in Quantifying Brain Development in HEALthy Brain and Child Development (HBCD) Study: The Magnetic Resonance Imaging and Spectroscopy Protocol. Developmental Cognitive Neuroscience.
- Dosenbach N.U.F., Koller J.M., Earl E.A., Miranda-Dominguez O., Klein R.L., Van A.N., Snyder A.Z., Nagel B.J., Nigg J.T., Nguyen A.L., Wesevich V., Greene D.J., Fair D.A. Real-time motion analytics during brain MRI improve data quality and reduce costs. Neuroimage. 2017;161:80–93. doi: 10.1016/j.neuroimage.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumornay N.M., Lebois L.A.M., Ressler K.J., Harnett N.G. Racial disparities in adversity during childhood and the false appearance of race-related differences in brain structure. Am. J. Psychiatry. 2023;180(2):127–138. doi: 10.1176/appi.ajp.21090961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, R.C., Planalp, E.M., Enlow, M.B., Akshoomoff, N., Bodison, S.C., Brennan, M.B., Ciciolla, L., Eiden, R.D., Fillipi, C.A., Gustafsson, H.C., McKelvey, L.M., Morris, A.S., Peralta-Carcelen, M., Poehlmann, J., Wakschlag, L.S., Wilson, S., & HBCD Child Behavior and Caregiver-Child Interaction Workgroup. (this issue). Capturing the complexity of child behavior and caregiver-child relationships in the HEALthy Brain and Child Development (HBCD) Study using a rigorous and equitable approach. Developmental Cognitive Neuroscience. [DOI] [PubMed]
- Elam J.S., Glasser M.F., Harms M.P., Sotiropoulos S.N., Andersson J.L.R., Burgess G.C., Curtiss S.W., Oostenveld R., Larson-Prior L.J., Schoffelen J.-M., Hodge M.R., Cler E.A., Marcus D.M., Barch D.M., Yacoub E., Smith S.M., Ugurbil K., Van Essen D.C. The Human Connectome Project: a retrospective. Neuroimage. 2021;244 doi: 10.1016/j.neuroimage.2021.118543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinor L. Sullivan, Ryan Bogdan, Ludmila Bakhireva, Pat Levitt, Joseph Jones, Michael Sheldon, Julie M. Croff, Moriah Thomason, Jamie O. Lo, Leigh MacIntyre, Susmita Shrivastava, Leigh-Anne Cioffredi, Andrea G. Edlow, Brittany R. Howell, Barbara H. Chaiyachati, Nicole Lashley-Simms, Kelly Molloy, Cris Lam, Anna Stoermann, … HBCD Study Biospecimens Workgroup. (this issue). Biospecimens in the HEALthy Brain and Child Development (HBCD) Study: Rationale and protocol. Developmental Cognitive Neuroscience.
- Evans A.C. The NIH MRI study of normal brain development. Neuroimage. 2006;30(1):184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Fordyce L., Berrigan D., Srinivasan S. Social determinants of health and the environmental exposures: a promising partnership. Transl. Toxicol. Ther.: Windows Dev. Susceptibility Reprod. Cancer. 2017:395–414. [Google Scholar]
- Fox, N.A., Pérez-Edgar, K., Morales, S., Campbell, A.M., Cavanagh, J.F., Gabard-Durnam, L.J., Hudac, C.M., Key, A.P., Larson-Prior, L.J., Pedapati, E.V., Norton, E.S., Reetzke, R., Roberts, T.P., Rutter, T.M., Scott, L.S., Shuffrey, L.C., Antúnez, M., Boylan, M.R., Garner, B.M., … HBCD Electroencephalography Workgroup. (this issue). The development and structure of the HEALthy Brain and Child Development (HBCD) Study EEG protocol. Developmental Cognitive Neuroscience.
- Gosdin L.K., Deputy N.P., Kim S.Y., Dang E.P., Denny C.H. Alcohol consumption and binge drinking during pregnancy among adults aged 18–49 Years — United States, 2018–2020. MMWR Morb. Mortal. Wkly Rep. 2022;(71):10–13. doi: 10.15585/mmwr.mm7101a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurka, K.K., Burris, H.H., Ciciolla, L., Coles, C.D., Massey, S.H., Newman, S., Rajagopalan, V., Smith, L.M., Zilverstand, A., Bandoli, G., & HBCD Pregnancy Exposures including Substances Workgroup. (this issue). Assessment of maternal health and behavior during pregnancy in the HEALthy Brain and Child Development Study: Rationale and approach. Developmental Cognitive Neuroscience.
- Haight S.C., Ko J.Y., Tong V.T., Bohm M.K., Callaghan W.M. Opioid use disorder documented at delivery hospitalization - United States, 1999-2014. MMWR Morb. Mortal. Wkly Rep. 2018;67(31):845–849. doi: 10.15585/mmwr.mm6731a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B., Compton W.M., Einstein E.B., Elder E., Volkow N.D. Pregnancy and postpartum drug overdose deaths in the us before and during the Covid-19 pandemic. JAMA Psychiatry. 2023 doi: 10.1001/jamapsychiatry.2023.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H., Parker C., Netherland J. Race as a ghost variable in (white) opioid research. Sci., Technol., Hum. Values. 2020;45(5):848–876. doi: 10.1177/0162243920912812. [DOI] [Google Scholar]
- Ho S.S., Muzik M., Rosenblum K.L., Morelen D., Nakamura Y., Swain J.E. Potential neural mediators of mom power parenting intervention effects on maternal intersubjectivity and stress resilience [original research] Front. Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.568824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J.T., Perrotte J.K., Flores K., Leong C., Nocito J.D., III, Howard K.J. Trends in binge drinking and heavy alcohol consumption among pregnant women in the US, 2011 to 2020. JAMA Netw. Open. 2022;5(8) doi: 10.1001/jamanetworkopen.2022.24846. e2224846-e2224846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.R., Styner M.A., Gao W., Yap P.-T., Wang L., Baluyot K., Yacoub E., Chen G., Potts T., Salzwedel A., Li G., Gilmore J.H., Piven J., Smith J.K., Shen D., Ugurbil K., Zhu H., Lin W., Elison J.T. The UNC/UMN Baby Connectome Project (BCP): an overview of the study design and protocol development. Neuroimage. 2019;185:891–905. doi: 10.1016/j.neuroimage.2018.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J.A. Kable A.S. Potter N. Akshoomoff P.M. Blasco S. Bodson L. Ciciolla S. DeGray Z. Hulce E.S. Kuschner B. Learnard M. Luciana A. Perez M.A. Novack T. Riggins S.Y. Shin S. Smith J. Vannest E.H. Zimak n.d. Neurocognitive and Language Workgroup. (this issue). Measurement of emerging neurocognitive and language skills in the HEALthy Brain and Child Development Study. Developmental Cognitive Neuroscience.
- Jernigan T.L., Brown T.T., Hagler D.J., Jr., Akshoomoff N., Bartsch H., Newman E., Thompson W.K., Bloss C.S., Murray S.S., Schork N., Kennedy D.N., Kuperman J.M., McCabe C., Chung Y., Libiger O., Maddox M., Casey B.J., Chang L., Ernst T.M., Dale A.M. The Pediatric Imaging, Neurocognition, and Genetics (PING) data repository. Neuroimage. 2016;124(Pt B):1149–1154. doi: 10.1016/j.neuroimage.2015.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C.J., Weiss S.R.B., Howlett K.D., Freund M.P. Introduction to the special issue on “informing longitudinal studies on the effects of maternal stress and substance use on child development: planning for the HEALthy Brain and Child Development (HBCD) study. Advers. Resil. Sci. 2020;1(4):217–221. doi: 10.1007/s42844-020-00022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp E.A., Kress A.M., Parker C.B., Page G.P., McArthur K., Gachigi K.K., Alshawabkeh A.N., Aschner J.L., Bastain T.M., Breton C.V., Bendixsen C.G., Brennan P.A., Bush N.R., Buss C., Camargo J., Carlos A., Catellier D., Cordero J.F., Croen L., Dabelea D., Influences on Child Health Outcomes, on behalf of program collaborators for Environmental The Environmental Influences on Child Health Outcomes (ECHO)-Wide Cohort. Am. J. Epidemiol. 2023;192(8):1249–1263. doi: 10.1093/aje/kwad071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lähdepuro A., Räikkönen K., Pham H., Thompson-Felix T., Eid R.S., O'Connor T.G., Glover V., Lahti J., Heinonen K., Wolford E., Lahti-Pulkkinen M., O'Donnell K.J. Maternal social support during and after pregnancy and child cognitive ability: examining timing effects in two cohorts. Psychol. Med. 2023:1–10. doi: 10.1017/s0033291723003550. [DOI] [PubMed] [Google Scholar]
- Landsbergis P.A. Assessing the contribution of working conditions to socioeconomic disparities in health: a commentary. Am. J. Ind. Med. 2010;53(2):95–103. doi: 10.1002/ajim.20766. [DOI] [PubMed] [Google Scholar]
- Leech A.A., Cooper W.O., McNeer E., Scott T.A., Patrick S.W. Neonatal abstinence syndrome in the United States, 2004-16. Health Aff. (Millwood) 2020;39(5):764–767. doi: 10.1377/hlthaff.2019.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, J.A., Osterman, M.J.K., & Driscoll, A.K. (2023). Declines in cigarette smoking during pregnancy in the United States, 2016–2021. NCHS Data Brief, Number 458, Hyattsville, MD: National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services. [PubMed]
- Mascarell Maričić L., Walter H., Rosenthal A., Ripke S., Quinlan E.B., Banaschewski T., Barker G.J., Bokde A.L.W., Bromberg U., Büchel C., Desrivières S., Flor H., Frouin V., Garavan H., Itterman B., Martinot J.-L., Martinot M.-L.P., Nees F., Orfanos D.P., Consortium I. The IMAGEN study: a decade of imaging genetics in adolescents. Mol. Psychiatry. 2020;25(11):2648–2671. doi: 10.1038/s41380-020-0822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick R. Does access to green space impact the mental well-being of children: a systematic review. J. Pediatr. Nurs. 2017;37:3–7. doi: 10.1016/j.pedn.2017.08.027. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Weissman D., Bitrán D. Childhood adversity and neural development: a systematic review. Annu Rev. Dev. Psychol. 2019;1:277–312. doi: 10.1146/annurev-devpsych-121318-084950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz T.D., Allshouse A.A., McMillin G.A., Greene T., Chung J.H., Grobman W.A., Haas D.M., Mercer B.M., Parry S., Reddy U.M., Saade G.R., Simhan H.N., Silver R.M. Cannabis exposure and adverse pregnancy outcomes related to placental function. JAMA. 2023;330(22):2191–2199. doi: 10.1001/jama.2023.21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos, C., Faucetta, K., Warren, A., & Mitchell, R. (2017). Evidence on the long-term effects of home visiting programs: laying the groundwork for Long-Term Follow-Up in the Mother and Infant Home Visiting Program Evaluation (MIHOPE). ORPE Report 2017-73, Office of Planning, Research and Evaluation, Administration for Children and Families (OPRE), U.S. Department of Health and Human Services.
- Mikati I., Benson A.F., Luben T.J., Sacks J.D., Richmond-Bryant J. Disparities in distribution of particulate matter emission sources by race and poverty status. Am. J. Public Health. 2018;108(4):480–485. doi: 10.2105/ajph.2017.304297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A.S., Wakschlag L., Krogh-Jespersen S., Fox N., Planalp B., Perlman S.B., Shuffrey L.C., Smith B., Lorenzo N.E., Amso D., Coles C.D., Johnson S.P. Principles for guiding the selection of early childhood neurodevelopmental risk and resilience measures: healthy brain and child development study as an exemplar. Advers. Resil. Sci. 2020;1(4):247–267. doi: 10.1007/s42844-020-00025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N. Pini W.P. Fifer J. Oh C. Nebeker J.M. Croff B.A. Smith (this issue). Remote data collection of infant activity and sleep patterns via wearable sensors in the HEALthy Brain and Child Development Study (HBCD) Developmental Cognitive Neuroscience.
- Nelson, C.A., Frankeberger, J., & Chambers, C.D. (this issue). An introduction to the HEALthy Brain and Child Development Study. Developmental Cognitive Neuroscience.
- Ni Y., Loftus C.T., Szpiro A.A., Young M.T., Hazlehurst M.F., Murphy L.E., Tylavsky F.A., Mason W.A., LeWinn K.Z., Sathyanarayana S., Barrett E.S., Bush N.R., Karr C.J. Associations of pre- and postnatal air pollution exposures with child behavioral problems and cognitive performance: A U.S. multi-cohort study. Environ. Health Perspect. 2022;130(6):67008. doi: 10.1289/EHP10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIDA. (2018a). Expert Panel Meeting to Discuss Study Design for a Longitudinal Study of the Impact of Prenatal Opioid and other Substance Exposure on Brain and Behavioral Development. U.S. Department of Health and Human Services, National Institutes of Health, National Institute on Drug Abuse. Retrieved 2024 February 12 from 〈https://archives.nida.nih.gov/meetings/2018/10/expert-panel-meeting-to-discuss-study-design-longitudinal-study-impact-prenatal-opioid-other〉.
- NIDA. (2018b). Research methodologies to understand long-term consequences of prenatal opioid and other substance exposure on brain and behavioral development. U.S. Department of Health and Human Services, National Institutes of Health, National Institute on Drug Abuse. Retrieved 2024 February 12 from 〈https://archives.nida.nih.gov/meetings/2018/09/research-methodologies-to-understand-long-term-consequences-prenatal-opioid-other-substance-exposure〉.
- Nunez C.C., Roussotte F., Sowell E.R. Focus on: structural and functional brain abnormalities in fetal alcohol spectrum disorders. Alcohol Res Health. 2011;34(1):121–131. [PMC free article] [PubMed] [Google Scholar]
- Nygaard E., Slinning K., Moe V., Fjell A., Walhovd K.B. Mental health in youth prenatally exposed to opioids and poly-drugs and raised in permanent foster/adoptive homes: a prospective longitudinal study. Early Hum. Dev. 2020;140 doi: 10.1016/j.earlhumdev.2019.104910. [DOI] [PubMed] [Google Scholar]
- Peterson B.S., Rosen T., Dingman S., Toth Z.R., Sawardekar S., Hao X., Liu F., Xu D., Dong Z., Peterson J.B., Ryoo J.H., Serino D., Branch C.A., Bansal R. Associations of maternal prenatal drug abuse with measures of newborn brain structure, tissue organization, and metabolite concentrations. JAMA Pediatr. 2020;174(9):831–842. doi: 10.1001/jamapediatrics.2020.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatou A., Filippi C.A., Aydin E., Vaughn K., Thompson D., Korom M., Dufford A.J., Howell B., Zöllei L., Martino A.D., Graham A., Scheinost D., Spann M.N. An ode to fetal, infant, and toddler neuroimaging: chronicling early clinical to research applications with MRI, and an introduction to an academic society connecting the field. Dev. Cogn. Neurosci. 2022;54 doi: 10.1016/j.dcn.2022.101083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado E.L., Dewey K.G. Nutrition and brain development in early life. Nutr. Rev. 2014;72(4):267–284. doi: 10.1111/nure.12102. [DOI] [PubMed] [Google Scholar]
- Raifman S., Biggs M.A., Rocca C., Roberts S.C.M. Is legal recreational cannabis associated with cannabis use during pregnancy, beliefs about safety, and perceived community stigma? Drug Alcohol Depend. 2024;255 doi: 10.1016/j.drugalcdep.2023.111079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle N., Zuk J., Ortiz-Mantilla S., Sliva D.D., Franceschi A., Grant P.E., Benasich A.A., Gaab N. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann. N. Y Acad. Sci. 2012;1252:43–50. doi: 10.1111/j.1749-6632.2012.06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard J.A., Parker T.C., Dhamala E., Kwasa J., Allsop A., Holmes A.J. Confronting racially exclusionary practices in the acquisition and analyses of neuroimaging data. Nat. Neurosci. 2023;26(1):4–11. doi: 10.1038/s41593-022-01218-y. [DOI] [PubMed] [Google Scholar]
- Ross E.J., Graham D.L., Money K.M., Stanwood G.D. Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology. 2015;40(1):61–87. doi: 10.1038/npp.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnake-Mahl A.S., Lazo M., Dureja K., Ehtesham N., Bilal U. Racial and ethnic inequities in occupational exposure across and between US cities. SSM Popul Health. 2021;16 doi: 10.1016/j.ssmph.2021.100959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff J.P., Slopen N., Williams D.R. Early childhood adversity, toxic stress, and the impacts of racism on the foundations of health. Annu. Rev. Public Health. 2021;42(1):115–134. doi: 10.1146/annurev-publhealth-090419-101940. [DOI] [PubMed] [Google Scholar]
- Si, Y., Bandoli, G., Cole, K.M., Fallin, D., Stuart, E.A., Gurka, K., Althoff, K.N., Thompson, W.K., & HBCD Design Workgroup and Biostatics Workgroup. (this issue). Advancing high quality longitudinal data collection: Implications for the HEALthy Brain and Child Development (HBCD) Study Design and Recruitment. Developmental Cognitive Neuroscience.
- Singer L.T., Chambers C., Coles C., Kable J. Fifty years of research on prenatal substances: lessons learned for the opioid epidemic. Advers. Resil. Sci. 2020;1(4):223–234. doi: 10.1007/s42844-020-00021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid M.C., Metz T.D., Gordon A.J. Stimulant use in pregnancy: an under-recognized epidemic among pregnant women. Clin. Obstet. Gynecol. 2019;62(1):168–184. doi: 10.1097/GRF.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J., Downey P., Elliott P., Green J., Landray M., Liu B., Matthews P., Ong G., Pell J., Silman A., Young A., Sprosen T., Peakman T., Collins R. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B.L., Levitt P., Stanwood G.D. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat. Rev. Neurosci. 2009;10(4):303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley U.A., Bassett D.S., Mackey A.P. Environmental influences on the pace of brain development. Nat. Rev. Neurosci. 2021;22(6):372–384. doi: 10.1038/s41583-021-00457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent M., Dooley D.G., Dougé J. The impact of racism on child and adolescent health. Pediatrics. 2019;144(2) doi: 10.1542/peds.2019-1765. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C., Ugurbil K., Auerbach E., Barch D., Behrens T.E., Bucholz R., Chang A., Chen L., Corbetta M., Curtiss S.W., Della Penna S., Feinberg D., Glasser M.F., Harel N., Heath A.C., Larson-Prior L., Marcus D., Michalareas G., Moeller S., Consortium W.U.-M.H. The Human Connectome Project: a data acquisition perspective. Neuroimage. 2012;62(4):2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataramani M., Ogunwole S.M., Caulfield L.E., Sharma R., Zhang A., Gross S.M., Hurley K.M., Lerman J.L., Bass E.B., Bennett W.L. Maternal, infant, and child health outcomes associated with the special supplemental nutrition program for women, infants, and children: a systematic review. Ann. Intern Med. 2022;175(10):1411–1422. doi: 10.7326/m22-0604. [DOI] [PubMed] [Google Scholar]
- Volkow, N.D. (2023). Pregnant People With Substance Use Disorders Need Treatment, Not Criminalization. U.S. Department of Health and Human Services, National Institutes of Health, National Institute on Drug Abuse. Retrieved 2024, February 12 from 〈https://nida.nih.gov/about-nida/noras-blog/2023/02/pregnant-people-substance-use-disorders-need-treatment-not-criminalization〉.
- Volkow N.D., Han B., Compton W.M., McCance-Katz E.F. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA. 2019;322(2):167–169. doi: 10.1001/jama.2019.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Koob G.F., Croyle R.T., Bianchi D.W., Gordon J.A., Koroshetz W.J., Pérez-Stable E.J., Riley W.T., Bloch M.H., Conway K., Deeds B.G., Dowling G.J., Grant S., Howlett K.D., Matochik J.A., Morgan G.D., Murray M.M., Noronha A., Spong C.Y., Weiss S.R.B. The conception of the ABCD Study: from substance use to a broad NIH collaboration. Dev. Cogn. Neurosci. 2018;32:4–7. doi: 10.1016/j.dcn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X., Chung M.V., Liszewski K.A., Todoro L.D., Giancarlo E.M., Zhang W., Berkelhamer S.K., Goniewicz M.L. cigarette smoking abstinence among pregnant individuals using e-cigarettes or nicotine replacement therapy. JAMA Netw. Open. 2023;6(9) doi: 10.1001/jamanetworkopen.2023.30249. e2330249-e2330249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X., Liu L., Moe A.A., Ormond I.K., Shuren C.C., Scott I.Y.N., Ozga J.E., Stanton C.A., Ruybal A.L., Hart J.L., Goniewicz M.L., Lee D., Vargees C. Use of E-cigarettes and cigarettes during late pregnancy among adolescents. JAMA Netw. Open. 2023;6(12) doi: 10.1001/jamanetworkopen.2023.47407. e2347407-e2347407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman T.N.A., Villapiano N., Kozhimannil K.B., Davis M.M., Patrick S.W. Incidence and costs of neonatal abstinence syndrome among infants with medicaid: 2004-2014. Pediatrics. 2018;141(4) doi: 10.1542/peds.2017-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh S.L., Eastwood J., Wright I.M., Morton R., Melhuish E., Ward M., Oei J.L. Cognitive and motor outcomes of children with prenatal opioid exposure: a systematic review and meta-analysis. JAMA Netw. Open. 2019;2(7) doi: 10.1001/jamanetworkopen.2019.7025. e197025-e197025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff K.C., Slama N.E., Sarovar V., Terplan M., Ansley D., Adams S.R., Alexeeff S.E. Trends in self-reported and biochemically verified cocaine and methamphetamine use among pregnant individuals in Northern California, 2011-2019. JAMA Netw. Open. 2022;5(12) doi: 10.1001/jamanetworkopen.2022.48055. e2248055-e2248055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou R., Boer O.D., Felix J.F., Muetzel R.L., Franken I.H.A., Cecil C.A.M., El Marroun H. Association of maternal tobacco use during pregnancy with preadolescent brain morphology among offspring. JAMA Netw. Open. 2022;5(8) doi: 10.1001/jamanetworkopen.2022.24701. e2224701-e2224701. [DOI] [PMC free article] [PubMed] [Google Scholar]