Abstract
Neurofibromatosis 1 (NF1) is a rare genetic syndrome that leads to the development of neurofibromas and increases the risk of malignancy, including malignant peripheral nerve sheath tumors. Patients with NF1 often have other orthopaedic manifestations, including short stature, osteopenia, and dysplasia. A 47-year-old patient with a history of NF1 and multiple neurofibromas of the right lower extremity presented with a severe valgus deformity, instability, and osteoarthritis of the right knee that was debilitating to daily life. Over time, the patient lost proprioception and potentially some sensation to the right knee with neurofibroma formation, leading to the development of Charcot arthropathy of the right knee with secondary osteoarthritis. The preoperative workup consisted of a magnetic resonance imaging of the knee to confirm no malignancy was present and templating to ensure the standard implant size was amenable for the patient. A primary total knee arthroplasty was performed with a cemented-stemmed hinged knee implant. At 6 months post-surgery, the patient had a dramatic improvement in her pain and quality of life.
Keywords: Neurofibromatosis, Neurofibroma, Charcot arthropathy, Osteoarthritis, Total knee arthroplasty, Deformity
Introduction
Neurofibromatosis 1 (NF1) is a genetic syndrome hallmarked by numerous tumors arising from the nervous system in association with a loss of function via mutation of the neurofibromin 1 gene (chromosome 17, band q11.2). Neurofibromin is a protein that has been linked to human development and is expressed in all tissue types but is particularly vital in the cell types of the nervous system [1]. The NF1 gene can undergo spontaneous mutation during early development; however, NF1 is generally an inherited autosomal dominant disorder with near 100% penetrance [2]. Neurofibromin functions as a negative regulator of the pathways of cellular proliferation and requires both wild-type allele copies for normal function. Loss of one allele via deletion or mutation leads to a high occurrence of malignancy involving the nervous system such as neurofibromas, which are usually benign [3]. There is a significantly increased risk of malignancy such as malignant nerve sheath tumors (MPNST), glioblastoma, meningioma, optic nerve gliomas, carcinomas, sarcomas, and leukemia. [3,4]. Therefore, patients with NF1 have a decreased life expectancy of approximately 10-15 years compared to the general population due to these malignancies [3,5,6].
Patients with NF1 also have an array of other clinical manifestations, such as café-au-lait spots, axillary or pubic freckling, iris hamartomas (Lisch nodules), cardiovascular abnormalities, and precocious/delayed puberty [4,7]. Orthopaedic manifestations are also common including congenital pseudoarthrosis of the tibia, scoliosis, dysplasia, cystic lesions, genu varum/valgus, osteopenia/osteoporosis, abnormal elongation of long bones, hemihypertrophy, and short stature [1,4,7,8]. Neurofibromatous neuropathy is another rare feature of NF1 (1.3%) that is more common in patients with NF2, who have a different mutation on chromosome 22 and bilateral vestibular schwannomas. Studies suggest this is caused by abnormal signaling in peripheral nerves between Schwann cells, fibroblasts, and perineural cells with a preference for sensory nerves [9]. There is limited literature discussing treatment of secondary complications of these orthopaedic manifestations of NF1, such as Charcot arthropathy, in patients with NF1. Charcot arthropathy is a progressive disease caused by chronic peripheral neuropathy that leads to the destruction of joints and adjacent bone, which often develops into severe deformity and dysfunction. The foot and ankle are by far the most affected with diabetes mellitus being the most common cause. However, Charcot arthropathy of the knee is rare, with a recent systemic review showing a total of 212 patients (259 knees total, 23% bilateral involvement) being reported in the literature. The most common cause of knee Charcot arthropathy was syphilis (85 patients), which has decreased in prevalence due to antibiotic treatment, so diabetes mellitus has become the most common cause. Other causes included spinal cord injury, idiopathic, Guillain-Barré, and syringomyelia. Valgus deformity of the knee (77%) is significantly more common than varus deformity, and the majority of patients have significant tibial plateau destruction [10].
Historically, Charcot arthropathy has been treated conservatively with bracing and immobilization initially. However, many patients progress over time, even with treatment of the underlying disease, such as penicillin for syphilis, later requiring surgical management. Although unlikely to be painful, Charcot arthropathy can be severely debilitating. Balaji et al. described a patient with NF1 who was treated with above knee amputation due to severe knee instability in the presence of multiple extra-articular neurofibromas [11]. Knee arthrodesis was commonly used 30+ years ago for surgical management, but advancement in arthroplasty implant technology has allowed a shift to treatment of knee Charcot arthropathy with total knee arthroplasty (TKA) and even unicompartmental knee arthroplasty if indicated. Patel et al. described a patient with severe deformity in the presence of congenital pseudoarthrosis of the tibia, leg length discrepancy, and 14° of valgus due to destruction of the lateral femoral condyle that was treated with unicompartmental knee arthroplasty since there was inadequate tibia bone stock to support a stemmed tibia component [12]. In this case report, we discuss a method of treating severe knee deformity secondary to Charcot arthropathy in a patient with the diagnosis of NF1 with TKA.
Case history
Initial presentation
A 47-year-old female presented to clinic, with complaints of right knee pain and dysfunction with standing, walking, and activities of daily living. Her past medical history was significant for her NF1 diagnosis at the age of 15. Her family history was notable for the diagnosis of NF1 in her three first cousins and her mother, who died of a MPNST. She underwent multiple procedures due to NF1 manifestations, including resection of multiple neurofibromas from the right leg, pelvis, and lumbar spine over the past 15 years. Therefore, she meets the revised diagnostic criteria for NF1 by being the child of a parent with NF1 and meeting one or more other diagnostic criteria, which include freckling of the axilla/inguinal area and multiple neurofibromas [13]. Furthermore, the various neurofibroma resections were associated with postoperative complications including peroneal nerve palsy (right foot drop), neurogenic bladder, and hypertrophic scar formation, in which she underwent excision at the buttock and lower leg with advancement flaps.
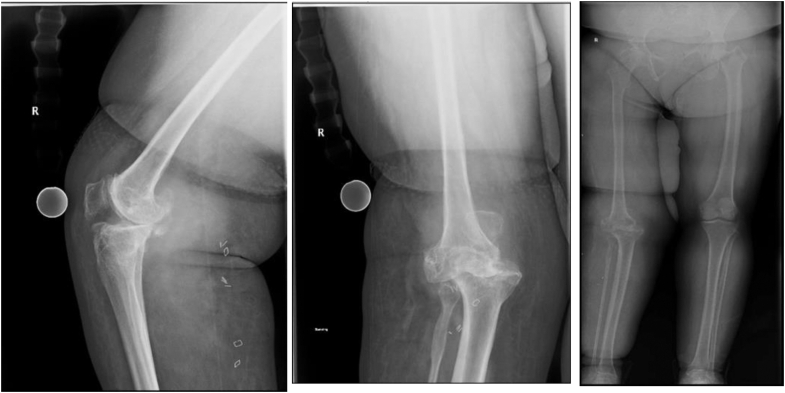
Initial physical exam demonstrated the patient to be of short stature (145 cm) with a body mass index of 38 kg/m2. Her gait was antalgic with notable instability of the right knee: 45° of valgus with weight bearing. Knee range of motion was −20° of extension (recurvatum) to 140° of flexion. There was significant knee instability in the coronal and sagittal planes. Leg length discrepancy of approximately 3 cm with the right longer than the left. She also had tenderness to palpation over the medial joint line and patellofemoral joint. Initial motor examination demonstrated 4/5 quadriceps, 4/5 hamstrings, and 3/5 anterior tibialis, consistent with her peroneal palsy. Anterior-posterior and lateral radiographs demonstrated severe osteophytosis, sclerosis, joint space narrowing, valgus deformity, and anterior subluxation of the tibia, coinciding with a Grade 4 knee osteoarthritis of the Kellgren and Lawrence Classification [14]. Full-length standing radiographs show elongation of the right femoral neck and shaft compared to the left, contributing to a 3 cm leg length discrepancy, which is partly due to pelvic obliquity and notable osteopenia (Fig. 1). To further evaluate the status of neurofibroma recurrence and to rule out any potential malignant transformation, a magnetic resonance imaging (MRI) with contrast of the knee was ordered, which showed no recurrence of neurofibromas of the knee. MRI was also notable for chronic medial collateral, anterior cruciate, and posterior cruciate ligmant tears with a partial lateral collateral ligmant tear.
Figure 1.
AP, lateral, and full-length standing radiographs of the right knee demonstrate significant valgus deformity with anterior medial subluxation of the tibia and severe degenerative changes of all compartments. Full-length standing X-rays demonstrate abnormal elongation of the right femur with smaller diameter of the femoral neck and shaft that attributes to a 3 cm leg length discrepancy. There is a notable decrease in cortical density compared to the left. AP, anterior-posterior.
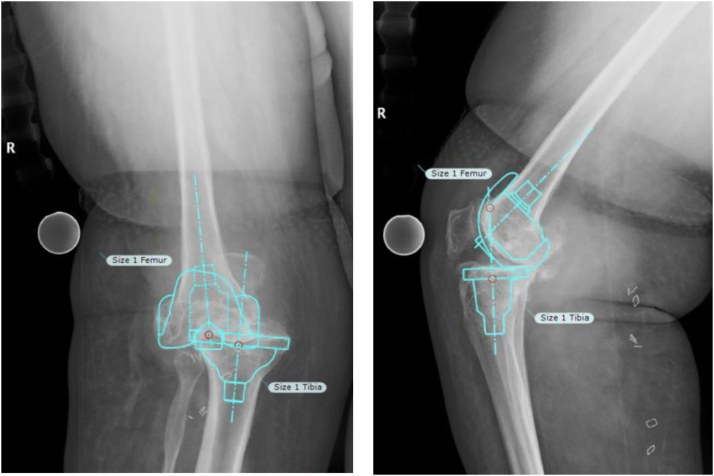
After a discussion with the patient, she elected to undergo TKA. Full-length standing and anterior-posterior/lateral radiographs were utilized to plan for measured resection of the distal femur and proximal tibia along with appropriate implant size using TraumaCad (Brainlab Ltd., 2016 Munich, Germany) templating software (Fig. 2).
Figure 2.
AP and lateral of the right knee showing templated components for preoperative planning (size 1 femoral and tibial components for Medacta GMK Hinge Knee). AP, anterior-posterior.
Procedure
A standard midline incision with a medial parapatellar approach was made. Lateral release was required to evert the patella and obtain the needed exposure. An intramedullary cutting guide was used to cut the femur in 5° of valgus, with the remaining cuts made using the standard epicondylar axis and measured resection. A box cut was also made using a cutting guide.
Next, an extramedullary guide was used to make the tibial cut to neutral in the coronal plane, following the mechanical axis. After trialing for the appropriate implant size, Size 1 femoral and tibial components for a rotating platform hinged knee prosthesis (Medacta GMK Sphere, Medacta International 2017-2024) were inserted with 11 × 30 mm stems and a 20 mm polyethylene tibial insert (Fig. 3). Femoral augments (4 mm each) were required posteriorly, both medial and lateral, due to the lack of bone stock. The patella was resurfaced with a size 1 polyethylene patella button. All components were cemented. The patient was placed in a hinged knee brace locked in extension immediately for postoperative restrictions. Also, a custom ankle-foot-orthosis brace was fitted and placed prior to discharge for her preexisting peroneal nerve palsy.
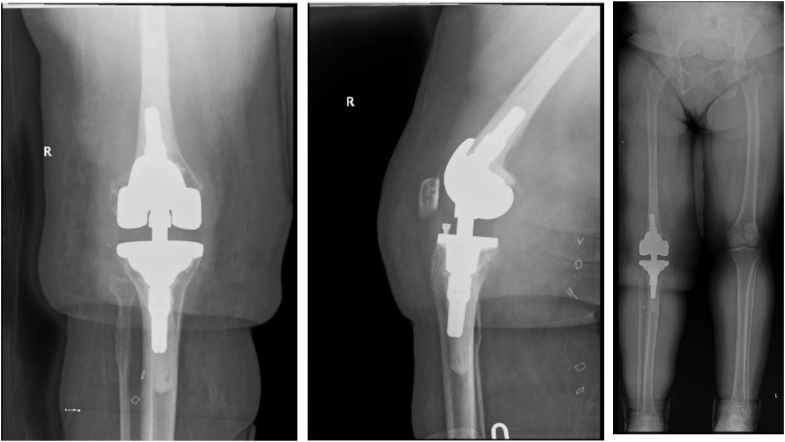
Figure 3.
Final AP, lateral, and full-length standing X-rays showing corrected alignment with a hinged knee implant. AP, anterior-posterior.
Postoperation
There were no notable postoperative complications in the acute period. However, at 2 months her range of motion was 0-80° and she was ambulating with a rolling walker. Her decreased range of motion was likely secondary to late initiation of formal physical therapy, as she was displaced from her home for several weeks after an accident resulting in the destruction of her house. At 6 months, she completed formal physical therapy with significant improvement. Her range of motion was 0-100° of flexion with no coronal or sagittal instability. She was ambulating well with minimal pain and used a rolling walker only as a safety measure in crowded places. She only complained of a leg-length discrepancy with the left leg being shorter than the right, which was present before surgery. Knee Society Scores drastically improved in nearly all components from preoperatively to 6 months postoperatively. Objective scores improved from 43/100 preoperatively to 84/100 postoperatively due to the provided stability of the implant, although there was some loss of range of motion: 140° of flexion preoperatively vs 100° of flexion postoperatively. Function scores also drastically improved from 30/100 preoperatively to 78/100 postoperatively, as the patient was able to ambulate and perform activities for longer periods of time with minimal pain. The patient’s satisfaction with her knee improved from 8/40 preoperatively to 30/40 postoperatively. Lastly, although the patient's expectation scores decreased postoperatively, 10/15 compared to 13/15 preoperatively, her assessment showed that her expectations of her knee pain and function were “just right.” At 9 months, she has continued to improve with knee range of motion at 0-120° of flexion and occasional use of a cane for prolonged ambulation.
Discussion
Neurofibromatosis is characterized by numerous neurofibromas that affect the various nerves and soft tissues of the extremities. When in the vicinity of joints, these tumors can deform soft tissues that support joint stability such as the capsule, collateral ligaments, and muscles/tendons, which serve as dynamic and static stabilizers of the knee joint [11]. Also, in this patient, the previously resected neurofibromas of the right leg may have disrupted sensory and/or proprioceptive nerve input of the knee joint leading to a cascade of repetitive microtrauma, cartilage loss, ligament tears/attenuation, bone erosion, and ultimately the development of Charcot neuroarthropathy [15,16]. X-rays and MRIs, along with surgical history of our patient, allude to the latter process, with Charcot arthropathy being the primary patient diagnosis and secondary osteoarthritis being the source of her pain. There are reports of similar pathology such as that of a 55-year-old woman who developed a large neurofibroma in the lower leg with resultant Charcot arthropathy of the hindfoot and midfoot including significant erosion of the calcaneus and cuboid. Interestingly, this patient did not develop pain in the foot until the removal of the neurofibroma, which further supports the neurofibroma’s ability to hinder sensory input to the adjacent joint [16]. Also more relevant to our case, a 30-year-old female with a recurrent neurofibroma of the right knee developed chronic edema, multidirectional knee instability, severe deformity, and eventual Charcot arthropathy. The authors attributed this to the chronic pressure and mass effect of the neurofibroma on the bones and ligaments. The patient underwent simultaneous resection of the recurrent neurofibroma and TKA with a rotating hinge knee prosthesis including cemented metadiaphyseal engaging stems. The patient’s severe deformity including a hypoplastic lateral femoral condyle, hypertrophic posterior femoral condyles, and large flexion gap were adequately addressed with appropriate implant placement and size selection [17].
For a complex disease with severe deformity to have a favorable outcome, a thorough preoperative workup with careful preoperative planning should be conducted. Due to our patient’s previous history of numerous neurofibromas, unknown surveillance of tumor recurrence, and associated risk of malignant transformation such as MPNST (up to 10%), an MRI of the operative extremity was conducted to rule out any malignancy and determine if any neurofibromas were present in the extraarticular tissues. We recommend MRI with contrast of the operative extremity be performed in all patients with neurofibromatosis, as the results of operating through an unknown malignant tumor can lead to contamination of multiple compartments and potentially lead to amputation. MRI also provides more information on soft tissue integrity to help determine potential constraint options, as in this patient the posterior cruciate ligmant, medial collateral, and lateral collateral ligmant were not intact, which indicated the need for a hinge prosthesis. We chose a rotating hinge implant due to the global instability of our patient’s knee. We also cemented our implant with 30 mm femoral and tibial stems due to the well-known increased risk of body mass index >30 and significant coronal deformity for aseptic loosening, in which recent studies show a decreased incidence of this complication with tibial stems [18,19]. Furthermore, there is no consensus on the most advantageous stem length or fixation method: cemented or press-fit, as there are advantages and disadvantages to both [20,21]. In the scenario of significant deformity, the use of cemented metaphyseal engaging stems allows flexibility in femoral and tibial component placement in the coronal and sagittal planes to achieve the best alignment and stability, particularly when there is significant bone loss. The longest stem was 30 mm that could be implanted in our patient without engaging the diaphysis, which would limit the ability to alter alignment of each component. On the femoral side, the implant can be translated medially or laterally and anterior or posterior to achieve the optimal flexion gap and a more native coronal balance such as 5° of valgus instead of the preset implant valgus of 7°, which is usually more tolerated in primary arthroplasty. On the tibial side, slope can be altered with slight flexion or extension of the base plate [20].
Templating is a useful element in preoperative planning with significant deformity. Our patient had a significant valgus deformity, which required careful consideration for measured resection without excess bone loss. Intramedullary or extramedullary cutting guides can be used on both the femur and tibia to obtain balanced alignment. In this case, the hinge prothesis is being used as a primary implant, so we opted to use an intramedullary alignment guide to cut the femur at 5° of valgus instead of the preset 7° of valgus normally used for this hinge implant. An extramedullary guide was used to cut the tibia with 2-3° of slope and neutral coronal alignment, also like a primary implant with careful consideration of not resecting too much proximal tibia. Using short, thin cemented femoral and tibial stems prevented potential placement of the implant in excessive valgus of the femur and allowed necessary adjustment of slope for proper flexion balancing of the tibia. Furthermore, in some cases, patients with NF1 have tibial bowing and other deformities that extend beyond the joint. These deformities may require extramedullary guides for the femur and/or osteotomies of the femur or tibia with fixation. Robotics may also be a useful tool to accommodate severe deformity [22,23]. Lastly, templating allows for close estimation of implant size. In many cases, NF1 patients are of short stature and may have a smaller bone structure such as our patient, who required the smallest implant size available. Other patients may require custom implants to be manufactured.
Summary
Overall, our patient had a favorable outcome with significant improvement in pain, function, and quality of life. Arthroplasty in patients with NF1 is a viable option even in the presence of severe deformity and instability; however, each patient should undergo a thorough workup with careful surgical planning and consideration of the type of resection guides, implant constraint, and implant size.
Conflicts of interest
W. Sherman is a Stryker consultant and is an editorial board member of Arthroplasty Today, the AAOS Knee Committee, and an associate editor of Orthopedic Reviews. F. Sanchez receives royalties from Medacta International; is a paid consultant for Medacta International; receives research support from Medacta International; receives other financial/material support from DePuy Synthes and J&J; and is a board/committee member of ABOS Part I and the AAOS OITE Linking Project Committee. All other authors declare no potential conflicts of interest.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2024.101453.
Informed patient consent
The author(s) confirm that written informed consent has been obtained from the involved patient(s) or if appropriate from the parent, guardian, power of attorney of the involved patient(s); and, they have given approval for this information to be published in this case report (series).
CRediT authorship contribution statement
Thomas Hodo: Writing – review & editing, Writing – original draft, Data curation. William Sherman: Writing – review & editing, Supervision. Santiago Sanchez: Writing – review & editing, Data curation. Edmund Anudu: Writing – review & editing, Data curation. Fernando Sanchez: Writing – review & editing, Supervision, Data curation, Conceptualization.
Appendix A. Supplementary data
References
- 1.Evans T.J., Wang X., Binitie O. Orthopaedic manifestations of neurofibromatosis type I. J Am Acad Orthop Surg. 2022;30:e1495–e1503. doi: 10.5435/JAAOS-D-22-00076. [DOI] [PubMed] [Google Scholar]
- 2.Evans D.G., Howard E., Giblin C., Clancy T., Spencer H., Huson S.M., et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A:327–332. doi: 10.1002/ajmg.a.33139. [DOI] [PubMed] [Google Scholar]
- 3.Patil S., Chamberlain R.S. Neoplasms associated with germline and somatic NF1 gene mutations. Oncol. 2012;17:101–116. doi: 10.1634/theoncologist.2010-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirbe A.C., Gutmann D.H. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834–843. doi: 10.1016/S1474-4422(14)70063-8. [DOI] [PubMed] [Google Scholar]
- 5.Duong T.A., Sbidian E., Valeyrie-Allanore L., Vialette C., Ferkal S., Hadj-Rabia S., et al. Mortality associated with neurofibromatosis 1: a cohort study of 1895 patients in 1980-2006 in France. Orphanet J Rare Dis. 2011;6:18. doi: 10.1186/1750-1172-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen S.A., Yang Q., Friedman J.M. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet. 2001;68:1110–1118. doi: 10.1086/320121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonsgard J.H. Clinical manifestations and management of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13:2–7. doi: 10.1016/j.spen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Năstase F., Radaschin D.S., Niculeț E., Brădeanu A.V., Verenca M.C., Nechita A., et al. Orthopaedic manifestations of neurofibromatosis type 1: a case report. Exp Ther Med. 2022;23:135. doi: 10.3892/etm.2021.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferner R.E., Hughes R.A., Hall S.M., Upadhyaya M., Johnson M.R. Neurofibromatous neuropathy in neurofibromatosis 1 (NF1) J Med Genet. 2004;41:837–841. doi: 10.1136/jmg.2004.021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu V., Zhang J., Thahir A., Zhou A., Krkovic M. Charcot knee - presentation, diagnosis, management - a scoping review. Clin Rheumatol. 2021;40:4445–4456. doi: 10.1007/s10067-021-05775-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balaji A., Toga A., Kano J., Fujimaru A., Matsumoto T., Katoh S. Unicompartmental knee arthroplasty for severe osteoarthritis and pseudarthrosis in a patient with neurofibromatosis. Orthop Res Rev. 2021;13:63–71. doi: 10.2147/ORR.S304651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel J., Whiting J., Jones D. Secondary knee osteoarthritis due to neurofibromatosis type 1 treated with above the knee amputation: a case report. Case Rep Orthop. 2013;2013 doi: 10.1155/2013/782106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legius E., Messiaen L., Wolkenstein P., Pancza P., Avery R.A., Berman Y., et al. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: an international consensus recommendation. Genet Med. 2021;23:1506–1513. doi: 10.1038/s41436-021-01170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Q.H., Hsu P., Gao Y.S., Zhang C.Q. Charcot neuroarthropathy of the knee due to idiopathic sensory peripheral neuropathy. BMC Muscoskel Disord. 2019;20:501. doi: 10.1186/s12891-019-2873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsukawa Y., Hara H., Ryu J., Nakano Y., Takeuchi M., Sasaki K., et al. Unilateral developments of osteoarthritis and Charcot's joint in a patient with neurofibromatosis. Med Sci Monit. 2009;15:CS113–C116. [PubMed] [Google Scholar]
- 17.Kenanidis E., Klonou E., Leonida I., Tsiridis E. Complex primary total knee arthroplasty in a young patient with neurofibromatosis type one and multidirectional knee instability: technical tips and outcome. Cureus. 2023;15 doi: 10.7759/cureus.39721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fournier G., Yener C., Gaillard R., Kenney R., Lustig S., Servien E. Increased survival rate in extension stemmed TKA in obese patients at minimum 2 years follow-up. Knee Surg Sports Traumatol Arthrosc. 2020;28:3919–3925. doi: 10.1007/s00167-020-05860-6. [DOI] [PubMed] [Google Scholar]
- 19.Hegde V., Bracey D.N., Brady A.C., Kleeman-Forsthuber L.T., Dennis D.A., Jennings J.M. A prophylactic tibial stem reduces rates of early aseptic loosening in patients with severe preoperative varus deformity in primary total knee arthroplasty. J Arthroplasty. 2021;36:2319–2324. doi: 10.1016/j.arth.2021.01.049. [DOI] [PubMed] [Google Scholar]
- 20.Jazrawi L.M., Bai B., Kummer F.J., Hiebert R., Stuchin S.A. The effect of stem modularity and mode of fixation on tibial component stability in revision total knee arthroplasty. J Arthroplasty. 2001;16:759–767. doi: 10.1054/arth.2001.25507. [DOI] [PubMed] [Google Scholar]
- 21.Patel A.R., Barlow B., Ranawat A.S. Stem length in revision total knee arthroplasty. Curr Rev Musculoskelet Med. 2015;8:407–412. doi: 10.1007/s12178-015-9297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sodhi N., Khlopas A., Ehiorobo J.O., Condrey C., Marchand K., Marchand R.C., et al. Robotic-Assisted total knee arthroplasty in the presence of extra-articular deformity. Surg Technol Int. 2019;34:497–502. [PubMed] [Google Scholar]
- 23.Alturki A.A., Alshammari N.A., Albassam A.L., Aljaafri Z.A., Almugren T.S. Robotic-assisted total knee arthroplasty for extra-articular femur deformity correction. J Surg Case Rep. 2023;2023 doi: 10.1093/jscr/rjad395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.