Abstract
The oligomeric state of the chicken liver receptor (chicken hepatic lectin), which mediates endocytosis of glycoproteins terminating with N-acetylglucosamine, has been investigated using physical methods as well as chemical cross-linking. Receptor isolated from liver and from transfected rat fibroblasts expressing the full-length polypeptide is a homotrimer immediately following solubilization in non-ionic detergent, but forms the previously observed hexamer during purification. These results are most consistent with the presence of a trimer of receptor polypeptides in liver membranes and in transfected cells. Analysis of truncated receptors reveals that the C-terminal extracellular portion of this type-II transmembrane protein does not form stable oligomers when isolated from the membrane anchor and cytoplasmic tail. The behaviour of chimeric receptors, in which the cytoplasmic tail of the glycoprotein receptor is replaced with the corresponding segments of rat liver asialoglycoprotein receptor or the beta-subunit of Na+,K(+)-ATPase, or with unrelated sequences from globin, indicates that the cytoplasmic tail influences oligomer stability. Replacement of N-terminal portions of the receptor with corresponding segments of influenza virus neuraminidase results in formation of tetramers, suggesting that the membrane anchor and flanking sequences are important determinants of oligomer formation.
Full text
PDF


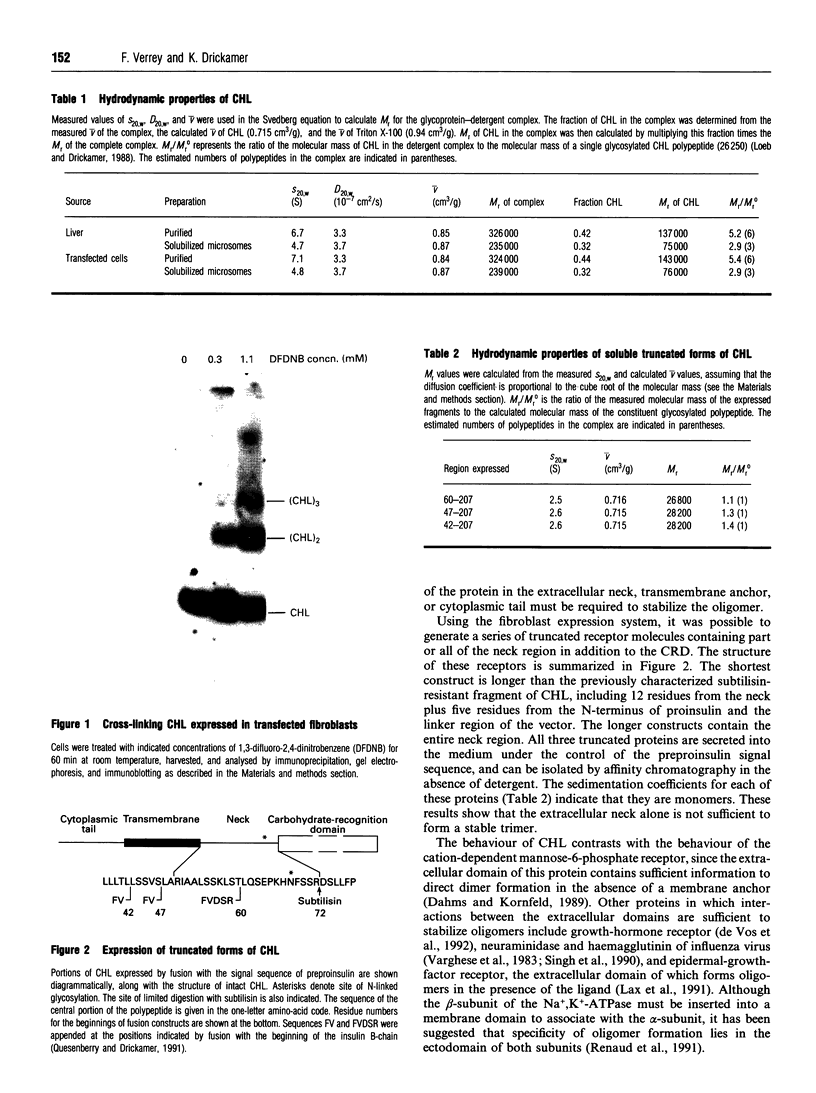
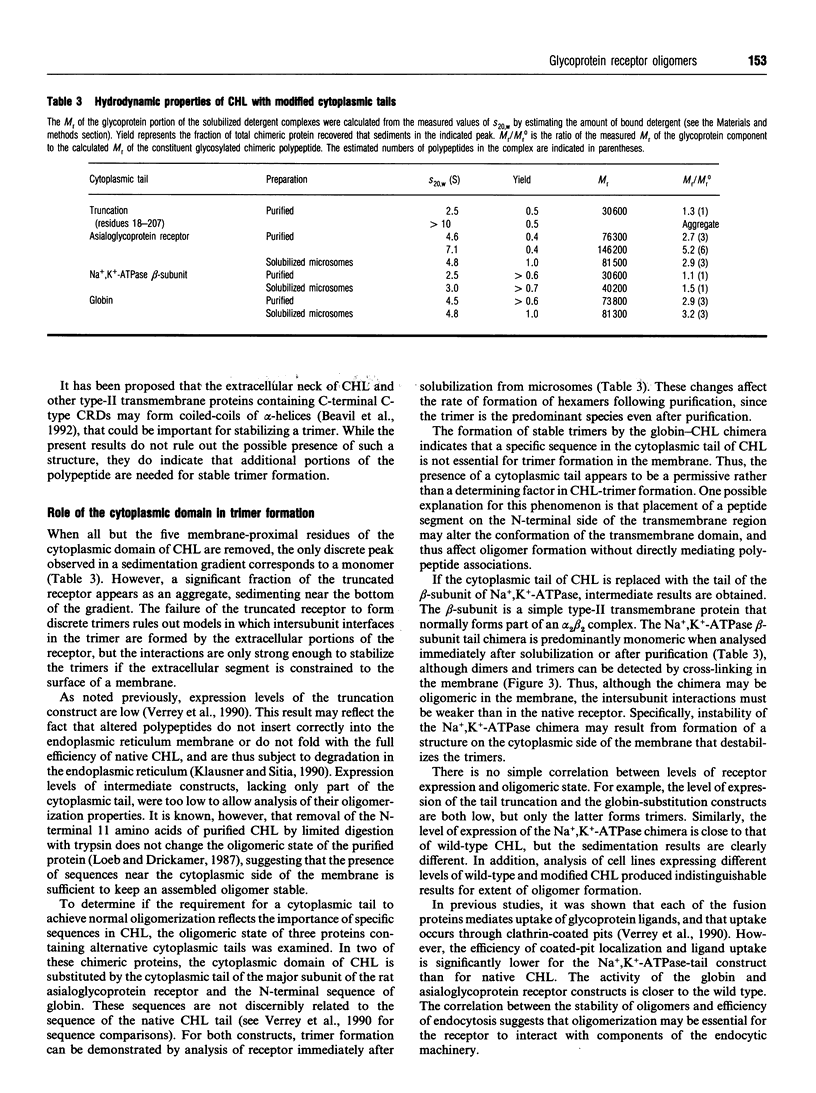
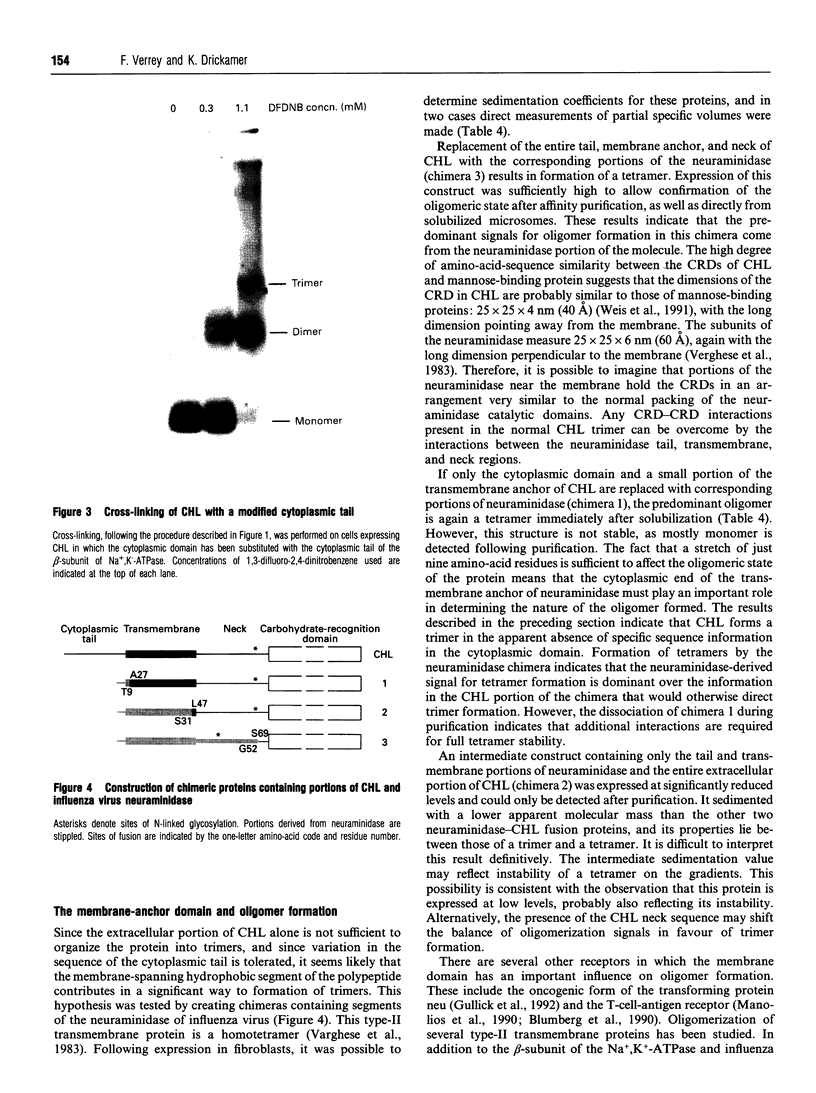

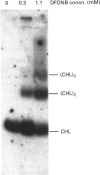
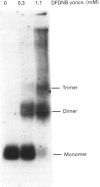
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavil A. J., Edmeades R. L., Gould H. J., Sutton B. J. Alpha-helical coiled-coil stalks in the low-affinity receptor for IgE (Fc epsilon RII/CD23) and related C-type lectins. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):753–757. doi: 10.1073/pnas.89.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezouska K., Crichlow G. V., Rose J. M., Taylor M. E., Drickamer K. Evolutionary conservation of intron position in a subfamily of genes encoding carbohydrate-recognition domains. J Biol Chem. 1991 Jun 25;266(18):11604–11609. [PubMed] [Google Scholar]
- Blumberg R. S., Alarcon B., Sancho J., McDermott F. V., Lopez P., Breitmeyer J., Terhorst C. Assembly and function of the T cell antigen receptor. Requirement of either the lysine or arginine residues in the transmembrane region of the alpha chain. J Biol Chem. 1990 Aug 15;265(23):14036–14043. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chiacchia K. B., Drickamer K. Direct evidence for the transmembrane orientation of the hepatic glycoprotein receptors. J Biol Chem. 1984 Dec 25;259(24):15440–15446. [PubMed] [Google Scholar]
- Colman P. M., Varghese J. N., Laver W. G. Structure of the catalytic and antigenic sites in influenza virus neuraminidase. Nature. 1983 May 5;303(5912):41–44. doi: 10.1038/303041a0. [DOI] [PubMed] [Google Scholar]
- Dahms N. M., Kornfeld S. The cation-dependent mannose 6-phosphate receptor. Structural requirements for mannose 6-phosphate binding and oligomerization. J Biol Chem. 1989 Jul 5;264(19):11458–11467. [PubMed] [Google Scholar]
- Doms R. W., Ruusala A., Machamer C., Helenius J., Helenius A., Rose J. K. Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. J Cell Biol. 1988 Jul;107(1):89–99. doi: 10.1083/jcb.107.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988 Jul 15;263(20):9557–9560. [PubMed] [Google Scholar]
- Fornstedt N., Porath J. Characterization studies on a new lectin found in seeds of Vicia ervilia. FEBS Lett. 1975 Sep 15;57(2):187–191. doi: 10.1016/0014-5793(75)80713-7. [DOI] [PubMed] [Google Scholar]
- Glick G. D., Toogood P. L., Wiley D. C., Skehel J. J., Knowles J. R. Ligand recognition by influenza virus. The binding of bivalent sialosides. J Biol Chem. 1991 Dec 15;266(35):23660–23669. [PubMed] [Google Scholar]
- Graeve L., Drickamer K., Rodriguez-Boulan E. Polarized endocytosis by Madin-Darby canine kidney cells transfected with functional chicken liver glycoprotein receptor. J Cell Biol. 1989 Dec;109(6 Pt 1):2809–2816. doi: 10.1083/jcb.109.6.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullick W. J., Bottomley A. C., Lofts F. J., Doak D. G., Mulvey D., Newman R., Crumpton M. J., Sternberg M. J., Campbell I. D. Three dimensional structure of the transmembrane region of the proto-oncogenic and oncogenic forms of the neu protein. EMBO J. 1992 Jan;11(1):43–48. doi: 10.1002/j.1460-2075.1992.tb05025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiti A. L., Nayak D. P. Complete nucleotide sequence of the neuraminidase gene of human influenza virus A/WSN/33. J Virol. 1982 Feb;41(2):730–734. doi: 10.1128/jvi.41.2.730-734.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland E. C., Drickamer K. Signal recognition particle mediates the insertion of a transmembrane protein which has a cytoplasmic NH2 terminus. J Biol Chem. 1986 Jan 25;261(3):1286–1292. [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Isolation and characterization of an avian hepatic binding protein specific for N-acetylglucosamine-terminated glycoproteins. J Biol Chem. 1977 Sep 25;252(18):6536–6543. [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Kundu A., Jabbar M. A., Nayak D. P. Cell surface transport, oligomerization, and endocytosis of chimeric type II glycoproteins: role of cytoplasmic and anchor domains. Mol Cell Biol. 1991 May;11(5):2675–2685. doi: 10.1128/mcb.11.5.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Lax I., Mitra A. K., Ravera C., Hurwitz D. R., Rubinstein M., Ullrich A., Stroud R. M., Schlessinger J. Epidermal growth factor (EGF) induces oligomerization of soluble, extracellular, ligand-binding domain of EGF receptor. A low resolution projection structure of the ligand-binding domain. J Biol Chem. 1991 Jul 25;266(21):13828–13833. [PubMed] [Google Scholar]
- Lobel L. I., Murphy J. E., Goff S. P. The palindromic LTR-LTR junction of Moloney murine leukemia virus is not an efficient substrate for proviral integration. J Virol. 1989 Jun;63(6):2629–2637. doi: 10.1128/jvi.63.6.2629-2637.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb J. A., Drickamer K. Conformational changes in the chicken receptor for endocytosis of glycoproteins. Modulation of ligand-binding activity by Ca2+ and pH. J Biol Chem. 1988 Jul 15;263(20):9752–9760. [PubMed] [Google Scholar]
- Loeb J. A., Drickamer K. The chicken receptor for endocytosis of glycoproteins contains a cluster of N-acetylglucosamine-binding sites. J Biol Chem. 1987 Mar 5;262(7):3022–3029. [PubMed] [Google Scholar]
- Manolios N., Bonifacino J. S., Klausner R. D. Transmembrane helical interactions and the assembly of the T cell receptor complex. Science. 1990 Jul 20;249(4966):274–277. doi: 10.1126/science.2142801. [DOI] [PubMed] [Google Scholar]
- Marks M. S., Blum J. S., Cresswell P. Invariant chain trimers are sequestered in the rough endoplasmic reticulum in the absence of association with HLA class II antigens. J Cell Biol. 1990 Sep;111(3):839–855. doi: 10.1083/jcb.111.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellow T. E., Halberg D., Drickamer K. Endocytosis of N-acetylglucosamine-containing glycoproteins by rat fibroblasts expressing a single species of chicken liver glycoprotein receptor. J Biol Chem. 1988 Apr 15;263(11):5468–5473. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moestrup S. K., Gliemann J. Analysis of ligand recognition by the purified alpha 2-macroglobulin receptor (low density lipoprotein receptor-related protein). Evidence that high affinity of alpha 2-macroglobulin-proteinase complex is achieved by binding to adjacent receptors. J Biol Chem. 1991 Jul 25;266(21):14011–14017. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Biosynthesis of the human transferrin receptor in cultured cells. J Biol Chem. 1981 Dec 25;256(24):12888–12892. [PubMed] [Google Scholar]
- Piskarev V. E., Navrátil J., Karásková H., Bezouska K., Kocourek J. Interaction of egg-white glycoproteins and their oligosaccharides with the monomer and the hexamer of chicken liver lectin. A multivalent oligosaccharide-combining site exists within the carbohydrate-recognition domain. Biochem J. 1990 Sep 15;270(3):755–760. doi: 10.1042/bj2700755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesenberry M. S., Drickamer K. Determination of the minimum carbohydrate-recognition domain in two C-type animal lectins. Glycobiology. 1991 Dec;1(6):615–621. doi: 10.1093/glycob/1.6.615. [DOI] [PubMed] [Google Scholar]
- Renaud K. J., Inman E. M., Fambrough D. M. Cytoplasmic and transmembrane domain deletions of Na,K-ATPase beta-subunit. Effects on subunit assembly and intracellular transport. J Biol Chem. 1991 Oct 25;266(30):20491–20497. [PubMed] [Google Scholar]
- Rice K. G., Weisz O. A., Barthel T., Lee R. T., Lee Y. C. Defined geometry of binding between triantennary glycopeptide and the asialoglycoprotein receptor of rat heptocytes. J Biol Chem. 1990 Oct 25;265(30):18429–18434. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shia M. A., Lodish H. F. The two subunits of the human asialoglycoprotein receptor have different fates when expressed alone in fibroblasts. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1158–1162. doi: 10.1073/pnas.86.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I., Doms R. W., Wagner K. R., Helenius A. Intracellular transport of soluble and membrane-bound glycoproteins: folding, assembly and secretion of anchor-free influenza hemagglutinin. EMBO J. 1990 Mar;9(3):631–639. doi: 10.1002/j.1460-2075.1990.tb08155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer C. J., Osborne J. C., Jr, Kempner E. S. Functional and physical molecular size of the chicken hepatic lectin determined by radiation inactivation and sedimentation equilibrium analysis. J Biol Chem. 1990 Mar 5;265(7):3744–3749. [PubMed] [Google Scholar]
- Verrey F., Gilbert T., Mellow T., Proulx G., Drickamer K. Endocytosis via coated pits mediated by glycoprotein receptor in which the cytoplasmic tail is replaced by unrelated sequences. Cell Regul. 1990 May;1(6):471–486. doi: 10.1091/mbc.1.6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis W. I., Kahn R., Fourme R., Drickamer K., Hendrickson W. A. Structure of the calcium-dependent lectin domain from a rat mannose-binding protein determined by MAD phasing. Science. 1991 Dec 13;254(5038):1608–1615. doi: 10.1126/science.1721241. [DOI] [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]