ABSTRACT
The 2021 Arctic Monitoring Assessment Program (AMAP)’s Human Health Assessment report presents a summary of the presence of contaminants in human populations across the circumpolar Arctic and provides an update to the previous assessment released in 2015. The primary objective of this paper is to summarise some of these findings by describing the current levels of metals across the Arctic, including key regional and temporal trends based on available national data and literature, and highlight knowledge gaps. Many Arctic populations continue to have elevated levels of these contaminants, and the highest levels of mercury (Hg) were observed in populations from Greenland, Faroe Islands, and Nunavik (Canada). Still, concentrations of several metals are declining in Arctic populations in regions where time trends data exist, although the declines are not consistent across all regions. The 2021 AMAP human health assessment report and this paper provide an extensive summary of levels of metals and trace elements in adults, pregnant women, and children across the Arctic.
KEYWORDS: Arctic, biomonitoring, contaminants, metals, mercury, lead
Introduction
Contaminants such as mercury have been widely detected in the Arctic environment, wildlife, and in Arctic human populations [1,2]. For some Arctic human populations, the hunting, harvesting and consumption of Arctic wildlife and other traditional foods (also commonly known as country foods or subsistence foods) can be a source of exposure for some metals and trace elements. Several species of Arctic wildlife, particularly marine mammals at the top of the Arctic food web, can have elevated levels of contaminants such as mercury [1]. As a result, human consumption of some parts of such marine mammals and other traditional foods can lead to elevated exposures to these contaminants as reported in the previous AMAP 2015 human health assessment [2].
Despite this, the harvesting of traditional foods has significant benefits to many Arctic Indigenous populations, including nutritional, social, cultural, economic and spiritual wellbeing. Consumption of traditional foods can also provide a source of nutrients and trace elements such as omega-3 fatty acids, iodine, vitamin D and selenium [3–5], some of which have been found to interact with mercury and/or potentially provide a protective effect against mercury toxicity [6,7].
Exposure to metals can also occur from several other sources. Use of lead shot for hunting has also been associated with exposure to lead, both from the consumption of wildlife killed with lead bullets but also from the use and storage of lead ammunition [8,9]. Cadmium is another heavy metal that is often detected in a number of terrestrial mammals, and the consumption of these traditional foods is a source of exposure to cadmium. Meanwhile, other major sources of exposure to cadmium and lead in Arctic populations include smoking and exposure to second-hand smoke (i.e. environmental tobacco smoke), as smoking is still quite prevalent in some circumpolar regions [10–13].
The collection of biomonitoring data is important for understanding exposure to contaminants and informing health officials, to better inform risk management and risk communication messaging. As contaminants in the Arctic remain a concern, human biomonitoring studies conducted over the last few decades have provided important data on current levels of these contaminants across the Arctic and identified a number of regional and temporal trends.
Materials and methods
This article presents a summary of human biomonitoring data from circumpolar Arctic countries as detailed in the 2021 AMAP Human Health Assessment Report [14]. The report provides an update to the AMAP 2015 report on the presence of metals and trace elements in human populations living in the Arctic. Biomonitoring data are presented for pregnant women, adult men and women, women of childbearing age, children and youth. These data have been collected as part of regional cross-sectional studies or ongoing cohort studies.
It has been reported in previous AMAP assessments that the mere participation in an external QA/QC programme has been shown to result in improved performance over time [15]. Data presented in this article are primarily from laboratories that are active participants in different QA/QC programmes, particularly the AMAP Ring test [14]. Only in a few cases, data from peer reviewed journal articles included in the AMAP report have come from laboratories which have used rigorous laboratory analysis procedures but have not participated in external QA/QC programmes. Information on the AMAP Ring and QA/QC methodology are described in greater detail in the AMAP human health assessments [2,14]. The data for metals presented are reported in blood on a wet weight basis (µg/L whole blood). Metals and trace elements that are presented across most Arctic regions include total mercury (Hg), lead (Pb), cadmium (Cd), selenium (Se), and in some regions data are also presented for arsenic (As), nickel (Ni), manganese (Mn), cobalt (Co), copper (Cu), and zinc (Zn). Comparisons made between populations or contaminants are descriptive comparisons only, unless specified in text as statistically significant comparisons and provided with original reference citations which detail the statistical methods used.
This article presents the main highlights of Arctic biomonitoring data from the latest AMAP human health assessment, and for a more extensive presentation of all available biomonitoring data readers should refer to Chapter 3 of the 2021 AMAP Human Health Assessment Report [14].
Results
The Arctic is populated by many different Indigenous and non-Indigenous peoples residing in eight different countries, with different cultural and dietary practices. Exposures to contaminants in each region are varied and unique, and therefore recent biomonitoring data and key findings from studies in Arctic regions are presented here in a regional manner by country.
Canada
Across the Canadian Arctic, multiple biomonitoring studies have collected data on blood concentrations of metals in both First Nations and Inuit populations, both of which rely on the consumption of country foods. In the Western Canadian Arctic, blood concentrations of metals in adult Gwich’in men and women from Old Crow (Yukon) are presented in Table 1 [16]. Concentrations of Hg and Pb were relatively higher among Gwich’in men than women, while concentrations of Cd and Se were similar between men and women. Concentrations of Hg and Cd in Old Crow are relatively similar to those in other First Nations communities and in the general population of Canada [16]. Concentrations of Pb, however, were elevated in Old Crow compared to non-Arctic Canadian populations [16], including more than twice as high as in the general Canadian population (CHMS Cycle 5, 2016–2017) [17] and First Nations communities represented by the First Nations Biomonitoring Initiative (FNBI) [18]. Complementary work was completed in the Mackenzie Valley of the Northwest Territories, for which blood concentrations of metals in adult men and women from nine First Nations communities from the Dehcho and Sahtú regions are presented in Table 2 [19]. Hg levels in blood were generally low in adult men and women, with a majority of participants having concentrations below the limit of detection. To measure Hg exposure over a 12-month period preceding sample collection, hair samples were also collected from participants in nine communities of the Dehcho and Sahtú regions. Segmental hair analysis showed significant differences among time periods, with the highest mean hair Hg levels occurring between July and October [20].
Table 1.
Blood concentrations of heavy metals in adults in Old Crow, Yukon, Canada. Data are presented as geometric means (10th −95th percentile) in whole blood (µg/l). Source: [16].
| |
Men |
Women |
||
| Year(s) | 2019 | 2019 | ||
| Mean age (range) | 43 (21–75) | 39 (20–72) | ||
| Sample size |
n = 26 |
n = 28 |
||
| µg/L whole blood |
% <LOD1 |
µg/L whole blood |
% <LOD1 |
|
| Cadmium | 0.85 (0.10–4.7) | 0 | 0.85 (0.19–4.3) | 0 |
| Lead | 30 (12–110) | 0 | 19 (6.4–110) | 0 |
| Mercury | 0.90 (0.11–6.2) | 3.8 | 0.66 (0.10–3.2) | 7.1 |
| Selenium | 170 (150–280) | 0 | 170 (150–220) | 0 |
1LOD: Limit of detection.
Table 2.
Blood concentrations of heavy metals in adults across multiple First Nations communities in the Northwest Territories, Canada. Data are presented as geometric means (10th −95th percentile) in whole blood (µg/l). Source: [19].
| |
Men |
Women |
||
| Year(s) | 2016–2018 | 2016–2018 | ||
| Mean age (range) | 47.3 (18–88) | 45.6 (18–80) | ||
| Sample size |
n = 128 |
n = 122 |
||
| µg/L whole blood |
% <LOD1 |
µg/L whole blood |
% <LOD1 |
|
| Cadmium | 0.373 (<0.006–3.58) | 10.2 | 0.404 (<0.006–3.15) | 9.8 |
| Lead | 21.0 (8.42–74.4) | 0 | 14.8 (5.36–73.6) | 0 |
| Mercury | nc2 (<0.03–7.68) | 55.5 | nc2 (<0.03–3.89) | 63.1 |
| Selenium | 170.1 (136–225) | 0 | 171 (140–229) | 0 |
1LOD: Limit of detection.
anc: not calculated as number of non-detects greater than 50%.
Blood Pb was higher in the population from the Sahtú region than the Dehcho region, while blood Cd and Hg levels were higher in the population from the Dehcho [19]. Country food consumption in Dene/Metis communities in the Northwest Territories was not associated with measured cadmium concentrations, however smoking was a determinant of cadmium exposure [10]. Similar to Gwich’in adults in the Yukon, blood Pb levels in the populations in the Dehcho and Sahtú region of the NWT were substantially higher than that observed in the general population of Canada, with a geometric mean and 95th percentile of blood Pb that was 1.7- and 2.8-fold higher than observed in the general Canadian population (CHMS Cycle 5, 2016–2017), respectively [19], although not as high as blood Pb levels observed among Gwich’in adults from the Yukon. Blood Cd levels in the populations from the Dehcho and Sahtú were also observed to have a higher geometric mean relative to the CHMS and FNBI.
In Nunavik, located in the eastern Canadian Arctic, concentrations of metals have been measured in youth, adults and pregnant women. Concentrations of metals in Inuit adults (18 years and above) and youth (16–17 years of age) were reported in the “Qanuilirpitaa? - How are we now?” 2017 Nunavik Inuit Health Survey (Lemire et al., 2021), which involved 1326 participants. Survey results are weighted by survey weights and presented in Tables 3 and 4. Geometric mean Hg concentrations were significantly higher in women than men (10 and 8.1 µg/L, respectively), as were concentrations of Se (330 and 280 µg/L, respectively). Concentrations of Pb, however, were significantly higher in men compared to women, and concentrations of Cd were not significantly different between men and women (Table 3) [21,22]. For most of the metals measured, concentrations appeared to increase with age with the highest geometric mean concentrations observed in the oldest age group (50+ years) for both men and women (Table 4). This was particularly evident for Hg where the mean concentrations in the oldest age group (50+) were almost double those of the younger adult group (18–49), and three-fold higher than the youngest age group (16–17) in men. It is also noted that mean Hg concentrations of women at 16–17 years of age were approximately double those of men in the same age range. Unlike Hg, Pb, and Se, geometric mean concentrations of Cd were lowest in the oldest age group for both men (0.97 µg/L) and women (1.5 µg/L). While concentrations of Cd in men were highest in the 18–49 year-old age group (1.8 µg/L), the highest concentrations of Cd in women occurred in the youngest at 16–17 years old (2.1 µg/L). Differences between age groups were statistically significant for mercury, lead, cadmium and selenium [21,22].
Table 3.
Blood concentrations of metals in Inuit adults (18 years old and older) from Nunavik, Canada, in the “Qanuilirpitaa? – how are we now?” 2017 Nunavik Inuit health survey. Data are presented as geometric means (10th − 95th percentile) in whole blood (µg/l) and results are weighted by survey weights. Source: [21,22].
| |
Men |
Women |
||
| Year | 2017 | 2017 | ||
| Mean age (range) |
38.9 (18–86) |
38.4 (18–81) |
||
| µg/L whole blood |
% <LOD1 |
µg/L whole blood |
% <LOD1 |
|
| Mercury | 8.1 (1.9-36) | 0 | 10 (2.6-42) | 0 |
| Lead | 28 (11-93) | 0 | 24 (9.3-98) | 0 |
| Cadmium | 1.6 (0.25-5.6) | 0 | 1.7 (0.32-5.1) | 0 |
| Selenium | 280 (160-670) | 0 | 330 (170-820) | 0 |
1LOD: Limit of detection.
Table 4.
Blood concentrations of metals in three different age groups of Inuit from Nunavik in the “Qanuilirpitaa? – how are we now?” 2017 Nunavik Inuit health survey. Data are presented as geometric means (10th −95th percentile) in whole blood (µg/l) and results are weighted by survey weights. Source: [21,22].
| |
Men |
Women |
||||
| Year | 2017 | 2017 | ||||
| Mean age (range) |
16.6 (16–17) |
31.4 (18–49) |
60.0 (50-86) |
16.6 (16–17) |
31.1 (18–49) |
58.7 (50-81) |
| Mercury | 3.5 (0.3–18) |
7.1 (1.8-29) |
12 (3-54) |
7.2 (2.1-23) |
8.7 (2.3-38) |
15 (5.4-51) |
| Lead | 18 (8.2-60) |
26 (11-81) |
38 (14-110) |
14 (6.9-54) |
21 (8.5-68) |
39 (17-110) |
| Cadmium | 1.6 (0.12-4.8) |
1.8 (0.25-5.8) |
0.97 (0.24-3.7) |
2.1 (0.24-6.1) |
1.8 (0.28-5.2) |
1.5 (0.33-4.9) |
| Selenium | 210 (120-390) |
270 (150-620) |
320 (160-750) |
260 (160-550) |
320 (160-790) |
370 (190-950) |
As further detailed in Lemire et al. [21], marine mammal and fish consumption was associated with higher blood mercury levels, confirming previous findings showing that beluga meat was the specific country food that contributed the most to mercury exposure [5]. Among adults of the 2017 Nunavik Inuit Health Survey, blood mercury levels were strongly and positively associated with long-chain omega-3 polyunsaturated fatty acids in red blood cells, and this supports the previous observation that the consumption of country foods, particularly marine foods, which are exceptionally rich in these high-quality fats, contribute to mercury exposure [21]. The use of lead ammunition for hunting has been previously associated with lead exposure among Inuits, including exposure to house dust containing lead [9,23]. Lead ammunition, and particularly lead pellets, might still contribute to lead exposure in Nunavik as blood lead levels were higher among Inuit who went hunting frequently, used lead pellets and reported living in houses where guns were cleaned indoors [21]. Blood lead levels were also higher among people who reported consuming cigarettes or cannabis as well as among those who declared being regularly exposed to second-hand smoke. Consumption of wild birds was associated with higher blood lead levels, and surprisingly higher blood lead levels were also associated with marine mammals [21]. Blood Cd concentrations have previously been associated with smoking rather than country food consumption [24], and similarly in this Inuit Health Survey current smokers exhibited elevated blood cadmium levels. Blood cadmium levels were also high in ex-smokers and people who reported consuming cannabis or being frequently exposed to second-hand smoke. No association was found between blood cadmium levels and country food consumption [21].
Blood concentrations of several metals in Nunavik adults have declined over the past 25 years, with Hg concentrations decreasing by 44%, lead levels by 71% and cadmium levels by 58% [21]. When comparing the most recent 2017 survey results to the earlier “Qanuippitaa? – How are we?” Nunavik Inuit Health Survey in 2004, Hg levels have declined only moderately (men from 9.2 to 8.1 µg/L; women from 12 to 10 µg/L), whereas lead levels have decreased markedly, primarily among men (men from 46 to 28 µg/L; women 34 to 24 µg/L) and cadmium levels by nearly half (from 3.0 to 1.6 µg/L in men and women). Still, blood mercury levels in Inuit adults in 2017 were 12-fold higher than those of the CHMS Cycle 4 (2014–2015) [25], while blood lead levels were two-fold higher and blood cadmium levels five-fold higher than in CHMS.
In addition to three health surveys among adult men and women (1992, 2004, and 2017), extensive time trends data series have been collected over several decades for blood concentrations of metals in pregnant women from Nunavik. Statistically significant declines have been observed for Hg, Pb, Cd and selenium between 1992 and 2017 (Table 5). Even though geometric mean blood concentrations of Hg have fluctuated over time, with temporary increases observed between 1998 and 2001, overall blood concentrations of Hg have declined by approximately half between 1992 and 2017. The greatest declines were observed between 1992 and 2007, while only small changes have been observed between 2007 and 2017. When studying monthly hair Hg levels of pregnant women in Nunavik in 2016–2017, seasonality was found to be an important factor as prenatal exposure to Hg was significantly higher in the summer months, especially in the Hudson Strait region of Nunavik which is when and where most of the beluga hunting takes place in Nunavik [26]. Beluga meat and nikku (air-dried meat) were identified as the most important dietary source of MeHg exposure for pregnant women, as well as adult Inuit [5,26].
Table 5.
Trends in metal concentrations in pregnant Inuit women from Nunavik, Canada. Data presented as geometric means (range) in µg/L whole blood. Results are presented only for contaminants with 60% and more of data detected. Source: [2,27].
| Year | 1992 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2004 | 2007 | 2012 | 2013 | 20171 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean age (range) |
24 (18–35) |
24 (16–33) |
24 (15–40) |
24 (14–33) |
24 (17–35) |
25 (16–35) |
26 (17–39) |
27 (19–37) |
23 (17–37) |
24 (18–39) |
24 (18–41) |
24 (15–38) |
p-value2 |
| Sample size | n = 11 | n = 25 | n = 53 | n = 27 | n = 16 | n = 29 | n = 18 | n = 25 | n = 42 | n = 111 | n = 95 | n = 123 | |
| Total mercury | 11 (3.6–33) |
13 (4.2–29) |
11 (3.8–44) |
6.9 (3.2–27) |
8.8 (2.6–31) |
8.6 (1.8–38) |
9.5 (1.6–33) |
7.4 (1.3–30) |
4.3 (0.68–24) |
5.1 (0.18–40) |
5.1 (0.28–32) |
5.4 (0.70–40) |
<0.0001 |
| Lead | 42 (8.3–170) |
48 (17–140) |
56 (10–260) |
53 (27–130) |
54 (19–110) |
43 (10–140) |
35 (10–130) |
17 (5.8–85) |
17 (6.6–77) |
13 (2.7–230) |
14 (4.2–62) |
13 (4.1–230) |
<0.0001 |
| Cadmium | 2.8 (0.36–7.5) |
na3 | na3 | na3 | na3 | na3 | na3 | 2.8 (0.36–8.0) |
2.0 (0.22–7.6) |
na3 | na3 | 1.5 (0.079–4.4)4 |
0.0046 |
| Selenium | na | 360 (190–620) |
320 (190–980) |
290 (180–460) |
300 (150–570) |
330 (190–1230) |
250 (200–390) |
260 (130–700) |
230 (130–710) |
320 (120–2990) |
300 (130–1420) |
250 (120–670) |
0.0033 |
1Weighted estimates for 2017 (Hg, Pb, Se).
ap-value based on orthogonal polynomial contrast for linear trend, using regression adjusted for age, smoking status (smoker vs. non-smoker) and multiparous woman (yes vs. no); for statistical purposes, values <LOD were replaced by LOD/2.
bna: not available.
cn = 32.
Blood Pb concentrations initially increased in pregnant women during the 1990s, but a steady decline has been observed since the late 1990s, which coincides with a voluntary ban on Pb pellets for hunting. Concentrations of Pb appear to have reached a plateau in 2012 with only slight fluctuations observed since then. Data points are sparser for Cd, however significant declines have also been observed between 1992 and 2017 as shown in Table 5. Statistically significant differences were observed for Se as well, however unlike Hg and Pb the changes in Se concentrations have been more modest. While concentrations appear to have declined throughout the 1990s and mid-2000s, this decline was followed by a brief increase in 2012 and 2013 before declining again in 2017. Country foods from the sea, particularly beluga mattaaq which is an Inuit delicacy made of whale skin and blubber, are particularly rich in Se. The fluctuations of blood Se concentrations in pregnant women may be due to time of blood sampling as well as the seasonality of marine mammal consumption by pregnant women.
It is also worth noting that recent studies have shown that the main selenocompound found in Nunavik Inuit blood is selenoneine, which has also been found to be the major form of Se in beluga mattaaq [3,4,28].
Greenland
Some of the highest concentrations of contaminants in the Arctic have historically been observed in Greenland especially in east Greenland, and concentrations of metals have been measured in Inuit children and pregnant women across several studies. The ACCEPT mother-child birth cohort (2010–2015) involved the recruitment of pregnant Inuit women from a total of 19 communities across Greenland and results are divided into 5 distinct regions for analysis purposes (see Table 6).
Table 6.
Blood concentrations of metals in the Greenland Inuit pregnant women of the ACCEPT cohort 2010–2015. Data are presented as geometric means (range) in whole blood. Source: [29].
| North | Disko Bay | West | South | East | p-value1 | Total | ||
|---|---|---|---|---|---|---|---|---|
| Mean age (range) |
28.4 (20–36) |
27.1 (18–41) |
27.4 (18–42) |
28.6 (20–41) |
28.2 (19–43) |
27.5 (18–43) |
||
| Sample size | n = 32 | n = 123 | n = 289 | n = 44 | n = 20 | n = 509 | ||
| Metal (µg/L) | % <LOQ2 | |||||||
| Mercury | 6.5 (1.28–54.5) |
4.48 (0.83–69.3) |
3.32 (0.32–73.0) |
3.44 (0.83–16.8) |
10.2 (2.10–29.3) |
<0.001 | 3.9 (0.32–73.0) |
21.6 |
| Lead | 7.67 (4.32–26.3) |
6.54 (1.58–31.5) |
7.12 (1.58–64.1) |
7.14 (2.72–48.4) |
9.76 (4.30–58.4) |
0.069 | 7.1 (1.58–64.1) |
37.8 |
| Cadmium | 0.87 (0.17–3.98) |
1.09 (0.11–10.8) |
0.92 (0.11–7.79) |
0.85 (0.11–4.75) |
1.61 (1.05–3.68) |
0.011 | 0.97 (0.11–10.8) |
65 |
| Selenium | 164 (59.8–1096) |
141 (62.0–2248) |
127 (45.4–2795) |
118 (74.4–259) |
145 (83.0–267) |
0.019 | 132 (45.4–2795) |
0 |
| Serum-selenium | 76.2 (29.4–157) |
69.1 (27.3–153) |
69.4 (5.25–177) |
71.2 (23.1–145) |
71.5 (37.8–105) |
0.785 | 70 (5.25–177) |
0.1 |
1difference among regions was tested by one-way ANOVA analysis.
aLOQ: limit of quantification.
Statistically significant regional differences were observed for several metals, with the highest geometric mean concentrations of Hg, Pb and Cd observed in eastern Greenland. Hg and Pb were positively correlated with marine food intake and the n-3/n-6 ratio. Pb and Cd also correlated positively with the current smoking biomarker (serum cotinine). Moreover, positive correlation was also found between age and levels of Se and Pb [29].
Due to repeated collection of blood metals data in pregnant women since the mid-1990s, trends of Hg and Pb blood levels in pregnant women are available and presented in Figure 1. Multiple time points from Disko Bay and Nuuk show a declining trend for Pb, but there does not appear to be a clear trend for Hg, although in Disko Bay approximately 50% lower Hg levels were found in the ACCEPT pregnant women 2010–2015 compared to pregnant women in 2006 [14,30].
Figure 1.
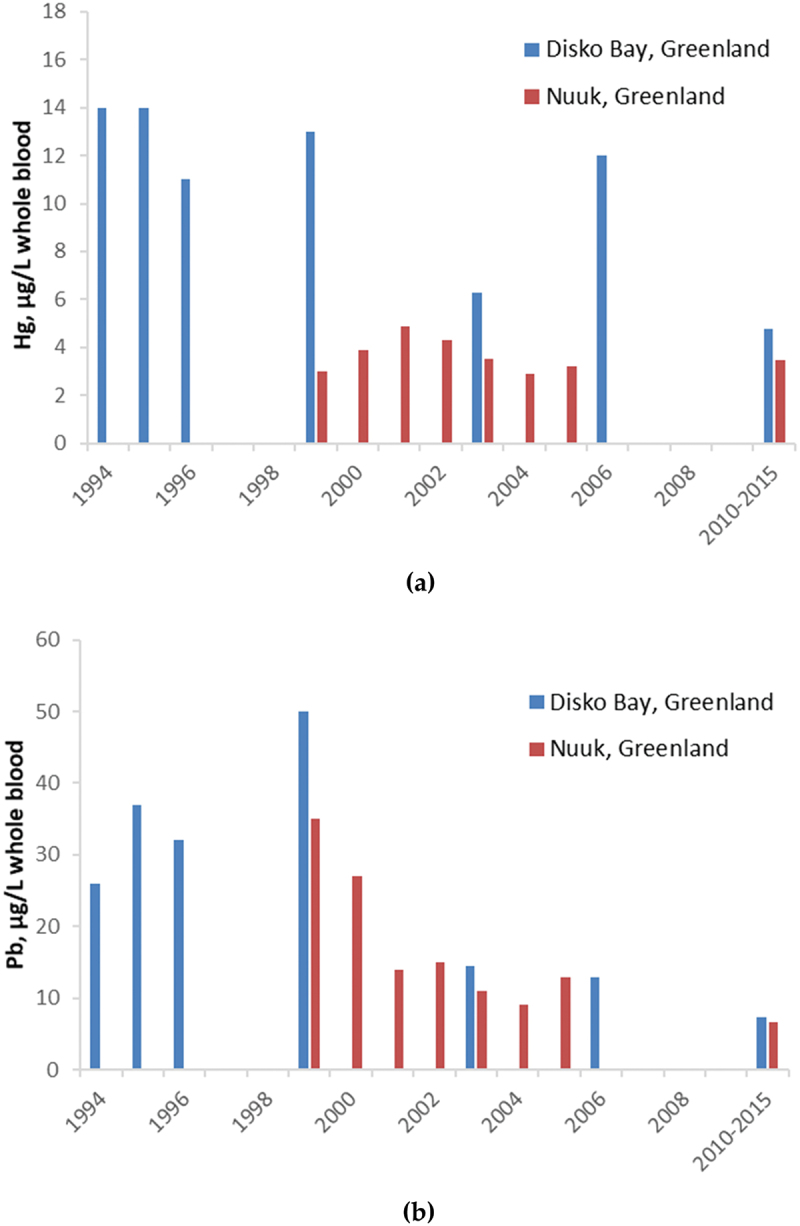
Temporal trends for (a) mercury and (b) lead in pregnant women from Disko Bay (blue) and Nuuk (red), Greenland. Metals presented in blood (µg/L whole blood). Source [14].
Blood Hg concentrations have also been measured in children (OCEANS study) between 2012 and 2015 [31]. The children resided in eight towns across the Disco Bay area, West Greenland and East Greenland. Hg was detected in all children, and girls had slightly higher geometric mean concentrations of Hg than boys (3.76 and 3.12 µg/L respectively) while also having a wider concentration range which was also reflected by the 5th−95th percentiles in girls (0.96–15.05 µg/L) compared to boys (0.75–12.97 µg/L). Concentrations of Hg were also significantly different (p < 0.001) between communities (Table 7), and the lowest geometric mean concentrations were found in Nuuk and western Greenland (Sisimiut and Maniitsoq), while the highest were in eastern Greenland (Tasiilaq) with a geometric mean of 8.73 µg/L in blood. These differences could be caused by differences in dietary intake, as frequent consumption of local Greenlandic food was associated with higher blood-Hg concentrations [31].
Table 7.
Regional comparisons of blood concentrations of contaminants in Greenlandic children between 2012 and 2015. Data are presented as geometric means (range); mercury in whole blood (µg/L). Source: [31].
| Region | Greenland (all) | Nuuk | Disko Bay1 | West2 | East3 | p-value4 |
|---|---|---|---|---|---|---|
| Mean age (range) |
9.8 (7.1–12.1) |
9.3 (7.3-11.0) |
9.5 (7.1–11.5) |
10.4 (8.1–11.6) |
10.5 (9.1–12.1) |
|
| Sample size | n = 333 | n = 81 | n = 128 | n = 100 | n = 24 | |
| Mercury | 3.41 (0.16–98.9) |
2.19 (0.32–8.06) |
4.34 (0.29–18.5) |
2.86 (0.16–25.7) |
8.73 (4.13–98.9) |
<0.001 |
aQeqertarsuaq, Aasiaat, and Ilulissat.
bSisimiut and Maniitsoq.
cTasiilaq.
ddifferences between areas were tested using Kruskal Wallis test.
Iceland
While extensive monitoring of POPs has been conducted in Iceland among pregnant women, there is relatively less information on the presence of metals in Icelandic people, although some biomonitoring data are available for Hg and Se among pregnant women. In 1995, pregnant women (age 18–41 years) in Reykjavik had geometric mean concentrations of 3.1 µg/L Hg in whole blood and 96.0 µg/L Se in whole blood [14]. Limited metals data are available for Iceland, so this represents baseline Hg and Se data for the region. Blood samples were also collected from pregnant women in 2014–2015 [32] and geometric mean Hg concentrations were 1.29 µg/L, which represents a decline from levels previously reported from 1995.
Faroe Islands
Due to concerns associated with pilot whale consumption, several cohorts have been initiated in the Faroe Islands since the late 1980s to investigate Faroese exposure to contaminants including mercury. Pregnant women and cohort children have been followed-up over multiple time points and concentrations of Hg from Cohorts 1 and 5 are shown in Table 8. Since cord blood samples were first collected in 1986–1987, total Hg concentrations in the blood of cohort 1 children have decreased from 8.4 µg/L in 1993–1994, to 4.1 µg/L in 2000–2001, to 2.5 µg/L in 2008–2009 when participants had reached adulthood at age 22 years. Since then, however, the trend for Hg has reversed, and in 2013–2016 the mean concentration in 28 year-olds had increased to 3.93 µg/L.
Table 8.
Time series of blood Hg in children from Faroe Islands Cohort 1 and Faroe Islands Cohort 5. Data are presented as geometric means (range) in whole blood (µg/L). Source: [2,14].
| |
Cohort 1 |
Cohort 5 |
|||||||
| Year(s) | 1986–1987 | 1993–1994 | 2000–2001 | 2008–2009 | 2013–2016 | 2007–2009 | 2009–2011 | 2012–2014 | 2016–2018 |
| Mean age | Cord blood | 7 | 13.8 | 22.1 | 28 | Cord blood | 1.5 | 5 | 9 |
| Sample size | n = 1022 | n = 922 | n = 792 | n = 849 | n = 703 | n = 500 | n = 363 | n = 349 | n = 390 |
| Total mercury | 22.3 (0.9–350) |
8.4 (0.1–62.8) |
4.1 (0.3–39.8) |
2.5 (0.1–46.3) |
3.93 (0.111–84.4) |
4.6 (0.8–44.5) |
1.4 (0.1–21.3) |
2.52 (0.21–40.9) |
1.40 (0.067–41.8) |
Concentrations of Hg are much lower in the fifth birth cohort of children, compared to Cohort 1 children of similar age groups. As the fifth cohort is more recent, fewer time points are available; however, these time points do indicate an increase in Hg between the ages of 1.5 years (1.4 µg/L in 2009–2011) and 5 years (up to 2.52 µg/L in 2012–2014). Further follow-up of Cohort 5 children at age 9 years in 2016–2018 showed concentrations had decreased to 1.4 µg/L. Whether this decline will continue has yet to be seen, however it will be many years before this cohort is old enough to be compared to adult children from the first Faroe Islands Cohort.
Sweden
The cross-sectional national dietary survey Riksmaten Adolescents 2016–2017 was undertaken by the Swedish Food Agency during the 2016–2017 school year [33] and included students in grades 5, 8 and 11, and concentrations of metals in whole blood from these adolescents (all) are presented in Table 9. Almost all adolescents (97.3–99.9%) had detectable concentrations of Cd, Hg and Pb in whole blood. Higher concentrations of Hg and Pb were observed among boys (0.77 µg Hg/L and 8.3 µg Pb/L) compared to girls (0.60 µg Hg/L and 6.7 µg Pb/L), while concentrations of Cd were higher in girls (0.13 µg/L) compared to boys (0.12 µg Hg/L) [34]. Higher fish consumption in boys may partly explain the observed difference between boys and girls [34], and higher Cd concentrations in girls may be due to lower iron stores in girls which leads to increased Cd absorption [35]. The results also showed that Hg concentrations were positively associated with fish consumption and with household education [34]. Further analysis of data by region found that there were no observable significant differences between regions in Sweden for Hg, Pb and Cd [36] and this is similar to results presented in a previous study among Swedish adults [37]. There were no significant associations between age and blood levels of Hg and Pb, however Cd concentrations increased with age with higher concentrations in adolescents 17–18 years of age (0.14 µg/L) than at 11–12 years of age (0.10 µg/L) [34].
Table 9.
Whole blood concentrations of metals in adolescents in Sweden. Metals data are presented as arithmetic means (5th −95th percentile) in whole blood (µg/L whole blood). Source: [36].
| Year(s) |
2016–2017 |
|
| Mean age (range) |
14 (10-21) |
|
| Sample size |
n = 1099 |
|
| µg/L whole blood |
% <LOD1 |
|
| Cadmium | 0.16 (0.06–0.35) |
2.7 |
| Mercury | 0.90 (0.13–2.06) |
0.7 |
| Lead | 8.35 (3.71–16.32) |
0.1 |
1LOD: limit of detection.
Data on several metals in Swedish adults go back several decades (1990–2014) for Hg, Pb and Cd [38–40] from the population-based Northern Sweden Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) study. Mercury was measured in erythrocytes from adults in 1990, 1994 and 1999 and a strong decline in Hg levels is clear in men (age 25–73 years) and women (age 25–74 years) (Figure 2). For men and women combined, this was a decrease of 5.8% per year [38]. From 2004 onwards, Hg was measured in whole blood, although in 2004 data were only available for women. Geometric mean concentrations of Hg in women in the younger and older age groups both declined between 2004 and 2009, with the decline slightly stronger in the older women (1.43 to 1.22 µg/L) than the younger women (0.725 to 0.662 µg/L). In 2009, concentrations of Hg in whole blood were measured in two age groups for men. The geometric mean concentrations for Hg in the younger men (0.583 µg/L) were approximately half that of the older men (1.10 µg/L).
Figure 2.
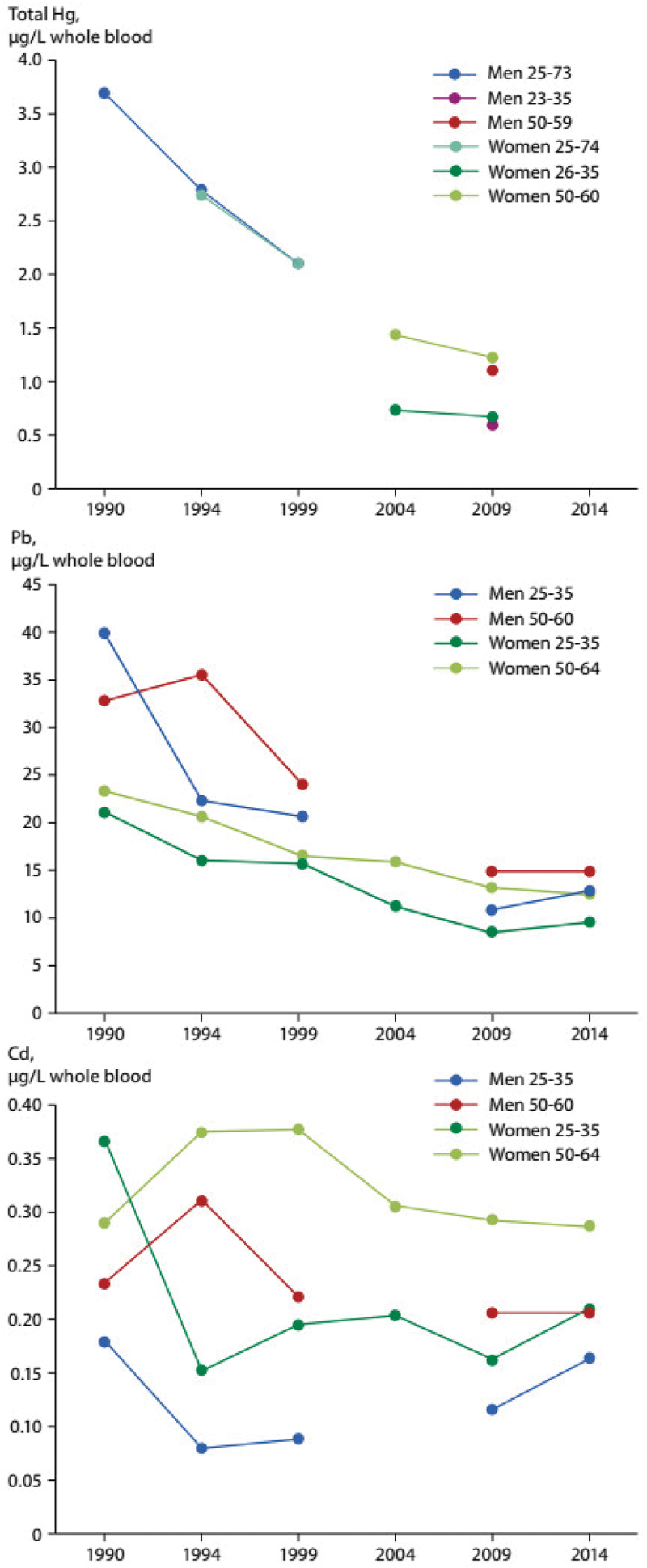
Time trends of total mercury, lead, and cadmium in the adult population of northern Sweden 1990–2014. Data presented as geometric means in whole blood (µg/l) except for mercury which is presented in erythrocytes (µg/l) in 1990, 1994 and 1999. Source [38–40].
Concentrations of Pb in whole blood were measured in men and women, in two different age groups, between 1990 and 2014, except for 2004 when only women were measured. A strong decline in Pb concentrations was seen in men and women of both age groups (Figure 2), with geometric mean concentrations in 2014 around 32–53% of those measured in 1990, however changes in Pb concentration were relatively minor after 2000. Between 1990 and 1999, Pb median levels decreased in men (all) and women (all) by 5.6% and 4.4% per year (age-adjusted), respectively [39]. In 2014, geometric mean concentrations of Pb in whole blood were 12.7 and 9.59 µg/L in the young age groups for men and women, respectively. At the same time, in the older age groups, men had a geometric mean concentration of 14.9 µg/L and women, 12.4 µg/L. Thus, in both men and women, higher concentrations were observed in the older age groups and in both age groups, men had slightly higher concentrations than women, which was also observed at earlier time points. Higher blood Pb associated with consumption of moose meat was demonstrated in men, but not women, raising the question of whether shooting itself contributes more to Pb exposure than eating moose meat shot with Pb pellets, since hunting and shooting was more common among men than women [39], and some studies have shown that shooting with Pb pellets can be a significant factor affecting blood Pb levels [41,42].
Cadmium was also measured in blood from 1990 to 2014 in the same two age groups as Pb. Cd levels in whole blood did not decrease over this period and an analysis of annual median concentration changes in never-smoking women even found an increase of 3% per year (age-adjusted) between 2009 and 2014 [39]. In Figure 2, geometric mean concentrations of Cd from 2014 in young men and women were 0.165 and 0.210 µg/L, respectively. Among men and women in the older age group, geometric mean concentrations in 2014 were higher at 0.206 and 0.287 µg/L, respectively. Smoking was confirmed as a major source of Cd in the Northern Sweden MONICA study [39].
Finland
The Northern Finland Birth Cohort programme (NFBC) was established in 1966 as a prospective longitudinal general population research programme and a follow-up of this cohort was conducted in 1997, which measured blood concentrations of metals including As, Cd, Pb, total Hg and Se (Table 10). Arsenic concentrations were not significantly different in men and women, and consumers of large amounts of fish did not have significantly higher concentrations of As than non-consumers [43]. Cadmium results indicated significantly higher concentrations in men than women (0.18 vs 0.12 µg/L) and levels in daily smokers were almost ten-fold higher than never-smokers (0.68 vs 0.07 µg/L) [43]. Lead concentrations were also higher in men than women (17.06 vs 9.06 µg/L) and daily consumers of reindeer or moose had higher concentrations than non-consumers (17.57 vs 11.17 µg/L) although the difference was not significant [43]. Daily consumers of sweetened drinks had significantly higher concentrations of Pb than non-consumers (17.80 vs 9.66 µg/L) [43]. The concentration of Hg was also significantly higher in men than women (2.18 vs 1.85 µg/L) and concentrations in regular consumers of fish (almost every day) were markedly higher than in those consuming fish less than once per month (4.91 vs 0.87 µg/L) [43]. Individuals with blood Hg concentrations <3 µg/L had on average 50% organic Hg while those with values >3 µg/L had 50–96% organic Hg. Correlations between concentrations of metals showed significant associations in women between As and Hg, Cd and Pb, Pb and Hg, Pb and calcium, Cd and Se, and Hg and Se. Among men, significant associations were found between Cd and Pb, Cd and Se, and Pb and Hg [43].
Table 10.
Blood concentrations of heavy metals in Finnish adults. Data presented as geometric means (range), in blood volume (µg/L whole blood). Source: [43].
| Men | Women | Combined | |
|---|---|---|---|
| Year | 1997 | 1997 | 1997 |
| Mean age | 31.1 | 31.1 | 31.1 |
| Sample size | n = 126 | n = 123 | n = 249 |
| Arsenic | 0.49 (0.08–18.02) |
0.44 (0.08–5.92) |
0.47 (0.08–18.02) |
| Cadmium | 0.18 (0.05–4.03) |
0.12 (0.05–3.37) |
0.15 (0.05–4.03) |
| Lead | 17.06 (3.9–145.5) |
9.06 (0.8–91.9) |
12.44 (0.8–145.5) |
| Mercury | 2.18 (0.32–14.54) |
1.85 (0.33–11) |
2.02 (0.32–14.54) |
| Selenium | 106.0 (49.5–195.2) |
94.34 (30–244.8) |
100.0 (30–244.8) |
Russia
Among residents in the Pechenga district of Murmansk Oblast, a number of metals were measured in the blood of men, women and pregnant women (Table 11) [44]. Participants from this study came from settlements located near a copper-nickel ore smelter (in Nickel) and briquetting facility (in Zapolyarny) and are therefore not reflective of contaminant exposures among Indigenous people (Sami) living in the Lovozersky district of Murmansk Oblast.
Table 11.
Metals in the population of the Pechenga district of Murmansk Oblast, Russia. Data presented as geometric means (range) in whole blood (µg/L whole blood). Source: [44].
| Men | Women | Pregnant women | |
|---|---|---|---|
| Year(s) | 2013 | 2013 | 2013–2014 |
| Mean age (range) |
39.9 (27–54) |
45.2 (26–65) |
29.2 (20–42) |
| Sample size | n = 18 | n = 32 | n = 50 |
| Mercury | 3.08 (0.75–25.2) |
2.18 (0.45–12.5) |
1.08 (0.3–3.99) |
| Lead | 23.06 (12.1–91) |
14.39 (4.42–108) |
8.21 (3.53–54) |
| Cadmium | 0.97 (0.44–2.4) |
0.59 (0.16–2.21) |
0.81 (0.24–2) |
| Arsenic | 4.43 (0.74–19.2) |
5.19 (0.86–28.7) |
1.5 (0.41–19) |
| Manganese | 45.97 (7.9–177) |
53.22 (9.7–305) |
12.17 (7.3–27.2) |
| Cobalt | 0.77 (0.31–2.1) |
0.91 (0.36–2.5) |
0.34 (0.2–0.79) |
| Nickel | 6.84 (1.02–85.6) |
7.2 (1.71–95.9) |
6.39 (4.49–20.4) |
| Copper | 1226 (912–1660) |
1436 (1040–2800) |
1416 (931–1950) |
| Zinc | 8103 (6590–9970) |
7659 (5580 –10,600) |
5369 (3830–6940) |
Concentrations of several metals including Pb, Cd, Zn, Ni and Hg were notably different among men, women and pregnant women. For most of these metals, concentrations were slightly higher among men than women, but these sex-based differences were even more noticeable between men and pregnant women. For geometric mean concentrations of Hg and Pb, concentrations were 2.6- and 2.8-fold higher in men than pregnant women. In contrast, men had lower geometric mean blood concentrations of Mn, Co and As, compared to women, although pregnant women still had the lowest mean concentrations.
Concentrations of metals also varied widely among participants. In particular, while the geometric mean concentrations of Mn in women were 53.22 µg/L, values ranged between 9.7 and 305 µg/L which is a wider range than for men (7.9–177 µg/L). The highest observed concentrations of Mn, Co, Ni, Cu, Zn, As and Pb were among women, while the highest observed concentrations of Cd and Hg were among men.
The higher geometric mean and individual maximum concentrations of the majority of metals in women could potentially indicate a unique source of exposure, as the women who participated in the study were not employed in local industry, and therefore not exposed directly to any “occupational” sources of exposure.
Sources of exposure to metals from local foods were also investigated [45]. Concentrations of metals were generally low in most foods measured, however mushrooms were observed to accumulate high concentrations of metals and should be considered as the main “sorbents” of the majority of metals measured, and as the main food contributor to human exposure to metals.
In the Chukotka Autonomous Okrug, located in the far northeast of the Russian Federation, an extensive collection of wild plants and seafood in the coastal Chukotka region were found to be an important dietary source of metals exposure for those Indigenous people consuming these local foods. High levels of metals were found in wild plants (Mn, Al, Ni, Ba, Sr), ascidians (Al, Cr, Sr), blue mussel (Cd, Al) and seaweed. Levels of As and Sr in seaweed were especially high, as well as levels of Al in ascidians and blue mussel [46].
Discussion
Extensive biomonitoring studies conducted over the past few decades have provided valuable information on the presence of contaminants including metals in human populations living in the Arctic, particularly among pregnant women but also among women of childbearing age, adults and children. The availability of recent biomonitoring data varies by country, with countries such as Canada, Greenland, and Sweden measuring concentrations of metals across several regions and among different population groups, while less extensive datasets are available for other countries.
Despite sparse data available in some Arctic regions, time trends data for some metals in the Arctic are extensive in several regions, with time series going back to the 1990s for some Arctic countries. In order to visualise both spatial and temporal trends across the Arctic, mean blood concentrations of Hg and Pb among women of childbearing age and pregnant women from across the circumpolar Arctic over several periods (1990–1999, 2000–2007, 2007–2013, 2013–2018) are illustrated in Figure 3. Compared to concentrations first measured in the 1990s, there appears to have been a decline of Hg and Pb over the last few decades, although trends are not clear in all regions. While not illustrated in Figure 3, multiple-cohort studies in the Faroe Islands also show that levels of Hg are decreasing, probably due to decreasing consumption of pilot whale meat, as concentrations among participants in Cohort 5 are much lower than those observed in the first Faroe Island cohort. From Figure 3, it is also clear that there are spatial differences in concentrations of Hg and Pb in pregnant women and women of childbearing age across Arctic regions. Blood concentrations of Hg in pregnant women were highest in Nunavik and Greenland and were 4- and 5-fold higher than Hg levels in other regions. It should also be noted that within Greenland, geometric mean Hg concentrations in pregnant women varied by region, between 3.3 and 10.2 µg/L, with the highest concentrations being observed in East Greenland. Blood concentrations of Pb were relatively similar across the Arctic, although the highest mean concentrations were in the Canadian Arctic.
Figure 3.
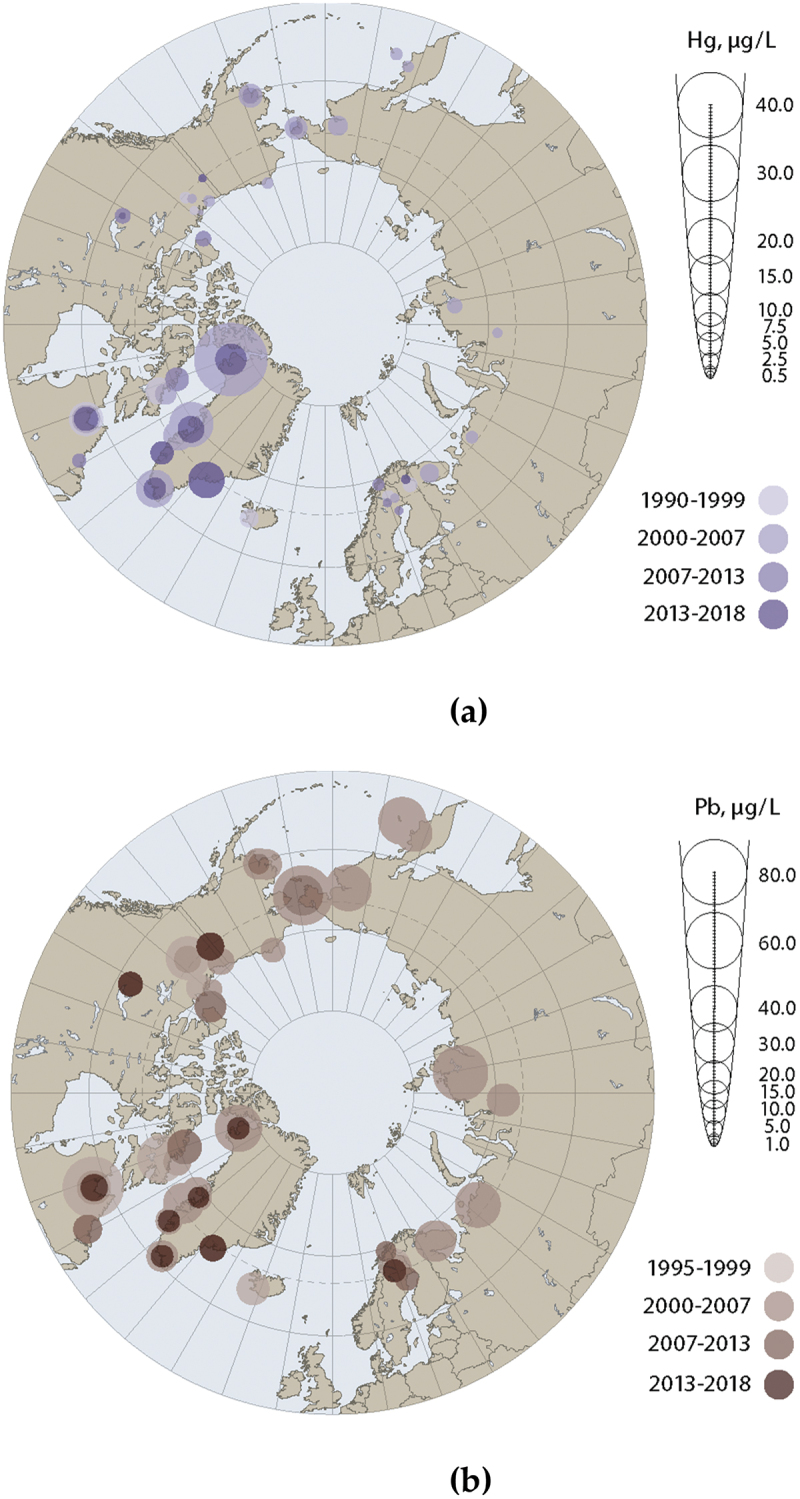
Circumpolar concentrations of (a) Hg, and (b) Pb. Metals presented in µg/L. Data from women of childbearing age (Yukon, DehCho/Sahtú region, inuvialuit settlement region, Nunavut and nunatsiavut [Canada]), maternal blood (Alaska, Faroe Islands, and coastal Chukotka [Russia]), blood of pregnant women (Nunavik [Canada], Greenland, Iceland, Norway, Sweden and the Pechenga district of Murmansk Oblast [Russia]). Source [14,40].
Biomonitoring data from studies conducted across the Arctic are presented in the results section above for each country, and despite differences in contaminant concentrations observed between regions, there were also many broad similarities observed across the Arctic. For example, it was noted in several Arctic regions that concentrations of Pb were generally higher among men than women, including in the Canadian Arctic, Sweden and in Murmansk Oblast (Russia). For mercury, concentrations of Hg were typically higher in men compared to women, except in Nunavik where geometric mean Hg concentrations in 2017 were slightly higher among women compared to men (10 and 8.1 µg/L respectively). In addition to sex differences, concentrations of Hg also varied among different age groups. In Nunavik, geometric mean concentrations among adult men and women were highest in the older age group (50–81 years) at 12 and 15 µg/L respectively, compared to the younger age group (18–49 years) at 7.1 and 8.7 µg/L respectively. Both adult age groups had higher Hg concentrations than the youngest age group (16–17 years). These are some of the highest mean concentrations of Hg reported in recent literature. Blood Hg concentrations in the Faroe Islands were also similarly elevated, with a geometric mean of 3.93 µg/L in 2013–2016 among 28-year-olds, while the geometric mean among 9 year-olds was only 1.4 µg/L in 2016–2018. In addition to descriptive comparisons of geometric mean concentrations, it is worth noting the maximum range values in many of these populations, as the individuals with the highest concentrations were much higher than the calculated mean values. Among the Faroe Island cohorts 1 and 5, the maximum concentration was over 80 µg/L in adults (2013–2016) and over 40 µg/L in children (2016–2018), while in pregnant women in Nunavik and Greenland maximum concentrations were 40 µg/L (2017) and over 70 µg/L (2010–2015), respectively. Exceedances of blood-based guidance values for Hg and Pb in Arctic countries are described in another article in this special issue [47]. Conversely, in several other regions of the Arctic, both mean and maximum concentrations of Hg are much lower. In Iceland, a small sample of pregnant women were reported to have a mean concentration of 1.29 µg/L [2]. Furthermore, in the western Canadian Arctic, concentrations of blood Hg were relatively low among First Nations communities (including below detection limits in some regions) and at levels similar to the general population of Canada (which predominantly reside in Southern Canada, outside of the Arctic).
Although concentrations of Hg have declined across some regions of the Arctic over the last three decades, concentrations of blood Hg in some Arctic regions remain elevated compared to the many populations outside of the Arctic (Table 12). Large survey samples of the general populations of Canada and the USA report geometric concentrations of Hg below 1 µg/L and occasionally below the detection limits [48,53]. It has also been noted in these regions that anglers have slightly elevated mean blood Hg concentrations compared to national averages, showing fish consumption is the principal source of Hg [54]. Mercury concentrations in the Arctic are more like concentrations reported in East and Southeast Asia, which are also elevated compared to North American and European populations, probably due to the high intake of marine-based foods, including large predatory species and/or marine mammals. A study of 6457 adult Koreans reported a mean concentration of 3.1 µg/L [50], while a study of 17,997 pregnant women in Japan reported a geometric mean concentration of 3.8 µg/L [52]. Both studies reported 95th percentile concentrations of just over 9 µg/L. In other populations around the world, a significant source of mercury exposure is gold mining activity. Median blood Hg concentration among pregnant women exposed to gold mining activity in Tanzania was 1.2 µg/L, while a similar study in Ghana reported a mean blood Hg concentration of 8 µg/L [55,56]. While the sources of exposure are different, these concentrations of total mercury are like the range reported in Greenland and Nunavik.
Table 12.
Comparisons of geometric mean mercury concentrations in adult men, women and pregnant women across the globe from 2010 to 2018 (µg/l).
| Country | Population | Mean age (range) | Years | n | Matrix | Hg | Source |
|---|---|---|---|---|---|---|---|
| Czech Republic | Men and women | (18–65) | 2015 | 302 | Whole blood | 0.689 | [46] |
| Canada | Men and women | (20–39) | 2016–2017 | 1037 | Whole blood | 0.55 | [17] |
| USA | Men and women | All | 2015–2016 | 4988 | Whole blood | 0.678 | [48] |
| New Zealand | Men and women | 19–64 | 2014–2016 | 304 | Whole blood | 1.646 | [49] |
| Korea | Men and women | 19–70+ | 2012–2014 | 6457 | Whole blood | 3.11 | [50] |
| China | Men and women | 0–60+ | 2016–2018 | 477 | Whole blood | 1.96 | [51] |
| Japan | Pregnant women | 31 (22–46) | 2015 | 17997 | Whole blood | 3.83 | [52] |
| Arctic | |||||||
| Greenland | Pregnant women | 27.5 (18–45) | 2010–2015 | 497 | Whole blood | 3.9 | [28] |
| Iceland | Pregnant women | 31.6 (22–43) | 2015 | 50 | Whole blood | 1.29 | [32] |
| Faroe Islands | Men and women | 28 | 2013–2016 | 703 | Whole blood | 3.93 | [14] |
| Russia (Murmansk Oblast) | Women | 45.2 (26–65) | 2013 | 32 | Whole blood | 2.18 | [44] |
| Canada (Nunavik) | Pregnant women | 24 (15–38) | 2017 | 123 | Whole blood | 5.4 | [27] |
In the Arctic, mercury exposure remains an important issue for Arctic populations, especially those that rely heavily on top predators in the marine food chain, such as Greenland and Nunavik. The Global Mercury Assessment 2018 [57] reported Hg levels in adults and children based on national biomonitoring programmes from around the world, and levels observed in Nunavik, Greenland and the Faroe Islands were elevated in comparison to these non-Arctic countries. The results of the 2021 AMAP Human Health in the Arctic also support this finding, as shown in this manuscript where levels of Hg in human populations living in Nunavik, Greenland, and the Faroe Islands, are several-fold higher than in other Arctic regions and many regions outside the Arctic.
Levels of several metals are declining in Arctic regions where time trend data exist, although these declines are not uniform across all regions. Large declines are observed when comparing current levels with levels first measured in the 1990s, although the declines observed in recent years have been much smaller in some regions. Despite extensive datasets in some regions, there are still regions of the Arctic with limited biomonitoring data available for metals, and further biomonitoring studies are needed to address this knowledge gap. Continued biomonitoring in the Arctic is needed to track any future changes in levels of contaminants, particularly as climate change can potentially impact levels of mercury in the Arctic environment [58].
Concentrations of metals such as mercury, lead and cadmium have been measured in several populations across the Arctic due to concerns with potential exposures resulting from the harvesting and consumption of certain country foods, and dietary risk messaging has been issued by relevant local health authorities in several Arctic regions where appropriate, as described in another article of this special issue [59]. While consumption of some marine species contributes to Hg exposure, these species can also be an excellent source of essential nutrients and minerals. Levels of Se have been measured in many marine foods, and analysis of beluga mattaq (a traditional food comprising a layer of beluga skin) in Nunavik has identified this as an important source of Se and selenoneine for Inuit in the region, which may offer some protective effects against Hg. In some Arctic regions, wild plants and seafood can also be an important dietary source of exposure for several metals as seen among Indigenous people in coastal Chukotka.
There is a need for biomonitoring data to support chemicals management under the Minamata Convention and subsequent effectiveness evaluations. Moreover, there is a need to address and closely monitor the effects of climate change on the exposure of Arctic wildlife and human populations to mercury, which can increase due to the release of mercury from thawing permafrost and melting glaciers and change according to the redistribution of species in food webs. Continued biomonitoring is needed in identified vulnerable populations to establish and monitor temporal changes of levels of these contaminants, ideally at the time of year when exposure to contaminants is highest. Particularly for contaminants such as mercury, seasonality can have an important impact on levels measured in Arctic human populations, due to large variations in Hg exposure throughout the year, thereby impacting interpretation of contaminant trends. While there have been several regional studies that have generated valuable information on contaminant levels in children and adults, there is still a need for maternal blood biomonitoring studies. Many of the earliest Arctic biomonitoring studies prioritised the sampling of pregnant women and this has provided AMAP with valuable time trend information, however it is still as important today to continue this work, either through maternal blood monitoring programmes or with new birth cohorts, to follow existing trends and improve understanding of the impact of Arctic contaminants on human health. Biomonitoring among Arctic populations coupled with dietary intake data and wildlife monitoring are essential to inform Arctic health authorities and support the health and wellbeing of Arctic populations.
Acknowledgments
This article is based on Chapter 3 “Human biomonitoring and exposure” of the AMAP Assessment 2021: Human Health in the Arctic, which was completed prior to February 2022.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Conceptualisation, B. A., E.B-J., A.D., K.O.; writing – original draft preparation, B. A., E.B-J., A.D., K.O., K.A., É.C-B., M.D., J.G-B., B.L., M.L., K.N., S.p-M., M.P., M.R., A.R., A.T., P.W., M.W.; writing – review and editing, P.A., I.G., S.L., M.L. All authors have read and agreed to the published version of the manuscript.
References
- [1].AMAP . AMAP assessment 2011: mercury in the Arctic. Oslo, Norway: Arctic Monitoring and Assessment Programme (AMAP); 2011. p. xiv + 193. [Google Scholar]
- [2].AMAP . AMAP assessment 2015: human health in the Arctic. Oslo, Norway: Arctic Monitoring and Assessment Programme (AMAP); 2015. [Google Scholar]
- [3].Achouba A, Dumas P, Ouellet N, et al. Plasma levels of selenium-containing proteins in Inuit adults from Nunavik. Environ Int. 2016;96:8–17. doi: 10.1016/j.envint.2016.08.015 [DOI] [PubMed] [Google Scholar]
- [4].Achouba A, Dumas P, Ouellet N, et al. Selenoneine is a major selenium species in beluga skin and red blood cells of Inuit from Nunavik. Chemosphere. 2019;229:549–558. doi: 10.1016/j.chemosphere.2019.04.191 [DOI] [PubMed] [Google Scholar]
- [5].Lemire M, Kwan M, Laouan-Sidi AE, et al. Local country food sources of methylmercury, selenium and omega-3 fatty acids in Nunavik, Northern Quebec. Sci Total Environ. 2015;509-510:248–259. doi: 10.1016/j.scitotenv.2014.07.102 [DOI] [PubMed] [Google Scholar]
- [6].Palmer JH, Parkin G.. Proteolytic cleavage of Hg–C bonds induced by 1-methyl-1,3-dihydro-2 H -benzimidazole-2-selone: synthesis and structural characterization of mercury complexes. J Am Chem Soc. 2015;137(13):4503–4516. doi: 10.1021/jacs.5b00840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yamashita M, Yamashita Y, Suzuki T, et al. Selenoneine, a novel selenium-containing compound, mediates detoxification mechanisms against methylmercury accumulation and toxicity in zebrafish embryo. Mar Biotechnol. 2013;15(5):559–570. doi: 10.1007/s10126-013-9508-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McAuley C, Ng C, McFarland C, et al. Lead exposure through consumption of small game harvested using lead-based ammunition and the corresponding health risks to first nations in Alberta, Canada. Cogent Environ Sci. 2018;4(1):1557316. doi: 10.1080/23311843.2018.1557316 [DOI] [Google Scholar]
- [9].Fillion M, Blais J, Yumvihoze E, et al. Identification of environmental sources of lead exposure in Nunavut (Canada) using stable isotope analyses. Environ Int. 2014;71:63–73. doi: 10.1016/j.envint.2014.06.004 [DOI] [PubMed] [Google Scholar]
- [10].Ratelle M, Li X, Laird B. Cadmium exposure in first nations communities of the Northwest Territories, Canada: smoking is a greater contributor than consumption of cadmium-accumulating organ meats. Environ Sci Processes Impacts. 2018;20(10):1441–1453. doi: 10.1039/C8EM00232K [DOI] [PubMed] [Google Scholar]
- [11].Dewailly É, Ayotte P, Bruneau S, et al. Exposure of the Inuit population of Nunavik (Arctic Quebec) to lead and mercury. Arch Environ Health. 2001;56(4):350–357. doi: 10.1080/00039890109604467 [DOI] [PubMed] [Google Scholar]
- [12].Fontaine J, Dewailly É, Benedetti J-L, et al. Re-evaluation of blood mercury, lead and cadmium concentrations in the Inuit population of Nunavik (québec): a cross-sectional study. Environ Health. 2008;7(1). doi: 10.1186/1476-069X-7-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Grandjean P. International perspectives of lead exposure and lead toxicity. Neurotoxicology. 1993 Summer-Fall;1993;14(2–3):9–14. PMID: 8247415. [PubMed] [Google Scholar]
- [14].AMAP . AMAP assessment 2021: human health in the Arctic. Arctic monitoring and assessment programme (AMAP). Tromsø, Norway; 2021. x+240pp. [Google Scholar]
- [15].Olafsdottir K, Sandandger T, Weber J-P. Analytical quality assurance and quality control. In: Wilson SJ, Symon C, editors. AMAP assessment 2009: human health in the Arctic. Oslo, Norway: Arctic Monitoring and Assessment Programme (AMAP); 2009. p. 49–60. [Google Scholar]
- [16].Drysdale M, Ratelle M, Skinner K, et al. Human biomonitoring results of contaminant and nutrient biomarkers in Old Crow, Yukon, Canada. Sci Total Environ. 2021;760:143339. doi: 10.1016/j.scitotenv.2020.143339 [DOI] [PubMed] [Google Scholar]
- [17].Health Canada . Fifth report on human biomonitoring of environmental chemicals in Canada. Results of the Canadian health measures survey cycle 5 (2016–2017). Health Canada, Ottawa (ON); 2019. [Google Scholar]
- [18].Assembly of First Nations . First nations biomonitoring initiative: national results (2011). 2013. Available from: www.afn.ca/uploads/files/afn_fnbi_en_-_2013-06-26.pdf
- [19].Ratelle M, Packull-McCormick S, Bouchard M, et al. Human biomonitoring of metals in sub-Arctic dene communities of the Northwest Territories, Canada. Environ Res. 2020;190:110008. doi: 10.1016/j.envres.2020.110008 [DOI] [PubMed] [Google Scholar]
- [20].Packull-McCormick S, Ratelle M, Lam C, et al. Hair to blood mercury concentration ratios and a retrospective hair segmental mercury analysis in the Northwest Territories, Canada. Environ Res. 2022;203:111800. doi: 10.1016/j.envres.2021.111800 [DOI] [PubMed] [Google Scholar]
- [21].Lemire M, Lavoie A, Pontual M, et al. Environmental contaminants: metals. Qanuilirpitaa? 2017 Nunavik Inuit Health Survey Nunavik Regional Board of Health and Social Services (NRBHSS) Institut Natl de santé Publique du Québec (INSPQ). 2021.
- [22].Allaire J, Johnson-Down L, Little M, et al. Country and market food consumption and nutritional status. Nunavik Inuit health survey 2017 qanuilirpitaa? how are we now? Quebec. Nunavik Regional Board of Health and Social Services (NRBHSS) & Institut national de santé publique du Québec (INSPQ); 2021. [Google Scholar]
- [23].Lévesque B, Duchesne J-F, Gariépy C, et al. Monitoring of umbilical cord blood lead levels and sources assessment among the inuit. Occup Environ Med. 2003;60(9):693–695. doi: 10.1136/oem.60.9.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fontaine J, Dewailly E, Benedetti J-L, et al. Re-evaluation of blood mercury, lead and cadmium concentrations in the Inuit population of Nunavik (québec): a cross-sectional study. Environ Health. 2008;7(1):25. doi: 10.1186/1476-069X-7-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Health Canada . Fifth report on human biomonitoring of environmental chemicals in Canada. Results of the Canadian health measures survey cycle 4 (2014–2015). Health Canada, Ottawa (ON); 2017. [Google Scholar]
- [26].Pontual MM, Ayotte P, Little M, et al. Seasonal variations in exposure to methylmercury and its dietary sources among pregnant Inuit women in Nunavik, Canada. Environment. 2021;755(Pt 2):143196. doi: 10.1016/j.scitotenv.2020.143196 [DOI] [PubMed] [Google Scholar]
- [27].Lemire M, Blanchette C. Personal communication. Québec, Canada: Axe Santé des populations et pratiques optimales en santé, Centre de Recherche du CHU de Québec - Université Laval; 2020. [Google Scholar]
- [28].Little M, Achouba A, Dumas P, et al. Determinants of selenoneine concentration in red blood cells of Inuit from Nunavik (Northern Quebec, Canada). Environ Int. 2019;127:243–252. doi: 10.1016/j.envint.2018.11.077 [DOI] [PubMed] [Google Scholar]
- [29].Bank-Nielsen PI, Long M, Bonefeld-Jorgensen EC. Pregnant Inuit women’s exposure to metals and association with fetal growth outcomes: ACCEPT 2010-2015. Int J Environ Res Public Health. 2019;16(7):1171. doi: 10.3390/ijerph16071171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Long M, Wielsøe M, Bonefeld-Jørgensen EC. Time trend of persistent organic pollutants and metals in Greenlandic Inuit during 1994–2015. Int K Environ Res Public Health. 2021;18(5):2774. doi: 10.3390/ijerph18052774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Timmermann CAG, Pedersen HS, Budtz-Jorgensen E, et al. Environmental chemical exposures among Greenlandic children in relation to diet and residence. Int J Circumpolar Health. 2019;78(1):1642090. doi: 10.1080/22423982.2019.1642090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Adlard B, Lemire M, Bonefeld-Jørgensen EC, et al. MercuNorth – monitoring mercury in pregnant women from the Arctic as a baseline to assess the effectiveness of the minamata convention. Int J Circumpolar Health. 2021;80(1):1. doi: 10.1080/22423982.2021.1881345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moraeus L, Lemming EW, Koivisto Hursti U-K, et al. Riksmaten adolescents 2016-17: a national dietary survey in Sweden - design, methods, and participation. Food Nutr Res. 2018;62:1381. doi: 10.29219/fnr.v62.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Almerud P, Zamaratskaia G, Lindroos AK, et al. Cadmium, total mercury, and lead in blood and associations with diet, sociodemographic factors, and smoking in Swedish adolescents. Environ Res. 2021;197(2021):110991. doi: 10.1016/j.envres.2021.110991 [DOI] [PubMed] [Google Scholar]
- [35].Vahter M, Åkesson A, Liden C, et al. Gender differences in the disposition and toxicity of metals. Environ Res. 2007;4(1):85–95. doi: 10.1016/j.envres.2006.08.003 [DOI] [PubMed] [Google Scholar]
- [36].Livsmedelsverket and Naturvårdsverket . Contaminants in blood and urine from adolescents in Sweden. Results from the national dietary survey riksmaten adolescents 2016-17. Livsmedelsverket, Naturvårdsverket. S 2020 nr 01. 2020.
- [37].Bjermo H, Sand S, Nalsen C, et al. Lead, mercury, and cadmium in blood and their relation to diet among Swedish adults. Food Chem Toxicol. 2013;57:161–169. doi: 10.1016/j.fct.2013.03.024 [DOI] [PubMed] [Google Scholar]
- [38].Wennberg M, Lundh T, Bergdahl IA, et al. Time trends in burdens of cadmium, lead, and mercury in the population of northern Sweden. Environ Res. 2006;100(3):330–338. doi: 10.1016/j.envres.2005.08.013 [DOI] [PubMed] [Google Scholar]
- [39].Wennberg M, Lundh T, Sommar JN, et al. Time trends and determinants of lead and cadmium in the adult population of northern Sweden 1990-2014. Environ Res. 2017;159:111–117. doi: 10.1016/j.envres.2017.07.029 [DOI] [PubMed] [Google Scholar]
- [40].Sundkvist A, Wennberg M, Rentschler G, et al. Time trends cadmium, lead and mercury in the population of Northern Sweden 1990-2009 and blood levels of rhodium and platinum in 2009. Swed Environ Protect Agency (EPA). 2011. [Google Scholar]
- [41].Tagne-Fotso R, Leroyer A, Howsam M, et al. Current sources of lead exposure and their relative contributions to the blood lead levels in the general adult population of northern France: the IMEPOGE study, 2008–2010. J Toxicol Environ Health A. 2016;79(6):245–265. doi: 10.1080/15287394.2016.1149131 [DOI] [PubMed] [Google Scholar]
- [42].Fustinoni S, Sucato S, Consonni D, et al. Blood lead levels following consumption of game meat in Italy. Environ Res. 2017;155:36–41. doi: 10.1016/j.envres.2017.01.041 [DOI] [PubMed] [Google Scholar]
- [43].Abass K, Koiranen M, Mazej D, et al. Arsenic, cadmium, lead and mercury levels in blood of Finnish adults and their relation to diet, lifestyle habits and sociodemographic variables. Environ Sci Pollut Res Int. 2017;24(2):1347–1362. doi: 10.1007/s11356-016-7824-5 [DOI] [PubMed] [Google Scholar]
- [44].Dudarev AA, Dushkina EV, Sladkova YN, et al. Levels of exposure to metals in population of Pechenga district of Murmansk region (Russian Federation). Med Tr Prom Ekol. 2016;6:11–16. [Article in Russian]. [PubMed] [Google Scholar]
- [45].Dudarev AA, Dushkina EV, Chupahin VS, et al. Metal content of local foods in Pechenga district of Murmansk region (Russian Federation). Med Tr Prom Ekol. 2015;2:35–40. [Article in Russian]. [PubMed] [Google Scholar]
- [46].Dudarev AA, Chupakhin VS, Vlasov SV, et al. Traditional diet and environmental contaminants in coastal Chukotka III: metals. Int J Environ Res Public Health. 2019;16(5):699. doi: 10.3390/ijerph16050699.NIPH [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Abass K, Dudarev AA, Adlard B, et al. Methodologies and challenges in Arctic human health risk assessment: case studies and evaluation of current practicesc. Int J Circumpolar Health. 2024;83. doi: 10.1080/22423982.2024.2361544 [DOI] [Google Scholar]
- [48].CDC . Fourth national report on human exposure to environmental chemicals, updated tables, January 2019. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC); 2019. [Google Scholar]
- [49].Mannetje A, Coakley J, Douwes J. Report on the biological monitoring of selected chemicals of concern: results of the New Zealand biological monitoring programme, 2014-2016. Technical report No. 2017-1. 2018.
- [50].Choi W, Kim S, Baek Y-W, et al. Exposure to environmental chemicals among Korean adults-updates from the second Korean national environmental health survey (2012–2014). Int J Hyg Environ Health. 2017;220:29–35. doi: 10.1016/j.ijheh.2016.10.002 [DOI] [PubMed] [Google Scholar]
- [51].Zeng H-L, Li H, Lu J. Assessment of 12 metals and metalloids in blood of general populations living in Wuhan of China by ICP-MS. Biol Trace Elem Res. 2019;189(2):344–353. [DOI] [PubMed] [Google Scholar]
- [52].Nakayama SF, Iwai-Shimada M, Oguri T, et al. Blood mercury, lead, cadmium, manganese and selenium levels in pregnant women and their determinants: the Japan environment and children’s study (JECS). J Expo Sci Environ Epidemiol. 2019;29(5):633–647. doi: 10.1038/s41370-019-0139-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Haines DA, Saravanabhavan G, Werry K, et al. An overview of human biomonitoring of environmental chemicals in the Canadian health measures survey: 2007–2019. Int J Hyg Environ Health. 2017;220(2):13–28. doi: 10.1016/j.ijheh.2016.08.002 [DOI] [PubMed] [Google Scholar]
- [54].Savadatti SS, Liu M, Caglayan C, et al. Biomonitoring of populations in western New York at risk for exposure to great lakes contaminants. Environ Res. 2019;179:108690. doi: 10.1016/j.envres.2019.108690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Afrifa J, Ogbordjor WD, Duku-Takyi R, et al. Variation in thyroid hormone levels is associated with elevated blood mercury levels among artisanal small-scale miners in Ghana. PLOS ONE. 2018;13(8):e0203335. doi: 10.1371/journal.pone.0203335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Nyanza EC, Dewey D, Manyama M, et al. Maternal exposure to arsenic and mercury in small-scale gold mining areas of Northern Tanzania. Environ Res. 2019;173:432–442. doi: 10.1016/j.envres.2019.03.031 [DOI] [PubMed] [Google Scholar]
- [57].AMAP/UN Environment . Technical background report for the global mercury assessment 2018. Arctic monitoring and assessment programme, Oslo, Norway/un environment programme. Geneva, Switzerland: Chemicals and Health Branch; 2019. [Google Scholar]
- [58].AMAP . AMAP arctic climate change update 2021: key trends and impacts. Arctic monitoring and assessment programme (AMAP). Tromsø, Norway; 2021. p. viii+148. [Google Scholar]
- [59].Krümmel EM, Boyd AD, Brandow D, et al. Updated review on contaminant communication experiences in the circumpolar arctic. Int J Circumpolar Health. 2024;83: (accepted). 1). doi: 10.1080/22423982.2024.2371623 [DOI] [PMC free article] [PubMed] [Google Scholar]


