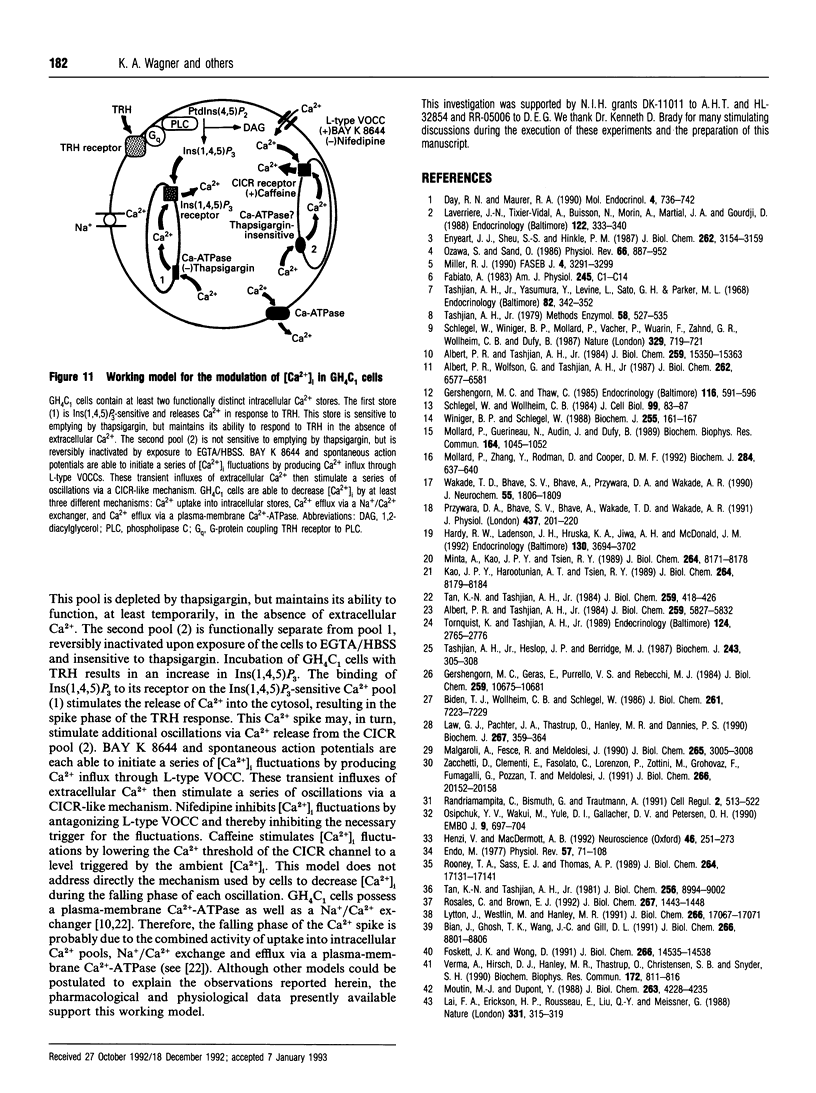
Abstract
Individual unstimulated GH4C1 cells exhibited spontaneous dynamic fluctuations in cytosolic free Ca2+ concentration ([Ca2+]i). Either chelation of extracellular Ca2+ with EGTA or treatment with nifedipine inhibited spontaneous [Ca2+]i fluctuations, indicating that the [Ca2+]i profile was dependent on the entry of extracellular Ca2+ via voltage-operated Ca2+ channels (VOCC). Spontaneous [Ca2+]i fluctuations did not resume immediately after exposure of EGTA-pretreated cells to extracellular Ca2+, supporting the hypothesis that the complex [Ca2+]i profiles observed in unstimulated cells required filling of an intracellular Ca2+ pool. BAY K 8644 elicited large rapid oscillations in [Ca2+]i. After chelation of extracellular Ca2+, however, re-addition of Ca2+ plus BAY K 8644 did not result in [Ca2+]i oscillations. The intracellular Ca2+ pool necessary for BAY K-induced oscillations was not the same Ins(1,4,5)P3-sensitive pool stimulated by thyrotropin-releasing hormone (TRH), because the TRH-stimulated Ins(1,4,5)P3-induced [Ca2+]i spike and the BAY K 8644-induced oscillations were differentially sensitive to chelation of extracellular Ca2+ and thapsigargin. Caffeine caused an increase in [Ca2+]i fluctuations in quiescent cells, supporting a role for Ca(2+)-induced Ca2+ release (CICR) in the generation of spontaneous [Ca2+]i fluctuations. In conclusion, the complex spontaneous changes in [Ca2+]i observed in single GH4C1 cells depend on both the influx of extracellular Ca2+ through VOCC and the action of an intracellular Ca2+ pool that increases [Ca2+]i through a CICR-like mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Tashjian A. H., Jr Relationship of thyrotropin-releasing hormone-induced spike and plateau phases in cytosolic free Ca2+ concentrations to hormone secretion. Selective blockade using ionomycin and nifedipine. J Biol Chem. 1984 Dec 25;259(24):15350–15363. [PubMed] [Google Scholar]
- Albert P. R., Tashjian A. H., Jr Thyrotropin-releasing hormone-induced spike and plateau in cytosolic free Ca2+ concentrations in pituitary cells. Relation to prolactin release. J Biol Chem. 1984 May 10;259(9):5827–5832. [PubMed] [Google Scholar]
- Albert P. R., Wolfson G., Tashjian A. H., Jr Diacylglycerol increases cytosolic free Ca2+ concentration in rat pituitary cells. Relationship to thyrotropin-releasing hormone action. J Biol Chem. 1987 May 15;262(14):6577–6581. [PubMed] [Google Scholar]
- Bian J. H., Ghosh T. K., Wang J. C., Gill D. L. Identification of intracellular calcium pools. Selective modification by thapsigargin. J Biol Chem. 1991 May 15;266(14):8801–8806. [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B., Schlegel W. Inositol 1,4,5-trisphosphate and intracellular Ca2+ homeostasis in clonal pituitary cells (GH3). Translocation of Ca2+ into mitochondria from a functionally discrete portion of the nonmitochondrial store. J Biol Chem. 1986 Jun 5;261(16):7223–7229. [PubMed] [Google Scholar]
- Day R. N., Maurer R. A. Pituitary calcium channel modulation and regulation of prolactin gene expression. Mol Endocrinol. 1990 May;4(5):736–742. doi: 10.1210/mend-4-5-736. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Enyeart J. J., Sheu S. S., Hinkle P. M. Dihydropyridine modulators of voltage-sensitive Ca2+ channels specifically regulate prolactin production by GH4C1 pituitary tumor cells. J Biol Chem. 1987 Mar 5;262(7):3154–3159. [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Wong D. Free cytoplasmic Ca2+ concentration oscillations in thapsigargin-treated parotid acinar cells are caffeine- and ryanodine-sensitive. J Biol Chem. 1991 Aug 5;266(22):14535–14538. [PubMed] [Google Scholar]
- Gershengorn M. C., Geras E., Purrello V. S., Rebecchi M. J. Inositol trisphosphate mediates thyrotropin-releasing hormone mobilization of nonmitochondrial calcium in rat mammotropic pituitary cells. J Biol Chem. 1984 Sep 10;259(17):10675–10681. [PubMed] [Google Scholar]
- Gershengorn M. C., Thaw C. Thyrotropin-releasing hormone (TRH) stimulates biphasic elevation of cytoplasmic free calcium in GH3 cells. Further evidence that TRH mobilizes cellular and extracellular Ca2+. Endocrinology. 1985 Feb;116(2):591–596. doi: 10.1210/endo-116-2-591. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Ladenson J. H., Hruska K. A., Jiwa A. H., McDonald J. M. The effects of extracellular calcium and epinephrine on cytosolic-free calcium in single rat adipocytes. Endocrinology. 1992 Jun;130(6):3694–3702. doi: 10.1210/endo.130.6.1597165. [DOI] [PubMed] [Google Scholar]
- Henzi V., MacDermott A. B. Characteristics and function of Ca(2+)- and inositol 1,4,5-trisphosphate-releasable stores of Ca2+ in neurons. Neuroscience. 1992;46(2):251–273. doi: 10.1016/0306-4522(92)90049-8. [DOI] [PubMed] [Google Scholar]
- Kao J. P., Harootunian A. T., Tsien R. Y. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J Biol Chem. 1989 May 15;264(14):8179–8184. [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Laverriere J. N., Tixier-Vidal A., Buisson N., Morin A., Martial J. A., Gourdji D. Preferential role of calcium in the regulation of prolactin gene transcription by thyrotropin-releasing hormone in GH3 pituitary cells. Endocrinology. 1988 Jan;122(1):333–340. doi: 10.1210/endo-122-1-333. [DOI] [PubMed] [Google Scholar]
- Law G. J., Pachter J. A., Thastrup O., Hanley M. R., Dannies P. S. Thapsigargin, but not caffeine, blocks the ability of thyrotropin-releasing hormone to release Ca2+ from an intracellular store in GH4C1 pituitary cells. Biochem J. 1990 Apr 15;267(2):359–364. doi: 10.1042/bj2670359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991 Sep 15;266(26):17067–17071. [PubMed] [Google Scholar]
- Malgaroli A., Fesce R., Meldolesi J. Spontaneous [Ca2+]i fluctuations in rat chromaffin cells do not require inositol 1,4,5-trisphosphate elevations but are generated by a caffeine- and ryanodine-sensitive intracellular Ca2+ store. J Biol Chem. 1990 Feb 25;265(6):3005–3008. [PubMed] [Google Scholar]
- Miller R. J. Receptor-mediated regulation of calcium channels and neurotransmitter release. FASEB J. 1990 Dec;4(15):3291–3299. [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Mollard P., Guerineau N., Audin J., Dufy B. Measurement of CA2+ transients using simultaneous dual-emission microspectrofluorimetry and electrophysiology in individual pituitary cells. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1045–1052. doi: 10.1016/0006-291x(89)91775-0. [DOI] [PubMed] [Google Scholar]
- Mollard P., Zhang Y., Rodman D., Cooper D. M. Limited accumulation of cyclic AMP underlies a modest vasoactive-intestinal-peptide-mediated increase in cytosolic [Ca2+] transients in GH3 pituitary cells. Biochem J. 1992 Jun 15;284(Pt 3):637–640. doi: 10.1042/bj2840637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutin M. J., Dupont Y. Rapid filtration studies of Ca2+-induced Ca2+ release from skeletal sarcoplasmic reticulum. Role of monovalent ions. J Biol Chem. 1988 Mar 25;263(9):4228–4235. [PubMed] [Google Scholar]
- Osipchuk Y. V., Wakui M., Yule D. I., Gallacher D. V., Petersen O. H. Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+ dependent Cl- current recording in single pancreatic acinar cells. EMBO J. 1990 Mar;9(3):697–704. doi: 10.1002/j.1460-2075.1990.tb08162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S., Sand O. Electrophysiology of excitable endocrine cells. Physiol Rev. 1986 Oct;66(4):887–952. doi: 10.1152/physrev.1986.66.4.887. [DOI] [PubMed] [Google Scholar]
- Przywara D. A., Bhave S. V., Bhave A., Wakade T. D., Wakade A. R. Dissociation between intracellular Ca2+ and modulation of [3H]noradrenaline release in chick sympathetic neurons. J Physiol. 1991 Jun;437:201–220. doi: 10.1113/jphysiol.1991.sp018591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriamampita C., Bismuth G., Trautmann A. Ca(2+)-induced Ca2+ release amplifies the Ca2+ response elicited by inositol trisphosphate in macrophages. Cell Regul. 1991 Jul;2(7):513–522. doi: 10.1091/mbc.2.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales C., Brown E. J. Calcium channel blockers nifedipine and diltiazem inhibit Ca2+ release from intracellular stores in neutrophils. J Biol Chem. 1992 Jan 25;267(3):1443–1448. [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Wollheim C. B. Thyrotropin-releasing hormone increases cytosolic free Ca2+ in clonal pituitary cells (GH3 cells): direct evidence for the mobilization of cellular calcium. J Cell Biol. 1984 Jul;99(1 Pt 1):83–87. doi: 10.1083/jcb.99.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. N., Tashjian A. H., Jr Receptor-mediated release of plasma membrane-associated calcium and stimulation of calcium uptake by thyrotropin-releasing hormone in pituitary cells in culture. J Biol Chem. 1981 Sep 10;256(17):8994–9002. [PubMed] [Google Scholar]
- Tan K. N., Tashjian A. H., Jr Voltage-dependent calcium channels in pituitary cells in culture. I. Characterization by 45Ca2+ fluxes. J Biol Chem. 1984 Jan 10;259(1):418–426. [PubMed] [Google Scholar]
- Tashjian A. H., Jr Clonal strains of hormone-producing pituitary cells. Methods Enzymol. 1979;58:527–535. doi: 10.1016/s0076-6879(79)58167-1. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Heslop J. P., Berridge M. J. Subsecond and second changes in inositol polyphosphates in GH4C1 cells induced by thyrotropin-releasing hormone. Biochem J. 1987 Apr 1;243(1):305–308. doi: 10.1042/bj2430305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Tornquist K., Tashjian A. H., Jr Dual actions of 1,25-dihydroxycholecalciferol on intracellular Ca2+ in GH4C1 cells: evidence for effects on voltage-operated Ca2+ channels and Na+/Ca2+ exchange. Endocrinology. 1989 Jun;124(6):2765–2776. doi: 10.1210/endo-124-6-2765. [DOI] [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Hanley M. R., Thastrup O., Christensen S. B., Snyder S. H. Inositol trisphosphate and thapsigargin discriminate endoplasmic reticulum stores of calcium in rat brain. Biochem Biophys Res Commun. 1990 Oct 30;172(2):811–816. doi: 10.1016/0006-291x(90)90747-b. [DOI] [PubMed] [Google Scholar]
- Wakade T. D., Bhave S. V., Bhave A., Przywara D. A., Wakade A. R. Ca2+ mobilized by caffeine from the inositol 1,4,5-trisphosphate-insensitive pool of Ca2+ in somatic regions of sympathetic neurons does not evoke [3H]norepinephrine release. J Neurochem. 1990 Nov;55(5):1806–1809. doi: 10.1111/j.1471-4159.1990.tb04972.x. [DOI] [PubMed] [Google Scholar]
- Winiger B. P., Schlegel W. Rapid transient elevations of cytosolic calcium triggered by thyrotropin releasing hormone in individual cells of the pituitary line GH3B6. Biochem J. 1988 Oct 1;255(1):161–167. doi: 10.1042/bj2550161. [DOI] [PMC free article] [PubMed] [Google Scholar]


