Highlights
-
•
Population studies report increased cardiovascular events, mortality in epilepsy.
-
•
Recurring seizures damage heart and vasculature, the “Epileptic Heart Syndrome.”
-
•
Myocardial infarction, arrhythmias, and depressed contractility are characteristic.
-
•
Certain antiseizure medications may exacerbate hyperlipidemia and/or arrhythmias.
-
•
Electrocardiography, echocardiography, lipid panels, etc., can improve detection.
Keywords: Antiseizure medications, Diastolic dysfunction, Ischemic heart disease/atherosclerosis, Sudden cardiac death/arrest, Sudden unexpected death in epilepsy, T-wave alternans
Abstract
Population studies report elevated incidence of cardiovascular events in patients with chronic epilepsy. Multiple pathophysiologic processes have been implicated, including accelerated atherosclerosis, myocardial infarction, altered autonomic tone, heart failure, atrial and ventricular arrhythmias, and hyperlipidemia. These deleterious influences on the cardiovascular system have been attributed to seizure-induced surges in catecholamines and hypoxemic damage to the heart and coronary vasculature. Certain antiseizure medications can accelerate heart disease through enzyme-inducing increases in plasma lipids and/or increasing risk for life-threatening ventricular arrhythmias as a result of sodium channel blockade. In this review, we propose that this suite of pathophysiologic processes constitutes “The Epileptic Heart Syndrome.” We further propose that this condition can be diagnosed using standard electrocardiography, echocardiography, and lipid panels. The ultimate goal of this syndromic approach is to evaluate cardiac risk in patients with chronic epilepsy and to promote improved diagnostic strategies to reduce premature cardiac death.
1. Cardiovascular disease in epilepsy
Extensive clinical studies indicate that chronic epileptic seizures exert a deleterious effect on electrical and mechanical function of the heart [1], [2], [3]. The lines of evidence are diverse, ranging from histology, echocardiography, and electrocardiography to population studies. Studies from extensive population databases, a type of investigation generally regarded as a gold standard mainly because of its complete case ascertainment, have reported evidence of increased incidence of cardiac events including cardiovascular death, ischemic heart disease/atherosclerosis, myocardial infarction, heart failure, sudden cardiac death (SCD)/cardiac arrest, and atrial and ventricular fibrillation (AF/VF), etc. (Table 1) [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]. This spectrum of cardiac abnormalities led us to formulate the concept of the “Epileptic Heart,” which we defined as “a heart and coronary vasculature damaged by chronic epilepsy as a result of repeated surges in catecholamines and hypoxia leading to electrical and mechanical dysfunction” [1].
Table 1.
Population Studies of Cardiovascular Morbidity and Mortality in Patients with Epilepsy.
| Cardiac condition | First author, year | Database | Study cohort | Significant Findings and Comparisons |
|---|---|---|---|---|
| Cardiovascular death, myocardial infarction | Janszky et al. 2009 [4] | Stockholm Heart Epidemiology Program | 1799 epilepsy patients with first MI; 2339 matched controls | 4.92-fold more MIs and 1.95-fold more cardiovascular deaths in patients with than without epilepsy |
| Cardiovascular death | Neligan et al. 2011 [5] | National General Practice Study of Epilepsy at the National Health Service Information Centre of the United Kingdom | >1000 patients with epilepsy | 3.3-fold more deaths in epilepsy patients with ischemic heart disease than without |
| Cardiovascular death | Nevalainen et al. 2013, 2016 [6], [7] | Social Insurance Institution of Finland | 10,818 patients with epilepsy and 43,894 subjects in reference cohort | 2.31-fold more cardiovascular deaths in patients with than without epilepsy |
| Cardiovascular death | DeGiorgio et al. 2020, 2021 [8], [9] | United States Centers for Disease Control and Prevention WONDER online database | 8052 deaths in patients with epilepsy | Cardiovascular deaths declined by 34 % to 43% of all deaths in patients with epilepsy |
| Cardiovascular death, heart failure, ventricular tachycardia/fibrillation, cardiac arrest | Mayer et al. 2024 [10] | French Hospital National Database from 2014 to 2022 | 682,349 patients with epilepsy plus 682,349 matched patients without epilepsy | 2.69-fold more all-cause deaths; 2.16-fold more cardiovascular deaths; 1.26-fold more heart failure cases; 2.08-fold more ischemic strokes; 1.10-fold more VT/VF events; 2.12-fold more cardiac arrests in patients with than without epilepsy |
| Ischemic heart disease | Chen et al. 2016 [11] | Census and Statistics Department, Hong Kong Special Administrative Region | 7461 patients with newly diagnosed epilepsy | 4.18-fold more ischemic heart disease cases in patients with newly diagnosed epilepsy |
| Ischemic heart disease/atherosclerosis | Zack and Luncheon 2018 [12] | US National Health Interview Survey | 95,196 respondents including 1705 with epilepsy | 1.8-fold more ischemic heart disease cases in patients with compared to without epilepsy, especially in families with incomes below poverty line |
| Ischemic heart disease | Husein et al. 2021 [13] | Canadian Longitudinal Study on Aging |
44,817 participants including 751 patients with epilepsy | 1.27-fold more ischemic heart disease cases, 1.88-fold more peripheral vascular disease cases in patients with than without epilepsy |
| Ischemic heart disease | Josephson et al. 2021 [14] | National Health Service hospitals in England | 10,916,166 adults; 31,479 adults with epilepsy free of cardiovascular disease at baseline | 1.21-fold more heart disease cases in patients with epilepsy receiving enzyme-inducing ASMs |
| Ischemic heart disease, heart failure, cardiac arrest, ventricular arrhythmia, atrial fibrillation | Bucci et al. 2023 [15] | TriNetX Global Federated Health Research Network | 271,172 patients with epilepsy | 3.23-fold more cardiovascular events at 5 years after seizure in 15,210 patients with compared to without 30-day events |
| Ischemic heart disease, sudden cardiac death, atrial fibrillation, ventricular arrhythmia | Shah et al. 2023 [16] | UK Biobank | 494,676 subjects without epilepsy; 7786 patients with epilepsy | 6.65-fold more SCDs, 3.9-fold more all-cause deaths in patients with than without epilepsy |
| Ischemic heart disease | Mayer et al. 2024 [17] | TriNetX Global Federated Health Research Network | 374,950 patients with epilepsy | Carbamazepine (1.39-fold) and sodium valproate (1.264-fold) were associated with more cardiac events compared to lamotrigine. Valproate was associated with a 10-year 1.226-fold higher risk of all-cause death than carbamazepine. |
| Myocardial infarction | Olesen et al. 2011 [18] | Danish National Patient Register | 48,602 patients with epilepsy | 1.09-fold more MIs in ASM-treated patients; 1.15-fold more MIs in non-ASM treated patients |
| Myocardial infarction | Renoux et al. 2015 [19] | U.K. Clinical Practice Research Datalink | 252,407 ASM users and matched control patients | 1.46-fold more MIs in patients using enzyme-inducing ASMs compared to noninducing ASMs; non-inducing ASMs were associated with fewer MIs (to 0.81-fold) |
| Myocardial infarction | Wilson et al. 2018 [20] | South Carolina hospital and emergency department encounter data | 39,203 patients with epilepsy, 119,559 patients without epilepsy | 1.24-fold more MIs in patients with compared to without epilepsy |
| Myocardial infarction | Wellejus Albertsen et al. 2020 [21] | DANish Comorbidity Index for Acute Myocardial Infarction (DANCAMI) | 36,685 patients with first MI | 1.26-fold more MIs in patients with compared to without epilepsy |
| Myocardial infarction, arrhythmia, SCD | Cheng et al. 2021 [22] | Taiwan National Health Insurance Research Database 1997–2013 | 27,055 patients including 5411 with epilepsy | 1.71-fold more MIs, 2.11-fold more arrhythmias, 1.83-fold more SCDs in patients with than without epilepsy |
| Heart failure | Doege et al. 2021 [23] | German outpatient cohort | 9646 patients with epilepsy and matched nonepileptic referents | 1.56-fold more heart failure cases |
| Heart failure deaths | Liang et al. 2022 [24] | Danish registries | 1345 patients with epilepsy and matched nonepilepsy referents | 2.35-fold more heart failure deaths,1.31-fold more all-cause deaths in 696 patients receiving valproate compared to 649 patients receiving lamotrigine or levetiracetam |
| Cardiac arrest/VF | Bardai et al. 2012 [25] | Amsterdam Resuscitation Studies (ARREST) | 1019 sudden cardiac arrest patients, 2834 referents without SCA | 2.9-fold more sudden cardiac arrests in patients with epilepsy |
| Cardiac arrest/VF | Stecker et al. 2013 [26] | Oregon Sudden Unexpected Death Study | ∼1,000,000-subject study population; 106 sudden cardiac arrests in patients with epilepsy; 2311 sudden cardiac arrests in patients without epilepsy | No seizure activity witnessed prior to 66 % of sudden cardiac arrests in patients with epilepsy |
| Cardiac arrest/VF | Bardai et al. 2015 [27] | Integrated Primary Care Information (IPCI) | 926 sudden cardiac deaths, 9832 referents without SCD | 5.8-fold more sudden cardiac arrests in patients with symptomatic epilepsy; 2.8-fold more sudden cardiac arrests in patients with sodium channel blocking ASMs |
| Cardiac arrest, arrhythmia | Rossi et al. 2021 [28] | New York, Florida, and California State Inpatient and Emergency Department Databases | 1,270,304 encounters for cardiac arrhythmias including 8717 in patients with epilepsy | 2.37- to 3.36-fold more encounters for cardiac arrest/arrhythmias across 180 days following seizure than in prior year |
| Out-of-hospital cardiac arrest (OHCA) | Eroglu et al. 2022 [29] | Danish registries | 35,195 OHCA cases and 351,950 matched non-OHCA controls | 1.76-fold more OHCAs in patients with epilepsy than in the general population |
| Ventricular arrhythmias, atrial fibrillation | Wang et al. 2023 [30] | UK Biobank | 329,432 subjects including 2699 with epilepsy | 1.80-fold more ventricular arrhythmias, 1.26-fold more atrial fibrillation events in patients with than without epilepsy |
| Atrial fibrillation | Desai et al. 2017 [31] | Nationwide Inpatient Sample (NIS) Database of patients with epilepsy | 1,424,320 hospitalized patients with epilepsy including 277,230 with cardiac arrhythmia | AF was most frequent cardiac arrhythmia (9.7 %). Incidence of sudden cardiac arrest was 1.4 %; incidence of VT was 1.0 % |
Key:
AF = atrial fibrillation
ASM = antiseizure medication
MI = myocardial infarction
OHCA = out-of-hospital cardiac arrest
SCD = sudden cardiac death
VF = ventricular fibrillation
VT = ventricular tachycardia
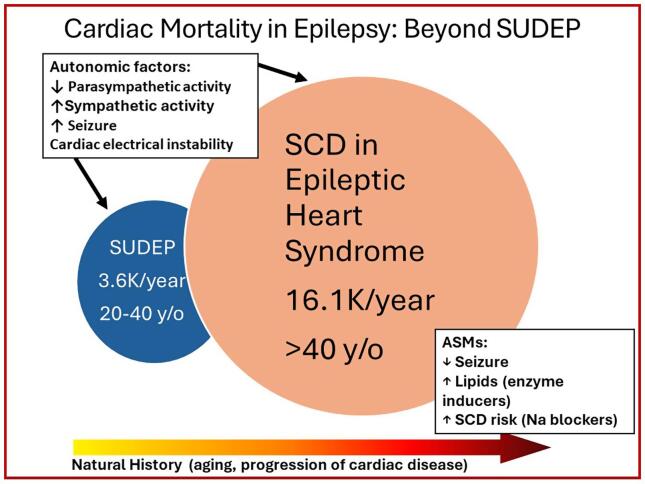
Premature demise in patients with epilepsy has generally been attributed to the clinical entity termed “sudden unexpected death in epilepsy” (SUDEP), which is due to respiratory failure in the post-ictal period leading to cardiac asystole [32]. There is growing evidence that cardiac mortality in patients with epilepsy may be 4.5-fold more frequent than SUDEP, which excludes deaths from known causes (Fig. 1) [1], [26], [33], [34], [35], [36], [37], [38].
Fig. 1.
Venn diagram of the interrelationship between sudden cardiac death (SCD) and sudden unexpected death in patients with epilepsy (SUDEP). SUDEP incidence data of 3600 cases/year are from Thurman et al. 2014 [35] and Harden et al. 2017 [37]. SCD incidence data are from Stecker et al. 2013 [26], Zack and Kobau 2015 [36], and Benjamin et al. 2018 [38]. ASMs = antiseizure medications. Republished with permission from Verrier et al. 2020 [1].
While deaths among patients with epilepsy nearly doubled in the United States between 1999 and 2017, the proportion of deaths due to ischemic heart disease in people with epilepsy declined by 34 % to 43% over the same time period [8], [9]. The former effect has been ascribed to increased cerebrovascular disease and neoplasms, while the latter change has been attributed to widespread implementation of techniques and treatments that reduce cardiovascular risk [9].
Currently, we are considering whether the “Epileptic Heart” condition constitutes a syndrome, with a constellation of manifestations that can be assessed with standard clinical tools on an individual patient basis [2]. The main objectives of this review are to characterize the pathophysiologic basis of the “Epileptic Heart Syndrome” and to define candidate clinical criteria that could be used in developing a systematic, practical approach to its detection.
2. “Epileptic Heart Syndrome”: Pathophysiology
The conceptual framework that characterizes the link between chronic epilepsy and the development of the “Epileptic Heart Syndrome” is illustrated in Fig. 2 [2]. The specific, well-documented abnormalities include changes in myocardial structure, with fibrosis, contraction-band necrosis, increased myocardial stiffness with diastolic dysfunction, and electrocardiographic abnormalities. The inciting factors include repeated seizure-induced hypoxemia [39], [40], [41] and myocardial ischemia [42], [43], the cardiotoxic effects of excess catecholamines [44], and the potential exacerbating effects of certain antiseizure medications (ASMs). The involvement of myocardial ischemia is supported by the report from Tigaran et al. [43] that ST-segment changes are evident in up to 40% of seizures. The cardiotoxic effects of catecholamines during seizures are underscored by reports of myocardial stunning with markedly reduced left ventricular ejection fraction in patients admitted to the Epilepsy Monitoring Unit [45]. The magnitude of this depression in cardiac contractility is akin to Takotsubo cardiomyopathy [46]. Secondary Takotsubo cardiomyopathy has been estimated to occur in ∼1 of 1000 in-hospital seizures, resulting in poor outcomes, including inpatient mortality (3.7%), arrhythmia (22.7%), cardiac arrest (3.9%), etc. [47]. This finding has led to the view that chronic epilepsy may be associated with a neurogenic cardiomyopathy phenotype.
Fig. 2.
Conceptual framework of the link between chronic epilepsy and development of the “Epileptic Heart Syndrome.” The factors include cardiotoxic effects of catecholamines, repeated hypoxemia, and increased cardiac electrical instability manifest as T-wave alternans, a repeating ABAB beat-to-beat pattern in the ST segment and T wave of the electrocardiogram. ASMs = antiseizure medications. Republished with permission from Verrier et al. 2021 [2].
Postmortem evidence of myocardial structural changes in patients with epilepsy has been reported by several groups. Falconer and Rajs [48] were the first to report postmortem evidence of cardiac fibrosis and myofibrillar degeneration. At autopsy, hearts of patients with epilepsy were characteristically dilated and were heavier than expected [48], [49]. Dasheiff et al. [50] observed mild ventricular hypertrophy and focal myocardial fibrosis in 20% of autopsy cases. Natelson and coworkers [51] noted myocardial vascularization and perivascular and interstitial myocardial fibrosis in a majority of autopsied hearts of patients with chronic epilepsy. Thus, a generalized pattern of microfocal interstitial and/or patchy myocardial fibrosis [52], [53] is a well-documented feature of the “Epileptic Heart Syndrome.” Devinsky and coworkers [54] have emphasized the need for further study to define more precisely the potential role of cardiac fibrosis in SUDEP.
Echocardiography studies have confirmed that patients with chronic epilepsy exhibit abnormally high levels of left ventricular stiffness, increased ventricular pressure, and greater left atrial volume compared to healthy age- and sex-matched controls [55]. The investigators proposed that recurring seizures could lead to heart failure with preserved left ventricular ejection fraction (HFpEF) [55], [56], [57], [58], a condition known to increase risk for premature death [57].
The convergence of the main pathologic pathways including myocardial stunning, histological changes involving myocardial vacuolization and interstitial fibrosis, and accelerated atherosclerosis lead to cardiac electrical instability of both the atria and ventricles. Increased incidence of atrial arrhythmias, particularly AF, has been documented in large populations [15], [16], [30], [31]. Yassin and colleagues [59] reported AF during routine EEG recordings in 6.3 % of patients. AF incidence in patients with epilepsy deserves further attention, especially because this arrhythmia carries increased risk for stroke and myocardial infarction. There is growing evidence that P-wave heterogeneity (PWH) precedes AF and can be used for risk assessment [60].
Significant increases in ventricular arrhythmia incidence including ventricular fibrillation in patients with epilepsy compared to general populations have been reported [10], [15], [22], [25], [26], [27], [28], [30]. Rossi and colleagues [28] reported a heightened incidence of cardiac arrhythmia/arrest across 180 days after a seizure. Sudden cardiac deaths and cardiac arrests were also more frequent in patients with than without epilepsy [10], [15], [16], [22], [25], [26], [27], [28], [29]. Seizure-induced electrical dysfunction of the ventricles is well-documented based on a number of clinically available electrocardiographic parameters such as QT interval prolongation and P-wave and T-wave alternans and heterogeneity.
It is well documented that epilepsy predisposes to cardiovascular death [4], [5], [6], [7], [8], [9], [10], ischemic heart disease [11], [12], [13], [14], [15], [16], [17], myocardial infarction [4], [18], [19], [20], [21], [22], and heart failure [10], [15], [23], [24].
An additional major compounding factor of the “Epileptic Heart Syndrome” is a role for certain ASMs. These agents introduce at least two potential major cardiac risks. First, as Mintzer and colleagues [61], [62] proposed, ASMs that induce the cytochrome P450 (CYP450) system are linked to elevations in lipids, Lpa, C-reactive protein, and homocysteine, contributors to atherosclerosis. Another important deleterious influence is that certain ASMs may increase risk for SCD and other cardiac events, notably, those with sodium channel-blocking effects, including carbamazepine, lamotrigine, and phenytoin [3], [17], [27], [63]. Blockade of the cardiac sodium channels, particularly in patients with cardiovascular disease, can promote conduction abnormalities and predispose patients to serious cardiac arrhythmias such as wide-complex ventricular tachycardia. The Cardiac Arrhythmia Suppression Trial (CAST), which enrolled post-myocardial infarction patients with cardiac arrhythmias, showed that the sodium channel blockers flecainide and encainide were severely proarrhythmic and predisposed to higher incidence of death [64]. In addition, in the Cardiac Arrest Study Hamburg (CASH) trial, conducted in patients with prior cardiac arrest, the sodium channel blocker propafenone was shown to increase all-cause mortality significantly in the first year of the study [65]. Myocardial ischemia during seizures, especially GTCSs, may enhance risk for life-threatening ventricular arrhythmias in individuals receiving chronic ASMs with sodium channel-blocking activity. It is noteworthy that certain ASMs such as carbamazepine and phenytoin, which have both enzyme-inducing effects and sodium-channel blocking properties, pose a “double-hit” by increasing plasma lipid levels and predisposing to malignant arrhythmias [27], [62].
3. Clinical criteria and methods for detection of the “Epileptic Heart Syndrome”
The candidate criteria and measurements that can be assessed with commercially available equipment are presented in Table 2. These criteria were derived from extensive Boolean searches of the medical literature using PubMed from National Library of Medicine (USA). We employed the terms “electrocardiographic,” “echocardiography,” “atherosclerosis,” “left ventricular function,” “heart rate variability,” “autonomics,” and “lipids.” Based on this comprehensive evaluation, we formulated five conditions and criteria that provide the basis for establishing the syndromic components of an “Epileptic Heart Syndrome” with these diagnostic tools [2].
Table 2.
Criteria for the Epileptic Heart Syndrome.*
|
Note: ILAE = International League Against Epilepsy.
Requires the presence of chronic epilepsy and any two other criteria.
3.1. Criterion #1: Presence of chronic epilepsy with or without drug resistance
Confirmation of chronic epilepsy is fundamental to the determination of the “Epileptic Heart Syndrome.” The basic rationale, as shown in our conceptual framework (Fig. 2) [2], is that seizures progressively injure the heart and coronary vasculature as a result of recurring bouts of myocardial ischemia and the cardiotoxic effects of excessive levels of catecholamines. A critical factor is age past 40 years [12], [66], when vulnerability to pathologic processes can be enhanced. Additionally, it is important to recognize the potential involvement of acquired channelopathies in epilepsy [67]. Experimental studies have shown that epilepsy can alter expression of ion channels in both the heart and brain, which in turn can predispose to cardiac dysfunction and mortality. This process may occur at younger ages than is the case for longer term progression of cardiovascular disease.
3.2. Criterion #2: Electrocardiographic markers of myocardial injury and risk for atrial and ventricular arrhythmias
With respect to atrial arrhythmia risk, the key clinical approach involves measurement of atrial enlargement in terms of P waves >2.5 mm tall or >110 ms wide. P-wave heterogeneity (PWH) may reveal increased susceptibility to AF and can be monitored from standard 12-lead EKGs. Recently, we have shown that patients with chronic epilepsy exhibit an increased PWH indicative of an arrhythmogenic atrial substrate that is comparable to levels observed in patients with AF [60]. The patients with epilepsy were ∼20 years younger than the patients with AF but without epilepsy, consistent with acceleration in structural atrial changes and/or cardiac electrical instability of the atria.
In terms of assessing ventricular injury and risk for ventricular arrhythmias, several measurements are available. These include presence of Q waves with large downward deflection, which may indicate prior myocardial infarction; QRS complex width >150 ms, which may indicate conduction abnormalities and electrical dyssynchrony; and severe QT interval prolongation (>450 ms in men, >470 ms in women), which may indicate repolarization abnormalities or ASM use. It has been shown, for example, that QT prolongation >448 ms was abnormal in nearly one-third of >18,000 patients with chronic epilepsy in the Mayo Clinic (Rochester MN) database and was associated with 1.48-fold increase in mortality [68].
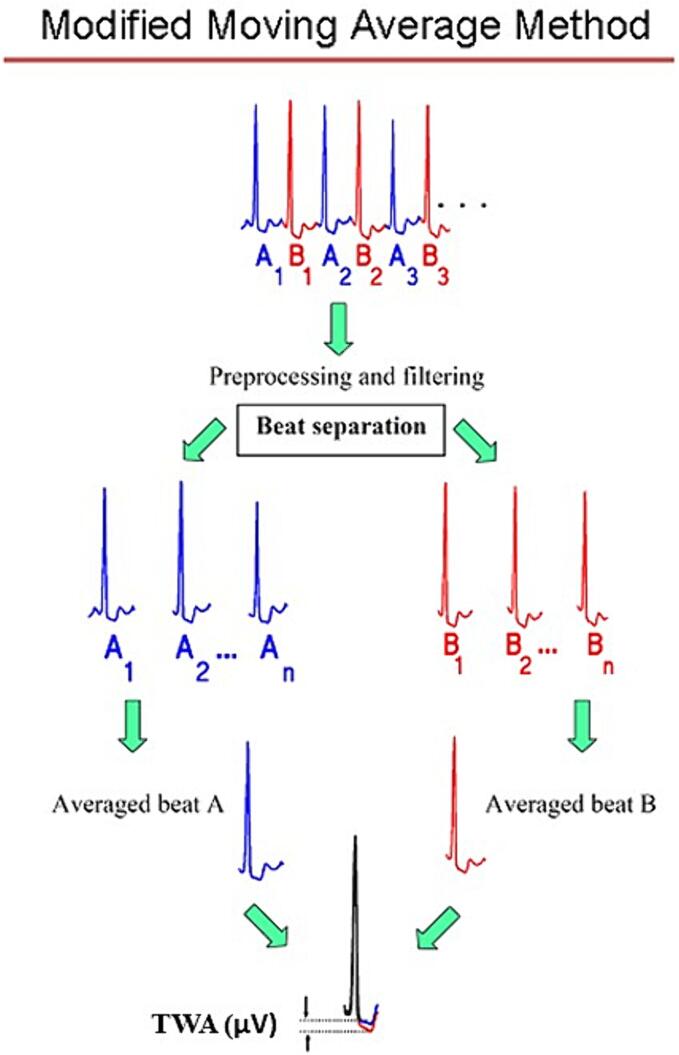
Our group found that a widely studied measure of arrhythmogenic repolarization abnormality, T-wave alternans (TWA), a beat-to-beat fluctuation in T-wave morphology (Fig. 3) [1], [69], [70], is elevated in individuals with chronic epilepsy to the level observed in patients with cardiomyopathy and myocardial infarction (Fig. 4) [1], [71]. TWA is a well-established marker of cardiac arrhythmia risk and is based on sound electrophysiologic principles, as it indicates heightened levels of heterogeneity of repolarization and sets the stage for unidirectional block and reentry. The capacity of TWA analysis to evaluate risk for malignant arrhythmias is supported by studies in ∼4800 patients with diverse cardiac conditions [70], specifically, cardiomyopathy, ischemic heart disease, and heart failure. TWA is also useful for detecting the influence of anti- and proarrhythmic agents [72], enabling clinicians to select nonarrhythmogenic treatment drugs. The FDA-cleared commercially available modified moving average method for TWA analysis is illustrated (Fig. 3).
Fig. 3.
Modified moving average technique for detection of T-wave alternans (TWA). Alternate beats are dichotomized into bins of “A” beats (blue) and “B” beats (red), and the T-wave morphologies in each bin are averaged. The averaged beats are then superimposed, and the difference in the magnitude of the T waves of the A and B averaged beats is quantified in microvolts. This difference is the TWA level, which indicates the degree of cardiac risk [67]. Republished with permission from Verrier et al. 2020 [1]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
T-wave alternans (TWA) ladder of risk. Patients with chronic epilepsy exhibit maximum 24-hour TWA in the severely abnormal range (>60 µV), similar to patients who experience ventricular tachycardia (VT) following ST-segment elevation myocardial infarction (STEMI). AECG, ambulatory electrocardiogram; MI, myocardial infarction. Republished with permission from Verrier et al. 2020 [1].
The utility of TWA in detecting latent arrhythmia risk is increased by EKG monitoring with multiday EKG patches, which are well-tolerated. Pang et al. [73] reported that TWA levels in chronic epilepsy patients were significantly higher than those in individuals with newly diagnosed epilepsy; the latter did not differ significantly from those observed in the healthy control group. In patients with chronic epilepsy, maximum TWA magnitude across 24 h exceeded the ≥47-μV TWA cut point for cardiac risk. It is of interest that during an interictal period, one of the patients experienced a crescendo in TWA level that heralded a brief run of ventricular tachycardia. This arrhythmia occurred on the fourth day of monitoring with an ambulatory EKG patch and would not have been detected by conventional Holter monitoring, which is typically limited to 1 or 2 days. Additional studies have indicated that heightened levels of T-wave alternans prior to EMU admission identify patients with greater likelihood of a seizure during their hospital stay [74]. We also found that T-wave heterogeneity, a precursor of TWA, can provide advance warning of seizure onset [75].
3.3. Criterion #3: Altered autonomic tone
An established feature of certain seizure types is the occurrence of bursts of sympathetic nerve activity. Heart rate variability (HRV) has proven to be a valuable marker of autonomic tone and has historically been used in ambulatory monitoring with 24-h EKG recorders. In recent years, HRV has been incorporated into multiday EKG patches and readers to characterize autonomic tone both intra- and interictally. The commonly employed metric of parasympathetic tone is “root mean square of successive differences of R-R intervals” (rMSSD). This time-domain HRV parameter appears to be inversely correlated with SCD risk in populations with cardiovascular disease [76], [77], [78]. A meta-analysis revealed that chronic epilepsy patients exhibit lower levels of rMSSD than are observed in the general population [79]. Our group [73] reported that during periods when rMSSD is reduced, TWA was reciprocally increased, indicating elevated levels of cardiac electrical instability. These findings point to an inverse relationship between parasympathetic autonomic activity and propensity for cardiac arrhythmias in patients with chronic epilepsy.
In Dravet syndrome, which is known to be associated with a heightened risk for premature death, HRV was found to be reduced, indicating relatively unopposed sympathetic nerve activity [80]. Also, markers of atrial and ventricular arrhythmia vulnerability, specifically, QT-interval and P-wave dispersion, were found to be abnormal.
3.4. Criterion #4: Echocardiographic evidence of myocardial stiffness and diastolic dysfunction
Several investigations applied state-of-the-art echocardiographic techniques to demonstrate that chronic epilepsy is associated with a type of stress-related cardiomyopathy typical of HFpEF [55], [57], [58], [81], [82], [83]. They attributed the resulting cardiac effects to recurring bouts of sympathetic nerve overstimulation and to the corresponding release of catecholamines at the neurocardiac junction, resulting in cardiac myofilament damage, extracellular matrix deposition, fibrosis, and inflammation. Other investigators have provided evidence that recurring epileptic seizures can set the stage for diastolic heart failure, with potential for left ventricular hypertrophy [50], [82]. Doege et al. [23] reported a 1.56-fold increase in heart failure diagnoses in patients with epilepsy. Collectively, these studies illustrate the potential for standard echocardiography to identify structural changes in the cardiac substrate that compromise cardiac mechanical function and promote risk for arrhythmia.
3.5. Criterion #5: Presence of hyperlipidemia and accelerated atherosclerosis
Growing evidence highlights the need for monitoring lipid levels and evidence of accelerated atherosclerosis in patients with chronic epilepsy. The normal limit for triglycerides is <149 mg/dl and for high-density lipoprotein (HDL) is >40 mg/dl, equivalent to the ranges established for ruling out the metabolic syndrome. In population studies enrolling >900 patients with epilepsy, hyperlipidemia was found to be ≥1.17-fold more common among patients with epilepsy than in comparison cohorts without epilepsy [84], [85]. It is germane that use of ASMs that induce the CYP450 system is associated with elevated serum lipid levels and C-reactive protein, and that use of CYP450-inhibiting ASMs is associated with a reduced risk for myocardial infarction [19]. Mintzer and colleagues [61], [62] also demonstrated that switching from CYP450-inducing agents (e.g., carbamazepine, phenytoin) to CYP450-noninducing ASMs (e.g., levetiracetam, lamotrigine, zonisamide) alone results in a persistent reduction in serum lipid and C-reactive protein levels and can decrease SCD risk by 3.2–5.7-fold. Liang and coworkers [24] reported that valproate was associated with 1.31-fold more all-cause deaths and 2.35-fold more heart failure deaths compared to treatment with lamotrigine or levetiracetam. The notable 34% to 43% decline in cardiovascular deaths in epilepsy patients from 1999 to 2017 has been attributed to primary prevention strategies and prescription of more modern ASMs [8], [9].
In individuals with hyperlipidemia, particularly those receiving enzyme-inducing ASMs, there is evidence that evaluating carotid intima media thickness (CIMT) with echocardiography could prove helpful [86], [87]. The presence of increased CIMT has the potential to signal more widespread atherosclerosis including coronary vascular stenosis and ischemic heart disease.
4. Diagnostic and therapeutic implications of the “Epileptic Heart Syndrome”
The proposed approach to the detection of the “Epileptic Heart Syndrome” in the adult population is rooted in sound pathophysiologic principles. The criteria include establishing chronic epilepsy with or without drug resistance as a centerpiece and is backed by electrocardiographic, autonomic, and echocardiographic measurements as well as plasma lipid level determinations. By combining these criteria, the soundness of diagnosis of the “Epileptic Heart Syndrome” is enhanced. Furthermore, the spectrum of variables assessed uncovers multiple potential therapeutic targets. The literature reviewed highlights the value of routine and multiday EKG-based assessment, which can help to disclose rhythm abnormalities, including atrial and ventricular arrhythmias. A number of attractive state-of-the-art wearable sensors including EKG patches and wristbands as well as minimally invasive insertable loop recorders hold promise to improve cardiac monitoring and to optimize therapeutic interventions in individuals afflicted with chronic epilepsy.
Ultimately, the body of evidence reviewed and the suggested syndromic approach provided [2] have the potential to impact day-to-day practice in patients suspected of an Epileptic Heart Syndrome. Particular attention to indications of cardiac dysfunction, including symptoms revealed in patient history such as palpitations, chest discomfort, and poor exercise performance, is recommended. The importance of hypertension, stroke, and diabetes mellitus in the morbidity and mortality of epilepsy patients has recently been emphasized with recommendation of cardiovascular risk estimation by American College of Cardiology’s atherosclerotic cardiovascular disease (ASCVD) estimator [88], [89], [90]. In addition to routine use of the 12-lead EKG during patient visits and hospital admission, we foresee that echocardiography could become an important additional tool in the armamentarium for determination of left ventricular ejection fraction and diastolic dysfunction, indicating a neurogenic cardiomyopathy phenotype [55], [57], [58].
The potential benefits of improved cardiovascular diagnostics and therapy in patients with epilepsy are supported by the reduction in cardiovascular deaths by primary prevention strategies [8], [9] and by eliminating use of enzyme-inducing agents [19], which can accelerate atherosclerosis. Avoidance of sodium channel blocking ASMs has also been shown to reduce risk for sudden arrhythmic death [17], [27], [61], [62], [63]. In the future, studies should be directed to evaluate the potential cardioprotective effect of reducing the impact of excess catecholamines. Promising therapies include cardioselective beta-adrenergic blockade and vagomimetic interventions such as vagus nerve stimulation with chronically implanted electrical stimulators [71], [91] or noninvasive tragus nerve stimulation [92], [93], based on principles emphasized in the current review.
Clearly, there is need for a comprehensive management plan that addresses not only epilepsy but also long-term cardiovascular risk.
Ethical statement
This is a review article. It provides no new data based on clinical studies or preclinical experiments.
Funding
This review article did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Richard L. Verrier: Writing – review & editing, Writing – original draft, Conceptualization. Steven C. Schachter: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Verrier is coinventor of United States Patent #10,022,060, which protects analytical methodologies used by Dr. Fialho and colleagues in Reference #60. Dr. Verrier is a member of the Medical Advisory Board of StratusNeuro, Inc. (Houston TX, USA) and has received consulting fees from Union Chimique Belge (UCB) Pharma S.A. (Brussels, Belgium). Dr. Schachter reports no conflicts of interest.
Footnotes
In: Epilepsy and Behavior Reports, Special Issue, Trudy D. Pang, MD, Guest Editor.
Contributor Information
Richard L. Verrier, Email: rverrier@bidmc.harvard.edu.
Steven C. Schachter, Email: sschacht@bidmc.harvard.edu.
References
- 1.Verrier R.L., Pang T.D., Nearing B.D., Schachter S.C. The epileptic heart: concept and clinical evidence. Epilepsy Behav. 2020;105 doi: 10.1016/j.yebeh.2020.106946. [DOI] [PubMed] [Google Scholar]
- 2.Verrier R.L., Pang T.D., Nearing B.D., Schachter S.C. Epileptic heart: a clinical syndromic approach. Epilepsia. 2021;62(8):1780–1789. doi: 10.1111/epi.16966. [DOI] [PubMed] [Google Scholar]
- 3.Surges R., Shmuely S., Dietze C., Ryvlin P., Thijs R.D. Identifying patients with epilepsy at high risk of cardiac death: signs, risk factors and initial management of high risk of cardiac death. Epileptic Disord. 2021;23:17–39. doi: 10.1684/epd.2021.1254. [DOI] [PubMed] [Google Scholar]
- 4.Janszky I., Hallqvist J., Tomson T., Ahlbom A., Mukamal K.J., Ahnve S. Increased risk and worse prognosis of myocardial infarction in patients with prior hospitalization for epilepsy—the Stockholm Heart Epidemiology Program. Brain. 2009;132:2798–2804. doi: 10.1093/brain/awp216. [DOI] [PubMed] [Google Scholar]
- 5.Neligan A., Bell G.S., Johnson A.L., Goodridge D.M., Shorvon S.D., Sander J.W. The long-term risk of premature mortality in people with epilepsy. Brain. 2011;134(Pt 2):388–395. doi: 10.1093/brain/awq378. [DOI] [PubMed] [Google Scholar]
- 6.Nevalainen O., Raitanen J., Ansakorpi H., Artama M., Isojärvi J., Auvinen A. Long-term mortality risk by cause of death in newly diagnosed patients with epilepsy in Finland: a nationwide register-based study. Eur J Epidemiol. 2013;28(12):981–990. doi: 10.1007/s10654-013-9848-1. [DOI] [PubMed] [Google Scholar]
- 7.Nevalainen O., Ansakorpi H., Sander J.W., Bell G.S., Keezer M.R. Cause of death and predictors of mortality in a community-based cohort of people with epilepsy. Neurology. 2016;87(8):852–853. doi: 10.1212/01.wnl.0000494744.36250.46. [DOI] [PubMed] [Google Scholar]
- 8.DeGiorgio C.M., Curtis A., Carapetian A., Hovsepian D., Krishnadasan A., Markovic D. Why are epilepsy mortality rates rising in the United States? A population-based multiple cause-of-death study. BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2019-035767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeGiorgio C.M., Curtis A.T., Hertling D., Kerr W.T., Markovic D. Changes in epilepsy causes of death: a US population study. Acta Neurol Scand. 2021;144(5):478–485. doi: 10.1111/ane.13500. [DOI] [PubMed] [Google Scholar]
- 10.Mayer J., Fawzy A.M., Bisson A., Pasi M., Bodin A., Vigny P., et al. Epilepsy and the risk of adverse cardiovascular events: a nationwide cohort study. Eur J Neurol. 2024;31(3) doi: 10.1111/ene.16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z., Liew D., Kwan P. Excess mortality and hospitalized morbidity in newly treated epilepsy patients. Neurology. 2016;87(7):718–725. doi: 10.1212/WNL.0000000000002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zack M., Luncheon C. Adults with an epilepsy history, notably those 45–64 years old or at the lowest income levels, more often report heart disease than adults without an epilepsy history. Epilepsy Behav. 2018;86:208–210. doi: 10.1016/j.yebeh.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husein N., Josephson C.B., Keezer M.R. Understanding cardiovascular disease in older adults with epilepsy. Epilepsia. 2021;62(9):2060–2071. doi: 10.1111/epi.16991. [DOI] [PubMed] [Google Scholar]
- 14.Josephson C.B., Wiebe S., Delgado-Garcia G., Gonzalez-Izquierdo A., Denaxas S., Sajobi T.T., et al. Association of enzyme-inducing antiseizure drug use with long-term cardiovascular disease. JAMA Neurol. 2021;78(11):1367–1374. doi: 10.1001/jamaneurol.2021.3424. [DOI] [PubMed] [Google Scholar]
- 15.Bucci T., Mbizvo G.K., Rivera-Caravaca J.M., Mayer J., Marson A.G., Abdul-Rahim A.H., et al. Epilepsy-heart syndrome: incidence and clinical outcomes of cardiac complications in patients with epilepsy. Curr Probl Cardiol. 2023;48(10) doi: 10.1016/j.cpcardiol.2023.101868. [DOI] [PubMed] [Google Scholar]
- 16.Shah R.A., Chahal C.A.A., Ranjha S., Sharaf Dabbagh G., Asatryan B., Limongelli I., et al. Cardiovascular disease burden, mortality, and sudden death risk in epilepsy: a UK Biobank Study. Can J Cardiol. 2024;40(4):688–695. doi: 10.1016/j.cjca.2023.11.02. [DOI] [PubMed] [Google Scholar]
- 17.Mayer J., Mbizvo G.K., Bucci T., Marson A., Lip G.Y.H. Association of antiseizure medications and adverse cardiovascular events: a global health federated network analysis. Epilepsia. 2024 doi: 10.1111/epi.17922. [DOI] [PubMed] [Google Scholar]
- 18.Olesen J.B., Abildstrøm S.Z., Erdal J., Gislason G.H., Weeke P., Andersson C., et al. Effects of epilepsy and selected antiepileptic drugs on risk of myocardial infarction, stroke, and death in patients with or without previous stroke: a nationwide cohort study. Pharmacoepidemiol Drug Saf. 2011;20(9):964–971. doi: 10.1002/pds.2186. [DOI] [PubMed] [Google Scholar]
- 19.Renoux C., Dell’Aniello S., Saarela O., Filion K.B., Boivin J.F. Antiepileptic drugs and the risk of ischaemic stroke and myocardial infarction: a population-based cohort study. BMJ Open. 2015;5(8):e008365. doi: 10.1136/bmjopen-2015-008365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson D.A., Wannamaker B.B., Malek A.M., Selassie A.W. Myocardial infarction after epilepsy onset: a population-based retrospective cohort study. Epilepsy Behav. 2018;88:181–188. doi: 10.1016/j.yebeh.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Wellejus Albertsen L., Heide-Jørgensen U., Schmidt S.A.J., Grey C., Jackson R., Sorensen H.T., et al. The DANish Comorbidity Index for Acute Myocardial Infarction (DANCAMI): development, validation and comparison with existing comorbidity indices. Clin Epidemiol. 2020;20(12):1299–1311. doi: 10.2147/CLEP.S277325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng C.Y., Hsu C.Y., Wang T.C., Liu C.Y., Yang Y.H., Yang W.H. Risk of cardiac morbidities and sudden death in patients with epilepsy and no history of cardiac disease: a population-based nationwide study. Mayo Clin Proc. 2021;96(4):964–974. doi: 10.1016/j.mayocp.2020.04.050. [DOI] [PubMed] [Google Scholar]
- 23.Doege C., Luedde M., Kostev K. Epilepsy is associated with an increased incidence of heart failure diagnoses. Epilepsy Behav. 2021;125 doi: 10.1016/j.yebeh.2021.108393. [DOI] [PubMed] [Google Scholar]
- 24.Liang D., Gardella E., Kragholm K., Polcwiartek C., Sessa M. The relationship between valproate and lamotrigine/levetiracetam use and prognosis in patients with epilepsy and heart failure: a Danish register-based study. J Card Fail. 2022;28(4):630–638. doi: 10.1016/j.cardfail.2021.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Bardai A., Lamberts R.J., Blom M.T., Spanjaart A.M., Berdowski J., van der Staal S.R., et al. Epilepsy is a risk factor for sudden cardiac arrest in the general population. PLoS One. 2012;7(8):e42749. doi: 10.1371/journal.pone.0042749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stecker E.C., Reinier K., Uy-Evanado A., Teodorescu C., Chugh H., Gunson K., et al. Relationship between seizure episode and sudden cardiac arrest in patients with epilepsy. Circ Arrhythm Electrophysiol. 2013;6:912–916. doi: 10.1161/CIRCEP.113.000544. [DOI] [PubMed] [Google Scholar]
- 27.Bardai A., Blom M.T., van Noord C., Verhamme K.M., Sturkenboom M.C.J.M., Tan H.L. Sudden cardiac death is associated both with epilepsy and with use of antiepileptic medications. Heart. 2015;101:17–22. doi: 10.1136/heartjnl-2014-305664. [DOI] [PubMed] [Google Scholar]
- 28.Rossi K.C., Gursky J.M., Pang T.D., Dhamoon M.S. Seizures and status epilepticus may be risk factor for cardiac arrhythmia or cardiac arrest across multiple time frames. Epilepsy Behav. 2021;120 doi: 10.1016/j.yebeh.2021.107998. [DOI] [PubMed] [Google Scholar]
- 29.Eroglu T.E., Folke F., Tan H.L., Torp-Pedersen C., Gislason G.H. Risk of out-of-hospital cardiac arrest in patients with epilepsy and users of antiepileptic drugs. Br J Clin Pharmacol. 2022;88(8):3709–3715. doi: 10.1111/bcp.15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J., Huang P., Yu Q., Lu J., Liu P., Yang Y., et al. Epilepsy and long-term risk of arrhythmias. Eur Heart J. 2023;44(35):3374–3382. doi: 10.1093/eurheartj/ehad523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desai R., Rupareliya C., Patel U., Naqvi S., Patel S., Lunagariya A., et al. Burden of arrhythmias in epilepsy patients: a national wide inpatient analysis of 1.4 million hospitalizations in the United States. Cureus. 2017;9:e1550. doi: 10.7759/cureus.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maguire MJ, Jackson CF, Marson AG, Nevitt SJ. Treatments for the prevention of sudden unexpected death in epilepsy (SUDEP). Cochrane Database Syst Rev. 2020 Apr 2;4(4):CD011792. doi: 10.1002/14651858.CD011792.pub3. [DOI] [PMC free article] [PubMed]
- 33.Walczak T.S., Leppik I.E., D'Amelio M., Rarick J., So E., Ahman P., et al. Incidence and risk factors in sudden unexpected death in epilepsy: a prospective cohort study. Neurology. 2001;56:519–525. doi: 10.1212/wnl.56.4.519. [DOI] [PubMed] [Google Scholar]
- 34.Nashef L., So E.L., Ryvlin P., Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia. 2012;53(2):227–233. doi: 10.1111/j.1528-1167.2011.03358.x. [DOI] [PubMed] [Google Scholar]
- 35.Thurman D.J., Hesdorffer D.C., French J.A. Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia. 2014;55:1479–1485. doi: 10.1111/epi.12666. [DOI] [PubMed] [Google Scholar]
- 36.Zack M.M., Kobau R. National and state estimates of the numbers of adults and children with active epilepsy– United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821–825. doi: 10.15585/mmwr.mm6631a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harden C., Tomson T., Gloss D., Buchhalter J., Cross J.H., Donner E., et al. Practice guideline summary: Sudden unexpected death in epilepsy incidence rates and risk factors: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2017;88(17):1674–1680. doi: 10.1212/WNL.0000000000003685. [DOI] [PubMed] [Google Scholar]
- 38.Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics— 2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 39.Seyal M., Pascual F., Lee C.Y., Li C.S., Bateman L.M. Seizure-related cardiac repolarization abnormalities are associated with ictal hypoxemia. Epilepsia. 2011;52(11):2105–2111. doi: 10.1111/j.1528-1167.2011.03262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li G., Bauer S., Nowak M., Norwood B., Tackenberg B., Rosenow F., et al. Cytokines and epilepsy. Seizure. 2011;20(3):249–256. doi: 10.1016/j.seizure.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Park K.J., Sharma G., Kennedy J.D., Seyal M. Potentially high-risk cardiac arrhythmias with focal to bilateral tonic-clonic seizures and generalized tonic-clonic seizures are associated with the duration of periictal hypoxemia. Epilepsia. 2017;58(12):2164–2171. doi: 10.1111/epi.13934. [DOI] [PubMed] [Google Scholar]
- 42.Tigaran S., Molgaard H., McClelland R., Dam M., Jaffe A.S. Evidence of cardiac ischemia during seizures in drug-refractory epilepsy patients. Neurology. 2003;60:491–495. doi: 10.1212/01.wnl.0000042090.13247.48. [DOI] [PubMed] [Google Scholar]
- 43.Tigaran S.P.C., Dalager-Pederson S., Baandrup U., Dam M., Vesterby-Charles A. Sudden unexpected death in epilepsy: is death by seizures a cardiac disease? Am J Forensic Med Pathol. 2005;26:99–105. [PubMed] [Google Scholar]
- 44.Nass R.D., Motloch L.J., Paar V., Lichtenauer M., Baumann J., Zur B., et al. Blood markers of cardiac stress after generalized convulsive seizures. Epilepsia. 2019;60:201–210. doi: 10.1111/epi.14637. [DOI] [PubMed] [Google Scholar]
- 45.Chin P.S., Branch K.R., Becker K.J. Postictal neurogenic stunned myocardium. Neurology. 2005;64(11):1977–1978. doi: 10.1212/01.WNL.0000163858.77494.7A. [DOI] [PubMed] [Google Scholar]
- 46.Stöllberger C., Wegner C., Finsterer J. Seizure-associated Takotsubo cardiomyopathy. Epilepsia. 2011;52(11):e160–e167. doi: 10.1111/j.1528-1167.2011.03185.x. [DOI] [PubMed] [Google Scholar]
- 47.Desai R., Singh S., Patel U., Fong H.K., Kaur V.P., Varma Y., et al. Frequency of Takotsubo cardiomyopathy in epilepsy-related hospitalizations among adults and its impact on in-hospital outcomes: a national standpoint. Int J Cardiol. 2020;299:67–70. doi: 10.1016/j.ijcard.2019.07.034. [DOI] [PubMed] [Google Scholar]
- 48.Falconer B., Rajs J. Post-mortem findings of cardiac lesions in epileptics: a preliminary report. Forensic Sci. 1976;8:63–71. doi: 10.1016/0300-9432(76)90048-0. [DOI] [PubMed] [Google Scholar]
- 49.Leestma J.E., Walczak T., Hughes J.R., Kalelkar M.B., Teas S.S. A prospective study on sudden unexpected death in epilepsy. Ann Neurol. 1989;26(2):195–203. doi: 10.1002/ana.410260203. [DOI] [PubMed] [Google Scholar]
- 50.Dasheiff R.M. Sudden unexpected death in epilepsy: a series from an epilepsy surgery program and speculation on the relationship to sudden cardiac death. J Clin Neurophysiol. 1991;8(2):216–222. [PubMed] [Google Scholar]
- 51.Natelson B.H., Suarez R.V., Terrence C.F., Turizo R. Patients with epilepsy who die suddenly have cardiac disease. Arch Neurol. 1998;55:857–860. doi: 10.1001/archneur.55.6.857. [DOI] [PubMed] [Google Scholar]
- 52.Särkioja T., Hirvonen J. Causes of sudden unexpected deaths in young and middle-aged persons. Forensic Sci Int. 1984;24:247–261. doi: 10.1016/0379-0738(84)90159-2. [DOI] [PubMed] [Google Scholar]
- 53.Earnest M.P., Thomas G.E., Eden R.A., Hossack K.F. The sudden unexplained death syndrome in epilepsy: demographic, clinical, and postmortem features. Epilepsia. 1992;33(2):310–316. doi: 10.1111/j.1528-1157.1992.tb02321.x. [DOI] [PubMed] [Google Scholar]
- 54.Devinsky O., Kim A., Friedman D., Bedigian A., Moffatt E., Tseng Z.H. Incidence of cardiac fibrosis in SUDEP and control cases. Neurology. 2018;91(1):e55–e61. doi: 10.1212/WNL.0000000000005740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fialho G.L., Wolf P., Walz R., Lin K. Increased cardiac stiffness is associated with autonomic dysfunction in patients with temporal lobe epilepsy. Epilepsia. 2018;59:e85–e90. doi: 10.1111/epi.14084. [DOI] [PubMed] [Google Scholar]
- 56.Chan M.M., Lam C.S. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail. 2013;15(6):604–613. doi: 10.1093/eurjhf/hft062. [DOI] [PubMed] [Google Scholar]
- 57.Fialho G.L., Pagani A.G., Wolf P., Walz R., Lin K. Echocardiographic risk markers of sudden death in patients with temporal lobe epilepsy. Epilepsy Res. 2018;140:192–197. doi: 10.1016/j.eplepsyres.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 58.Fialho G.L., Wolf P., Walz R., Lin K. Epilepsy and ultra-structural heart changes: The role of catecholaminergic toxicity and myocardial fibrosis. What can we learn from cardiology? Seizure. 2019;71:105–109. doi: 10.1016/j.seizure.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Yassin A., El-Salem K., Khassawneh B.Y., Al-Mistarehi A.H., Jarrah M., Zein Alaabdin A.M., et al. Diagnostic value of electrocardiogram during routine electroencephalogram. Seizure. 2021;89:19–23. doi: 10.1016/j.seizure.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Fialho G.L., Pang T.D., Kong W.Y., Tran A.P., Yu C.G., Rodriguez I.D., et al. Individuals with chronic epilepsy have elevated P-wave heterogeneity comparable to patients with atrial fibrillation. Epilepsia. 2023;64(9):2361–2372. doi: 10.1111/epi.17686. [DOI] [PubMed] [Google Scholar]
- 61.Mintzer S., Miller R., Shah K., Chervoneva I., Nei M., Skidmore C., et al. Long-term effect of antiepileptic drug switch on serum lipids and C-reactive protein. Epilepsy Behav. 2016;58:127–132. doi: 10.1016/j.yebeh.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mintzer S., Trinka E., Kraemer G., Chervoneva I., Werhahn K.J. Impact of carbamazepine, lamotrigine, and levetiracetam on vascular risk markers and lipid-lowering agents in the elderly. Epilepsia. 2018;59:1899–1907. doi: 10.1111/epi.14554. [DOI] [PubMed] [Google Scholar]
- 63.Zaccara G., Lattanzi S. Comorbidity between epilepsy and cardiac arrhythmias: Implication for treatment. Epilepsy Behav. 2019;97:304–312. doi: 10.1016/j.yebeh.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 64.Echt D.S., Liebson P.R., Mitchell L.B., Peters R.W., Obias-Manno D., Barker A.H., et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 65.Kuck K.H., Cappato R., Siebels J., Rüppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH) Circulation. 2000;102(7):748–754. doi: 10.1161/01.cir.102.7.748. [DOI] [PubMed] [Google Scholar]
- 66.Hamed S.A. Atherosclerosis in epilepsy: its causes and implications. Epilepsy Behav. 2014;41:290–296. doi: 10.1016/j.yebeh.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Li M.C.H., O'Brien T.J., Todaro M., Powell K.L. Acquired cardiac channelopathies in epilepsy: evidence, mechanisms, and clinical significance. Epilepsia. 2019;60(9):1753–1767. doi: 10.1111/epi.16301. [DOI] [PubMed] [Google Scholar]
- 68.Chahal C.A.A., Gottwald J.A., St Louis E.K., Xie J., Brady P.A., Alhurani R.E., et al. QT prolongation in patients with index evaluation for seizure or epilepsy is predictive of all-cause mortality. Heart Rhythm. 2022;19(4):578–584. doi: 10.1016/j.hrthm.2021.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nearing B.D., Verrier R.L. Modified moving average method for T-wave alternans analysis with high accuracy to predict ventricular fibrillation. J Appl Physiol. 2002;92:541–549. doi: 10.1152/japplphysiol.00592.2001. [DOI] [PubMed] [Google Scholar]
- 70.Verrier R.L., Klingenheben T., Malik M., El-Sherif N., Exner D., Hohnloser S., et al. Microvolt T-wave alternans: physiologic basis, methods of measurement, and clinical utility. Consensus guideline by the International Society for Holter and Noninvasive Electrocardiology. J Am Coll Cardiol. 2011;44:1309–1324. doi: 10.1016/j.jacc.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schomer A.C., Nearing B.D., Schachter S.C., Verrier R.L. Vagus nerve stimulation reduces cardiac electrical instability assessed by quantitative T-wave alternans analysis in patients with drug-resistant focal epilepsy. Epilepsia. 2014;55:1996–2002. doi: 10.1111/epi.12855. [DOI] [PubMed] [Google Scholar]
- 72.Verrier R.L., Nieminen T. T-wave alternans as a therapeutic marker for antiarrhythmic agents. J Cardiovasc Pharmacol. 2010;55(6):544–554. doi: 10.1097/FJC.0b013e3181d6b781. [DOI] [PubMed] [Google Scholar]
- 73.Pang T.D., Nearing B.D., Krishnamurthy K.B., Olin B., Schachter S.C., Verrier R.L. Cardiac electrical instability in newly diagnosed/chronic epilepsy tracked by Holter and EKG patch. Neurology. 2019;93:450–458. doi: 10.1212/WNL.0000000000008077. [DOI] [PubMed] [Google Scholar]
- 74.Pang T.D., Nearing B.D., Verrier R.L., Schachter S.C. Epileptic seizures and epilepsy monitoring unit admission disclose latent cardiac electrical instability. Epilepsy Behav. 2022;135:10881. doi: 10.1016/j.yebeh.2022.108881. [DOI] [PubMed] [Google Scholar]
- 75.Pang T.D., Nearing B.D., Verrier R.L., Schachter S.C. T-wave heterogeneity crescendo in the surface EKG is superior to heart rate acceleration for seizure prediction. Epilepsy Behav. 2022;130 doi: 10.1016/j.yebeh.2022.108670. [DOI] [PubMed] [Google Scholar]
- 76.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–81. [PubMed]
- 77.DeGiorgio C.M., Miller P., Meymandi S., Chin A., Epps J., Gordon S., et al. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory. Epilepsy Behav. 2010;19(1):78–81. doi: 10.1016/j.yebeh.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Myers K.A., Bello-Espinosa L.E., Symonds J.D., Zuberi S.M., Clegg R., Sadleir L.G., et al. Heart rate variability in epilepsy: a potential biomarker of sudden unexpected death in epilepsy risk. Epilepsia. 2018;59:1372–1380. doi: 10.1111/epi.14438. [DOI] [PubMed] [Google Scholar]
- 79.Lotufo P.A., Valiengo L., Benseñor I.M., Brunoni A.R. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia. 2012;53(2):272–282. doi: 10.1111/j.1528-1167.2011.03361.x. [DOI] [PubMed] [Google Scholar]
- 80.Ergul Y., Ekici B., Tatli B., Nisli K., Ozmen M. QT and P wave dispersion and heart rate variability in patients with Dravet syndrome. Acta Neurol Belg. 2013;113:161–166. doi: 10.1007/s13760-012-0140-z. [DOI] [PubMed] [Google Scholar]
- 81.Bybee K.A., Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008;118:397–409. doi: 10.1161/CIRCULATIONAHA.106.677625. [DOI] [PubMed] [Google Scholar]
- 82.Bilgi M., Yerdelen D., Calkesen Y., Maderrisoylu H. Evaluation of left ventricular diastolic function by tissue Doppler imaging in patients with newly diagnosed and untreated primary generalized epilepsy. Seizure. 2013;22:537–541. doi: 10.1016/j.seizure.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 83.Al-Najafi S., Rosman H. Seizure-induced myocardial stunning: a possible cardiac link to sudden unexpected death in epilepsy (SUDEP) Seizure. 2015;24:137–139. doi: 10.1016/j.seizure.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 84.Harnod T., Chen H.J., Li T.C., Sung F.C., Kao C.H. A high risk of hyperlipidemia in epilepsy patients: a nationwide population-based cohort study. Ann Epidemiol. 2014;24(12):910–914. doi: 10.1016/j.annepidem.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 85.Mintzer S., Yi M., Hegarty S., Maio V., Keith S. Hyperlipidemia in patients newly treated with anticonvulsants: a population study. Epilepsia. 2020;61:259–266. doi: 10.1111/epi.16420. [DOI] [PubMed] [Google Scholar]
- 86.Lai Q., Shen C., Zheng Y., Zhang Y., Guo Y., Ding M. Effects of antiepileptic drugs on the carotid artery intima-media thickness in epileptic patients. J Clin Neurol. 2017;13(4):371–379. doi: 10.3988/jcn.2017.13.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Safarpour Lima B., Mohamadzadeh A., Dadras M., Mahdavi A., Mansouri B., Farazdaghi M. Carotid intima-media and epicardial adipose tissue thickness in adult patients with epilepsy taking anti-seizure medication and its long-term significance. Epilepsy Behav. 2021;125 doi: 10.1016/j.yebeh.2021.108432. [DOI] [PubMed] [Google Scholar]
- 88.Lloyd-Jones D.M., Braun L.T., Ndumele C.E., Smith S.C., Jr, Sperling L.S., Virani S.S., et al. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. Circulation. 2019;139(25):e1162–e1177. doi: 10.1161/CIR.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 89.Shlobin N.A., Sander J.W. Drivers for the comorbidity of type 2 diabetes mellitus and epilepsy: a scoping review. Epilepsy Behav. 2020;106 doi: 10.1016/j.yebeh.2020.107043. [DOI] [PubMed] [Google Scholar]
- 90.Gaertner M.L., Mintzer S., DeGiorgio C.M. Increased cardiovascular risk in epilepsy. Front Neurol. 2024;15 doi: 10.3389/fneur.2024.1339276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Verrier R.L., Nearing B.D., Olin B., Boon P., Schachter S.C. Baseline elevation and reduction in cardiac electrical instability assessed by quantitative T-wave alternans in patients with drug-resistant epilepsy treated with vagus nerve stimulation in the AspireSR E-36 trial. Epilepsy Behav. 2016;62:85–89. doi: 10.1016/j.yebeh.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 92.Stefan H., Kreiselmeyer G., Kerling F., Kurzbuch K., Rauch C., Heers M., et al. Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: a proof of concept trial. Epilepsia. 2012;53(7):e115–e118. doi: 10.1111/j.1528-1167.2012.03492.x. [DOI] [PubMed] [Google Scholar]
- 93.Stavrakis S., Kulkarni K., Singh J.P., Katritsis D.G., Armoundas A.A. Autonomic modulation of cardiac arrhythmias: methods to assess treatment and outcomes. JACC Clin Electrophysiol. 2020;6(5):467–483. doi: 10.1016/j.jacep.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]