Abstract
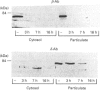
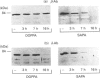
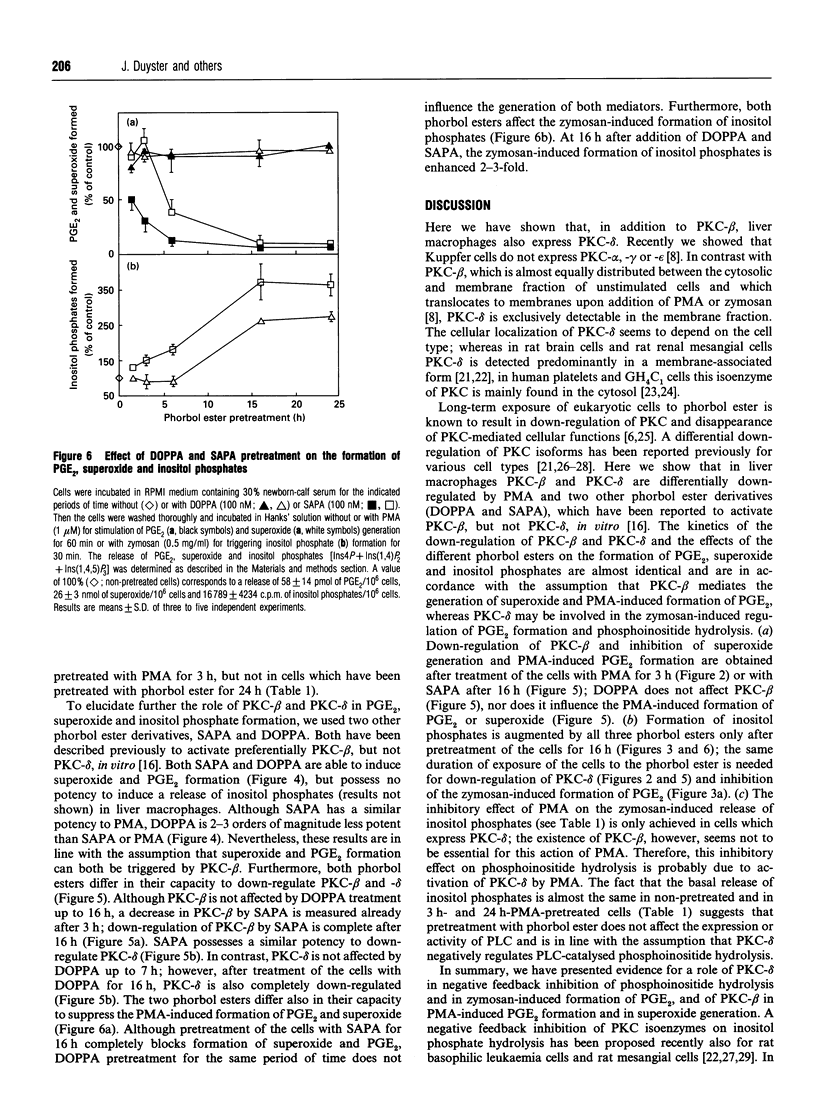
In contrast with protein kinase C (PKC)-beta, PKC-delta is exclusively detectable in the membrane fraction of liver macrophages. After long-term treatment with phorbol 12-myristate 13-acetate (PMA) PKC-beta is depleted faster (within 3 h) than PKC-delta (> 7h). Simultaneously, pretreatment with PMA for 3 h inhibits the PMA- and zymosan-induced generation of superoxide and the PMA-induced formation of prostaglandin (PG) E2, whereas a preincubation of more than 7 h is required to affect the zymosan-induced release of PGE2 and inositol phosphates. These results support an involvement of PKC-beta in the PMA-induced activation of the arachidonic acid cascade and in superoxide formation and imply an involvement of PKC-delta in zymosan-induced phosphoinositide hydrolysis and PGE2 formation. Two phorbol ester derivates, sapintoxin A (SAPA) and 12-deoxyphorbol 13-phenylacetate 20-acetate (DOPPA), which have been previously reported to activate preferentially PLC-beta but not PKC-delta in vitro [Ryves, Evans, Olivier, Parker and Evans (1992) FEBS Lett. 288, 5-9], induce the formation of PGE2 and superoxide, down-regulate PKC-delta and potentiate inositol phosphate formation in parallel SAPA, but not DOPPA, down-regulates PKC-beta and inhibits the PMA-induced formation of eicosanoids and superoxide.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacher N., Zisman Y., Berent E., Livneh E. Isolation and characterization of PKC-L, a new member of the protein kinase C-related gene family specifically expressed in lung, skin, and heart. Mol Cell Biol. 1991 Jan;11(1):126–133. doi: 10.1128/mcb.11.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Wymann M. P. Turning on the respiratory burst. Trends Biochem Sci. 1990 Feb;15(2):69–72. doi: 10.1016/0968-0004(90)90179-f. [DOI] [PubMed] [Google Scholar]
- Baldassare J. J., Henderson P. A., Burns D., Loomis C., Fisher G. J. Translocation of protein kinase C isozymes in thrombin-stimulated human platelets. Correlation with 1,2-diacylglycerol levels. J Biol Chem. 1992 Aug 5;267(22):15585–15590. [PubMed] [Google Scholar]
- Borner C., Guadagno S. N., Fabbro D., Weinstein I. B. Expression of four protein kinase C isoforms in rat fibroblasts. Distinct subcellular distribution and regulation by calcium and phorbol esters. J Biol Chem. 1992 Jun 25;267(18):12892–12899. [PubMed] [Google Scholar]
- Borner C., Guadagno S. N., Fabbro D., Weinstein I. B. Expression of four protein kinase C isoforms in rat fibroblasts. Distinct subcellular distribution and regulation by calcium and phorbol esters. J Biol Chem. 1992 Jun 25;267(18):12892–12899. [PubMed] [Google Scholar]
- Decker K. Biologically active products of stimulated liver macrophages (Kupffer cells). Eur J Biochem. 1990 Sep 11;192(2):245–261. doi: 10.1111/j.1432-1033.1990.tb19222.x. [DOI] [PubMed] [Google Scholar]
- Dieter P., Fitzke E. RO 31-8220 and RO 31-7549 show improved selectivity for protein kinase C over staurosporine in macrophages. Biochem Biophys Res Commun. 1991 Nov 27;181(1):396–401. doi: 10.1016/s0006-291x(05)81432-9. [DOI] [PubMed] [Google Scholar]
- Dieter P., Schulze-Specking A., Decker K. Differential inhibition of prostaglandin and superoxide production by dexamethasone in primary cultures of rat Kupffer cells. Eur J Biochem. 1986 Sep 15;159(3):451–457. doi: 10.1111/j.1432-1033.1986.tb09907.x. [DOI] [PubMed] [Google Scholar]
- Dieter P., Schulze-Specking A., Decker K. Release of lysosomal enzymes is not correlated with superoxide and prostaglandin production by stimulated rat Kupffer cells in primary culture. J Hepatol. 1988 Apr;6(2):167–174. doi: 10.1016/s0168-8278(88)80028-x. [DOI] [PubMed] [Google Scholar]
- Dieter P., Schulze-Specking A., Fitzke E. Activation of phospholipase C is not correlated to the formation of prostaglandins and superoxide in cultured rat liver macrophages. Cell Signal. 1991;3(1):65–71. doi: 10.1016/0898-6568(91)90009-j. [DOI] [PubMed] [Google Scholar]
- Duyster J., Hidaka H., Decker K., Dieter P. Proteinkinase C beta-isoform triggers the formation of prostanoids and superoxide in liver macrophages. Biochem Biophys Res Commun. 1992 Mar 31;183(3):1247–1253. doi: 10.1016/s0006-291x(05)80324-9. [DOI] [PubMed] [Google Scholar]
- Duyster J., Schulze-Specking A., Fitzke E., Dieter P. Protein kinase C involved in zymosan-induced release of arachidonic acid and superoxide but not in calcium ionophore-elicited arachidonic acid release or formation of prostaglandin E2 from added arachidonate. J Cell Biochem. 1992 Mar;48(3):288–295. doi: 10.1002/jcb.240480309. [DOI] [PubMed] [Google Scholar]
- Eyhorn S., Schlayer H. J., Henninger H. P., Dieter P., Hermann R., Woort-Menker M., Becker H., Schaefer H. E., Decker K. Rat hepatic sinusoidal endothelial cells in monolayer culture. Biochemical and ultrastructural characteristics. J Hepatol. 1988 Feb;6(1):23–35. doi: 10.1016/s0168-8278(88)80459-8. [DOI] [PubMed] [Google Scholar]
- Fiebich B., Hug H., Marmé D. High-efficiency expression of rat protein kinase C-gamma in baculovirus-infected insect cells. FEBS Lett. 1990 Dec 17;277(1-2):15–18. doi: 10.1016/0014-5793(90)80798-n. [DOI] [PubMed] [Google Scholar]
- Gat-Yablonski G., Sagi-Eisenberg R. Differential down-regulation of protein kinase C selectively affects IgE-dependent exocytosis and inositol trisphosphate formation. Biochem J. 1990 Sep 15;270(3):679–684. doi: 10.1042/bj2700679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Tanaka T., Onoda K., Hagiwara M., Watanabe M., Ohta H., Ito Y., Tsurudome M., Yoshida T. Cell type-specific expression of protein kinase C isozymes in the rabbit cerebellum. J Biol Chem. 1988 Apr 5;263(10):4523–4526. [PubMed] [Google Scholar]
- Huwiler A., Fabbro D., Pfeilschifter J. Possible regulatory functions of protein kinase C-alpha and -epsilon isoenzymes in rat renal mesangial cells. Stimulation of prostaglandin synthesis and feedback inhibition of angiotensin II-stimulated phosphoinositide hydrolysis. Biochem J. 1991 Oct 15;279(Pt 2):441–445. doi: 10.1042/bj2790441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwiler A., Fabbro D., Stabel S., Pfeilschifter J. Immunocharacterization of delta- and zeta-isoenzymes of protein kinase C in rat renal mesangial cells. FEBS Lett. 1992 Apr 6;300(3):259–262. doi: 10.1016/0014-5793(92)80858-e. [DOI] [PubMed] [Google Scholar]
- Kiley S. C., Parker P. J., Fabbro D., Jaken S. Differential regulation of protein kinase C isozymes by thyrotropin-releasing hormone in GH4C1 cells. J Biol Chem. 1991 Dec 15;266(35):23761–23768. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Krause H., Dieter P., Schulze-Specking A., Ballhorn A., Ferber E., Decker K. Synergistic effect of magnesium and calcium ions in the activation of phospholipase A2 of liver macrophages. Biochem Biophys Res Commun. 1991 Mar 15;175(2):532–536. doi: 10.1016/0006-291x(91)91597-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liyanage M., Frith D., Livneh E., Stabel S. Protein kinase C group B members PKC-delta, -epsilon, -zeta and PKC-L(eta). Comparison of properties of recombinant proteins in vitro and in vivo. Biochem J. 1992 May 1;283(Pt 3):781–787. doi: 10.1042/bj2830781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Ogita K., Miyamoto S., Yamaguchi K., Koide H., Fujisawa N., Kikkawa U., Sahara S., Fukami Y., Nishizuka Y. Isolation and characterization of delta-subspecies of protein kinase C from rat brain. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1592–1596. doi: 10.1073/pnas.89.5.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier A. R., Parker P. J. Identification of multiple PKC isoforms in Swiss 3T3 cells: differential down-regulation by phorbol ester. J Cell Physiol. 1992 Aug;152(2):240–244. doi: 10.1002/jcp.1041520204. [DOI] [PubMed] [Google Scholar]
- Osada S., Mizuno K., Saido T. C., Akita Y., Suzuki K., Kuroki T., Ohno S. A phorbol ester receptor/protein kinase, nPKC eta, a new member of the protein kinase C family predominantly expressed in lung and skin. J Biol Chem. 1990 Dec 25;265(36):22434–22440. [PubMed] [Google Scholar]
- Osada S., Mizuno K., Saido T. C., Suzuki K., Kuroki T., Ohno S. A new member of the protein kinase C family, nPKC theta, predominantly expressed in skeletal muscle. Mol Cell Biol. 1992 Sep;12(9):3930–3938. doi: 10.1128/mcb.12.9.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryves W. J., Evans A. T., Olivier A. R., Parker P. J., Evans F. J. Activation of the PKC-isotypes alpha, beta 1, gamma, delta and epsilon by phorbol esters of different biological activities. FEBS Lett. 1991 Aug 19;288(1-2):5–9. doi: 10.1016/0014-5793(91)80989-g. [DOI] [PubMed] [Google Scholar]