Abstract
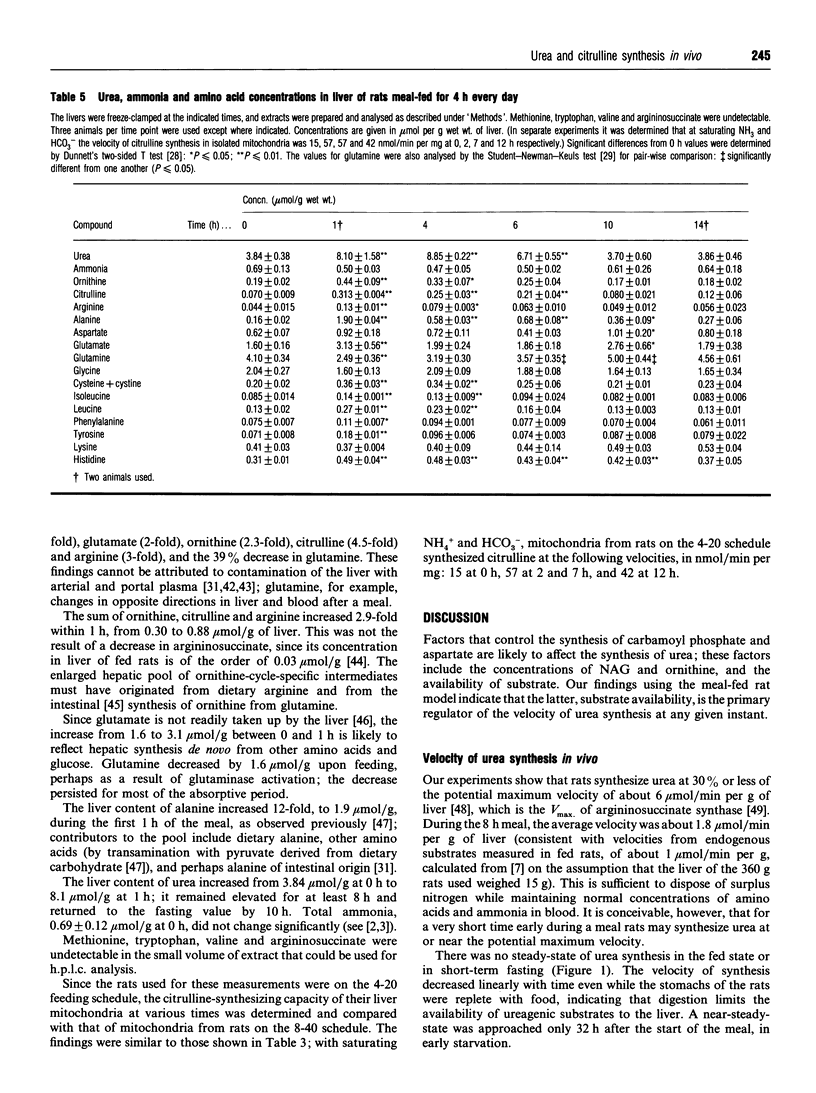
Information on the regulation of urea synthesis in vivo was obtained by examining the relationship between ureagenesis in vivo, citrulline synthesis in vitro, and two factors currently hypothesized to exert short-term regulation of this pathway: the liver mitochondrial content of N-acetylglutamate (NAG) and substrate availability. Rats meal-fed for 4 h every day (4-20 schedule) or for 8 h every other day (8-40 schedule) were used. (1) The citrulline-synthesizing capacity of mitochondria from livers of rats on the 8-40 schedule exceeded the corresponding velocity of urea synthesis in vivo at all time points studied. (2) Mitochondrial NAG in these livers increased from 127 +/- 32 pmol/mg of protein at 0 h to 486 +/- 205 pmol/mg at 3 h after the start of a meal, and decreased thereafter, but the correlation between NAG content and the velocity of citrulline synthesis was not simple, suggesting that NAG is not the only determinant of the state of activation of carbamoyl phosphate synthase I. (3) In rats on the 4-20 schedule killed 1 h after the start of the meal, the liver content of ornithine, citrulline, arginine, glutamate, alanine and urea increased 2.1-12-fold with respect to the values at 0 h; glutamine decreased by 39%. (4) The combined findings indicate that in vivo, moment-to-moment control of the velocity of urea synthesis is exerted by substrate availability. (5) Digestion limits the supply of substrate to the liver, and prevents its ureagenic capacity from being overwhelmed following a protein-containing meal.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa T., Matsutaka H., Yamamoto H., Okuda T., Ishikawa E. Gluconeogenesis and amino acid metabolism. II. Inter-organal relations and roles of glutamine and alanine in the amino acid metabolism of fasted rats. J Biochem. 1973 Nov;74(5):1003–1017. [PubMed] [Google Scholar]
- Alonso E., Rubio V. Determination of N-acetyl-L-glutamate using high-performance liquid chromatography. Anal Biochem. 1985 Apr;146(1):252–259. doi: 10.1016/0003-2697(85)90423-3. [DOI] [PubMed] [Google Scholar]
- Bean E. S., Atkinson D. E. Regulation of the rate of urea synthesis in liver by extracellular pH. A major factor in pH homeostasis in mammals. J Biol Chem. 1984 Feb 10;259(3):1552–1559. [PubMed] [Google Scholar]
- Brosnan J. T., Krebs H. A., Williamson D. H. Effects of ischaemia on metabolite concentrations in rat liver. Biochem J. 1970 Mar;117(1):91–96. doi: 10.1042/bj1170091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan J. T., Williamson D. H. Mechanisms for the formation of alanine and aspartate on rat liver in vivo after administration of ammonium chloride. Biochem J. 1974 Mar;138(3):453–462. doi: 10.1042/bj1380453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusilow S. W., Batshaw M. L., Waber L. Neonatal hyperammonemic coma. Adv Pediatr. 1982;29:69–103. [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Cheung C. W., Raijman L. Arginine, mitochondrial arginase, and the control of carbamyl phosphate synthesis. Arch Biochem Biophys. 1981 Jul;209(2):643–649. doi: 10.1016/0003-9861(81)90324-6. [DOI] [PubMed] [Google Scholar]
- Clarke S. A major polypeptide component of rat liver mitochondria: carbamyl phosphate synthetase. J Biol Chem. 1976 Feb 25;251(4):950–961. [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W. Differential effects of N-acetylglutamate on citrulline synthesis by coupled and uncoupled mitochondria. Arch Biochem Biophys. 1984 Oct;234(1):31–44. doi: 10.1016/0003-9861(84)90321-7. [DOI] [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Kyan F. S., Jones E. E., Raijman L. Mitochondrial carbamyl phosphate and citrulline synthesis at high matrix acetylglutamate. J Biol Chem. 1982 Jun 25;257(12):6898–6907. [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Raijman L. Altered enzyme activities and citrulline synthesis in liver mitochondria from ornithine carbamoyltransferase-deficient sparse-furash mice. Biochem J. 1989 Jan 1;257(1):251–257. doi: 10.1042/bj2570251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Raijman L. Channeling of extramitochondrial ornithine to matrix ornithine transcarbamylase. J Biol Chem. 1987 Jan 5;262(1):203–208. [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Raijman L. Measurements of mitochondrial volumes are affected by the amount of mitochondria used in the determinations. Biochem J. 1987 Jul 15;245(2):375–379. doi: 10.1042/bj2450375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Raijman L. The effects of ornithine on mitochondrial carbamyl phosphate synthesis. J Biol Chem. 1980 Nov 10;255(21):10248–10255. [PubMed] [Google Scholar]
- Cohen N. S., Kyan F. S., Kyan S. S., Cheung C. W., Raijman L. The apparent Km of ammonia for carbamoyl phosphate synthetase (ammonia) in situ. Biochem J. 1985 Jul 1;229(1):205–211. doi: 10.1042/bj2290205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K. R., Tipton K. F. Kinetic studies of bovine liver carbamoyl phosphate synthetase. Biochem J. 1974 Sep;141(3):807–816. doi: 10.1042/bj1410807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin M. L., Chen C. B., Cheema-Dhadli S., West M. L., Jungas R. L. Is urea formation regulated primarily by acid-base balance in vivo? Am J Physiol. 1986 Apr;250(4 Pt 2):F605–F612. doi: 10.1152/ajprenal.1986.250.4.F605. [DOI] [PubMed] [Google Scholar]
- Hems R., Stubbs M., Krebs H. A. Restricted permeability of rat liver for glutamate and succinate. Biochem J. 1968 May;107(6):807–815. doi: 10.1042/bj1070807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensgens H. E., Verhoeven A. J., Meijer A. J. The relationship between intramitochondrial N-acetylglutamate and activity of carbamoyl-phosphate synthetase (ammonia). The effect of glucagon. Eur J Biochem. 1980;107(1):197–205. doi: 10.1111/j.1432-1033.1980.tb04640.x. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Lund P. Some regulatory mechanisms in the synthesis of urea in the mammalian liver. Adv Enzyme Regul. 1973;11:361–377. doi: 10.1016/0065-2571(73)90024-1. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. The role of chemical equilibria in organ function. Adv Enzyme Regul. 1975;13:449–472. doi: 10.1016/0065-2571(75)90030-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lund P., Wiggins D. Inhibition of carbamoyl-phosphate synthase (ammonia) by Tris and Hepes. Effect on Ka for N-acetylglutamate. Biochem J. 1987 Apr 1;243(1):273–276. doi: 10.1042/bj2430273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund P., Wiggins D. The ornithine requirement of urea synthesis. Formation of ornithine from glutamine in hepatocytes. Biochem J. 1986 Nov 1;239(3):773–776. doi: 10.1042/bj2390773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer A. J., Lamers W. H., Chamuleau R. A. Nitrogen metabolism and ornithine cycle function. Physiol Rev. 1990 Jul;70(3):701–748. doi: 10.1152/physrev.1990.70.3.701. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Lof C., Ramos I. C., Verhoeven A. J. Control of ureogenesis. Eur J Biochem. 1985 Apr 1;148(1):189–196. doi: 10.1111/j.1432-1033.1985.tb08824.x. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Verhoeven A. J. N-acetylglutamate and urea synthesis. Biochem J. 1984 Oct 15;223(2):559–560. doi: 10.1042/bj2230559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G., Rogers Q. R. Arginine: an essential amino acid for the cat. J Nutr. 1978 Dec;108(12):1944–1953. doi: 10.1093/jn/108.12.1944. [DOI] [PubMed] [Google Scholar]
- Pedersen P. L., Greenawalt J. W., Reynafarje B., Hullihen J., Decker G. L., Soper J. W., Bustamente E. Preparation and characterization of mitochondria and submitochondrial particles of rat liver and liver-derived tissues. Methods Cell Biol. 1978;20:411–481. doi: 10.1016/s0091-679x(08)62030-0. [DOI] [PubMed] [Google Scholar]
- Potter V. R., Baril E. F., Watanabe M., Whittle E. D. Systematic oscillations in metabolic functions in liver from rats adapted to controlled feeding schedules. Fed Proc. 1968 Sep-Oct;27(5):1238–1245. [PubMed] [Google Scholar]
- Prescott A., Mangnall D. Interference by sucrose in the chemical determination of citrulline with diacetyl monoxime. Anal Biochem. 1976 Dec;76(2):551–555. doi: 10.1016/0003-2697(76)90348-1. [DOI] [PubMed] [Google Scholar]
- Raijman L., Jones M. E. Purification, composition, and some properties of rat liver carbamyl phosphate synthetase (ammonia). Arch Biochem Biophys. 1976 Jul;175(1):270–278. doi: 10.1016/0003-9861(76)90508-7. [DOI] [PubMed] [Google Scholar]
- Ratner S. A radiochemical assay for argininosuccinate synthetase with [U-14C]aspartate. Anal Biochem. 1983 Dec;135(2):479–488. doi: 10.1016/0003-2697(83)90716-9. [DOI] [PubMed] [Google Scholar]
- Rochovansky O., Kodowaki H., Ratner S. Biosynthesis of urea. Molecular and regulatory properties of crystalline argininosuccinate synthetase. J Biol Chem. 1977 Aug 10;252(15):5287–5294. [PubMed] [Google Scholar]
- Ross G., Dunn D., Jones M. E. Ornithine synthesis from glutamate in rat intestinal mucosa homogenates: evidence for the reduction of glutamate to gamma-glutamyl semialdehyde. Biochem Biophys Res Commun. 1978 Nov 14;85(1):140–147. doi: 10.1016/s0006-291x(78)80021-7. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T. Adaptive characteristics of urea cycle enzymes in the rat. J Biol Chem. 1962 Feb;237:459–468. [PubMed] [Google Scholar]
- SCHIMKE R. T. Differential effects of fasting and protein-free diets on levels of urea cycle enzymes in rat liver. J Biol Chem. 1962 Jun;237:1921–1924. [PubMed] [Google Scholar]
- Saheki T., Katsunuma T., Sase M. Regulation of urea synthesis in rat liver. Changes of ornithine and acetylglutamate concentrations in the livers of rats subjected to dietary transitions. J Biochem. 1977 Aug;82(2):551–558. [PubMed] [Google Scholar]
- Shigesada K., Tatibana M. Role of acetylglutamate in ureotelism. I. Occurrence and biosynthesis of acetylglutamate in mouse and rat tissues. J Biol Chem. 1971 Sep 25;246(18):5588–5595. [PubMed] [Google Scholar]
- Stewart P. M., Walser M. Short term regulation of ureagenesis. J Biol Chem. 1980 Jun 10;255(11):5270–5280. [PubMed] [Google Scholar]
- Varin F., Huet P. M. Hepatic microcirculation in the perfused cirrhotic rat liver. J Clin Invest. 1985 Nov;76(5):1904–1912. doi: 10.1172/JCI112186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSER M., BODENLOS L. J. Urea metabolism in man. J Clin Invest. 1959 Sep;38:1617–1626. doi: 10.1172/JCI103940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders R. J., Van Roermund C. W., Meijer A. J. Analysis of the control of citrulline synthesis in isolated rat-liver mitochondria. Eur J Biochem. 1984 Jul 16;142(2):247–254. doi: 10.1111/j.1432-1033.1984.tb08278.x. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lopes-Vieira O., Walker B. Concentrations of free glucogenic amino acids in livers of rats subjected to various metabolic stresses. Biochem J. 1967 Aug;104(2):497–502. doi: 10.1042/bj1040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Source and fate of circulating citrulline. Am J Physiol. 1981 Dec;241(6):E473–E480. doi: 10.1152/ajpendo.1981.241.6.E473. [DOI] [PubMed] [Google Scholar]
- Wolpert E., Phillips S. F., Summerskill W. H. Transport of urea and ammonia production in the human colon. Lancet. 1971 Dec 25;2(7739):1387–1390. doi: 10.1016/s0140-6736(71)90667-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Aikawa T., Matsutaka H., Okuda T., Ishikawa E. Interorganal relationships of amino acid metabolism in fed rats. Am J Physiol. 1974 Jun;226(6):1428–1433. doi: 10.1152/ajplegacy.1974.226.6.1428. [DOI] [PubMed] [Google Scholar]


