Abstract
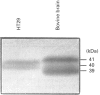
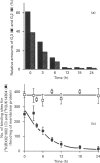
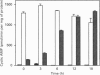
Previous studies have established that the human colon carcinoma cell line HT29 expresses an alpha 2-adrenergic receptor of the alpha 2A subtype, which is negatively coupled to adenylate cyclase. The purpose of the present study was to examine the mechanisms of alpha 2-adrenergic signal transduction in these cells. [32P]ADP-ribosylation with pertussis toxin and immunoblots using antibodies specific for the Gi alpha-subunits indicated that two distinct Gi-proteins (Gi2 and Gi3) were present in HT29-cell membranes. Treatment of intact cells with pertussis toxin resulted in a time-dependent decrease in the amount of [32P]ADP-ribosylatable Gi2 and Gi3, which coincided with a diminution in the number of alpha 2-adrenergic receptors in high-affinity state for agonists and with a progressive loss of ability of UK14304 to inhibit forskolin-stimulated accumulation of cyclic AMP. When membranes were [32P]ADP-ribosylated with cholera toxin in the absence of exogenous added guanine nucleotides, radioactivity was incorporated into a 45 kDa polypeptide representing Gs, as well as into 40-41 kDa polypeptides corresponding to Gi3 and Gi2. The amount of radioactivity incorporated into the two GiS under basal conditions was decreased by addition of the alpha 2-antagonist RX821002. It was not significantly affected by addition of clonidine (partial alpha 2-agonist), but was doubled by the addition of UK14304 (full alpha 2-agonist). This effect was blocked by RX821002. Study of adenylate cyclase activity indicated that preincubation of HT29 membranes with the antibody AS/7 (anti-alpha i1/alpha i2), but not with the antibody EC/2 (anti-alpha i3), attenuated the inhibitory effect of UK14304 on forskolin-stimulated adenylate cyclase. These data demonstrate that the alpha 2A-adrenergic receptor is coupled to both Gi2 and Gi3, and identify Gi2 as the major mediator of inhibition of adenylate cyclase in HT29 cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez R., Daniels D. V. A single column method for the assay of adenylate cyclase. Anal Biochem. 1990 May 15;187(1):98–103. doi: 10.1016/0003-2697(90)90423-7. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Berlan M., Montastruc J. L., Lafontan M. Pharmacological prospects for alpha 2-adrenoceptor antagonist therapy. Trends Pharmacol Sci. 1992 Jul;13(7):277–282. doi: 10.1016/0165-6147(92)90085-k. [DOI] [PubMed] [Google Scholar]
- Bouscarel B., Cortinovis C., Carpene C., Murat J. C., Paris H. alpha 2-Adrenoceptors in the HT 29 human colon adenocarcinoma cell line: characterization with [3H]clonidine; effects on cyclic AMP accumulation. Eur J Pharmacol. 1985 Jan 2;107(2):223–231. doi: 10.1016/0014-2999(85)90062-7. [DOI] [PubMed] [Google Scholar]
- Couvineau A., Darmoul D., Blais A., Rouyer-Fessard C., Daviaud D., Voisin T., Paris H., Rouot B., Laburthe M. Gs and Gi protein subunits during cell differentiation in intestinal crypt-villus axis: regulation at the mRNA level. Am J Physiol. 1992 Jun;262(6 Pt 1):C1478–C1484. doi: 10.1152/ajpcell.1992.262.6.C1478. [DOI] [PubMed] [Google Scholar]
- Denis C., Paris H., Murat J. C. Hormonal control of fructose 2,6-bisphosphate concentration in the HT29 human colon adenocarcinoma cell line. Alpha 2-adrenergic agonists counteract effect of vasoactive intestinal peptide. Biochem J. 1986 Nov 1;239(3):531–536. doi: 10.1042/bj2390531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devedjian J. C., Fargues M., Denis-Pouxviel C., Daviaud D., Prats H., Paris H. Regulation of the alpha 2A-adrenergic receptor in the HT29 cell line. Effects of insulin and growth factors. J Biol Chem. 1991 Aug 5;266(22):14359–14366. [PubMed] [Google Scholar]
- Gerhardt M. A., Neubig R. R. Multiple Gi protein subtypes regulate a single effector mechanism. Mol Pharmacol. 1991 Nov;40(5):707–711. [PubMed] [Google Scholar]
- Homburger V., Brabet P., Audigier Y., Pantaloni C., Bockaert J., Rouot B. Immunological localization of the GTP-binding protein Go in different tissues of vertebrates and invertebrates. Mol Pharmacol. 1987 Apr;31(4):313–319. [PubMed] [Google Scholar]
- Itoh H., Toyama R., Kozasa T., Tsukamoto T., Matsuoka M., Kaziro Y. Presence of three distinct molecular species of Gi protein alpha subunit. Structure of rat cDNAs and human genomic DNAs. J Biol Chem. 1988 May 15;263(14):6656–6664. [PubMed] [Google Scholar]
- Jones S. B., Bylund D. B. Characterization and possible mechanisms of alpha 2-adrenergic receptor-mediated sensitization of forskolin-stimulated cyclic AMP production in HT29 cells. J Biol Chem. 1988 Oct 5;263(28):14236–14244. [PubMed] [Google Scholar]
- Kurose H., Regan J. W., Caron M. G., Lefkowitz R. J. Functional interactions of recombinant alpha 2 adrenergic receptor subtypes and G proteins in reconstituted phospholipid vesicles. Biochemistry. 1991 Apr 2;30(13):3335–3341. doi: 10.1021/bi00227a024. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langin D., Lafontan M., Stillings M. R., Paris H. [3H]RX821002: a new tool for the identification of alpha 2A-adrenoceptors. Eur J Pharmacol. 1989 Aug 11;167(1):95–104. doi: 10.1016/0014-2999(89)90751-6. [DOI] [PubMed] [Google Scholar]
- Limbird L. E. Receptors linked to inhibition of adenylate cyclase: additional signaling mechanisms. FASEB J. 1988 Aug;2(11):2686–2695. doi: 10.1096/fasebj.2.11.2840317. [DOI] [PubMed] [Google Scholar]
- McClue S. J., Milligan G. Molecular interaction of the human alpha 2-C10-adrenergic receptor, when expressed in Rat-1 fibroblasts, with multiple pertussis toxin-sensitive guanine nucleotide-binding proteins: studies with site-directed antisera. Mol Pharmacol. 1991 Nov;40(5):627–632. [PubMed] [Google Scholar]
- McClue S. J., Selzer E., Freissmuth M., Milligan G. Gi3 does not contribute to the inhibition of adenylate cyclase when stimulation of an alpha 2-adrenergic receptor causes activation of both Gi2 and Gi3. Biochem J. 1992 Jun 1;284(Pt 2):565–568. doi: 10.1042/bj2840565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson G. A. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985 Nov;14(3):213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- Milligan G., Carr C., Gould G. W., Mullaney I., Lavan B. E. Agonist-dependent, cholera toxin-catalyzed ADP-ribosylation of pertussis toxin-sensitive G-proteins following transfection of the human alpha 2-C10 adrenergic receptor into rat 1 fibroblasts. Evidence for the direct interaction of a single receptor with two pertussis toxin-sensitive G-proteins, Gi2 and Gi3. J Biol Chem. 1991 Apr 5;266(10):6447–6455. [PubMed] [Google Scholar]
- Milligan G. Guanine nucleotide regulation of the pertussis and cholera toxin substrates of rat glioma C6 BU1 cells. Biochim Biophys Acta. 1987 Jul 6;929(2):197–202. doi: 10.1016/0167-4889(87)90176-5. [DOI] [PubMed] [Google Scholar]
- Milligan G., McKenzie F. R. Opioid peptides promote cholera-toxin-catalysed ADP-ribosylation of the inhibitory guanine-nucleotide-binding protein (Gi) in membranes of neuroblastoma x glioma hybrid cells. Biochem J. 1988 Jun 1;252(2):369–373. doi: 10.1042/bj2520369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. Tissue distribution and subcellular location of guanine nucleotide binding proteins: implications for cellular signalling. Cell Signal. 1989;1(5):411–419. doi: 10.1016/0898-6568(89)90027-2. [DOI] [PubMed] [Google Scholar]
- Nakaki T., Nakadate T., Yamamoto S., Kato R. Alpha-2 adrenergic inhibition of intestinal secretion induced by prostaglandin E1, vasoactive intestinal peptide and dibutyryl cyclic AMP in rat jejunum. J Pharmacol Exp Ther. 1982 Mar;220(3):637–641. [PubMed] [Google Scholar]
- Neubig R. R., Gantzos R. D., Thomsen W. J. Mechanism of agonist and antagonist binding to alpha 2 adrenergic receptors: evidence for a precoupled receptor-guanine nucleotide protein complex. Biochemistry. 1988 Apr 5;27(7):2374–2384. doi: 10.1021/bi00407a019. [DOI] [PubMed] [Google Scholar]
- Paris H., Galitzky J., Senard J. M. Interactions of full and partial agonists with HT29 cell alpha 2-adrenoceptor: comparative study of [3H]UK-14,304 and [3H]clonidine binding. Mol Pharmacol. 1989 Mar;35(3):345–354. [PubMed] [Google Scholar]
- Paris H., Taouis M., Galitzky J. In vitro study of alpha 2-adrenoceptor turnover and metabolism using the adenocarcinoma cell line HT29. Mol Pharmacol. 1987 Nov;32(5):646–654. [PubMed] [Google Scholar]
- Ribeiro-Neto F. A., Mattera R., Hildebrandt J. D., Codina J., Field J. B., Birnbaumer L., Sekura R. D. ADP-ribosylation of membrane components by pertussis and cholera toxin. Methods Enzymol. 1985;109:566–572. doi: 10.1016/0076-6879(85)09115-7. [DOI] [PubMed] [Google Scholar]
- Rouot B., Carrette J., Lafontan M., Lan Tran P., Fehrentz J. A., Bockaert J., Toutant M. The adipocyte Go alpha-immunoreactive polypeptide is different from the alpha subunit of the brain Go protein. Biochem J. 1989 May 15;260(1):307–310. doi: 10.1042/bj2600307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Codina J., Unson C. G., Spiegel A. M. Gi2 mediates alpha 2-adrenergic inhibition of adenylyl cyclase in platelet membranes: in situ identification with G alpha C-terminal antibodies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7809–7813. doi: 10.1073/pnas.86.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Tilly B. C., Kansen M., van Gageldonk P. G., van den Berghe N., Galjaard H., Bijman J., de Jonge H. R. G-proteins mediate intestinal chloride channel activation. J Biol Chem. 1991 Feb 5;266(4):2036–2040. [PubMed] [Google Scholar]
- Tsukamoto T., Toyama R., Itoh H., Kozasa T., Matsuoka M., Kaziro Y. Structure of the human gene and two rat cDNAs encoding the alpha chain of GTP-binding regulatory protein Go: two different mRNAs are generated by alternative splicing. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):2974–2978. doi: 10.1073/pnas.88.8.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ui M., Katada T. Bacterial toxins as probe for receptor-Gi coupling. Adv Second Messenger Phosphoprotein Res. 1990;24:63–69. [PubMed] [Google Scholar]
- van den Berghe N., Nieuwkoop N. J., Vaandrager A. B., de Jonge H. R. Asymmetrical distribution of G-proteins among the apical and basolateral membranes of rat enterocytes. Biochem J. 1991 Sep 1;278(Pt 2):565–571. doi: 10.1042/bj2780565. [DOI] [PMC free article] [PubMed] [Google Scholar]