Abstract
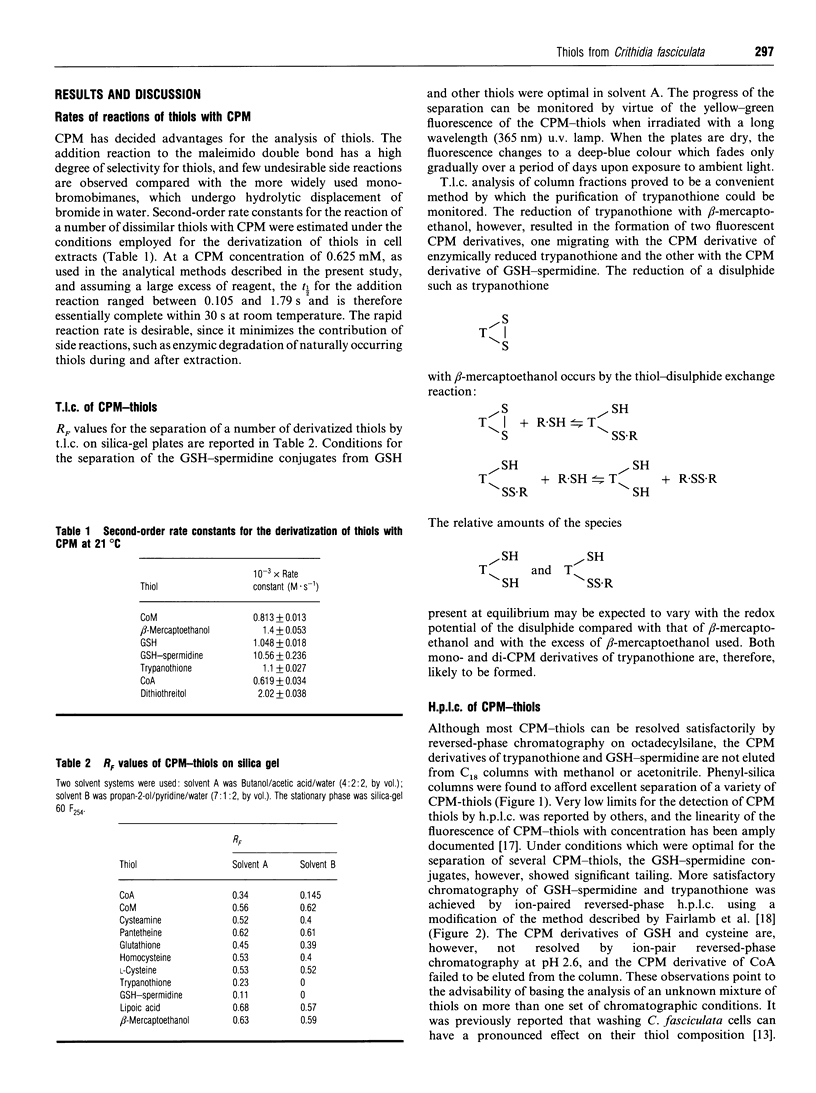
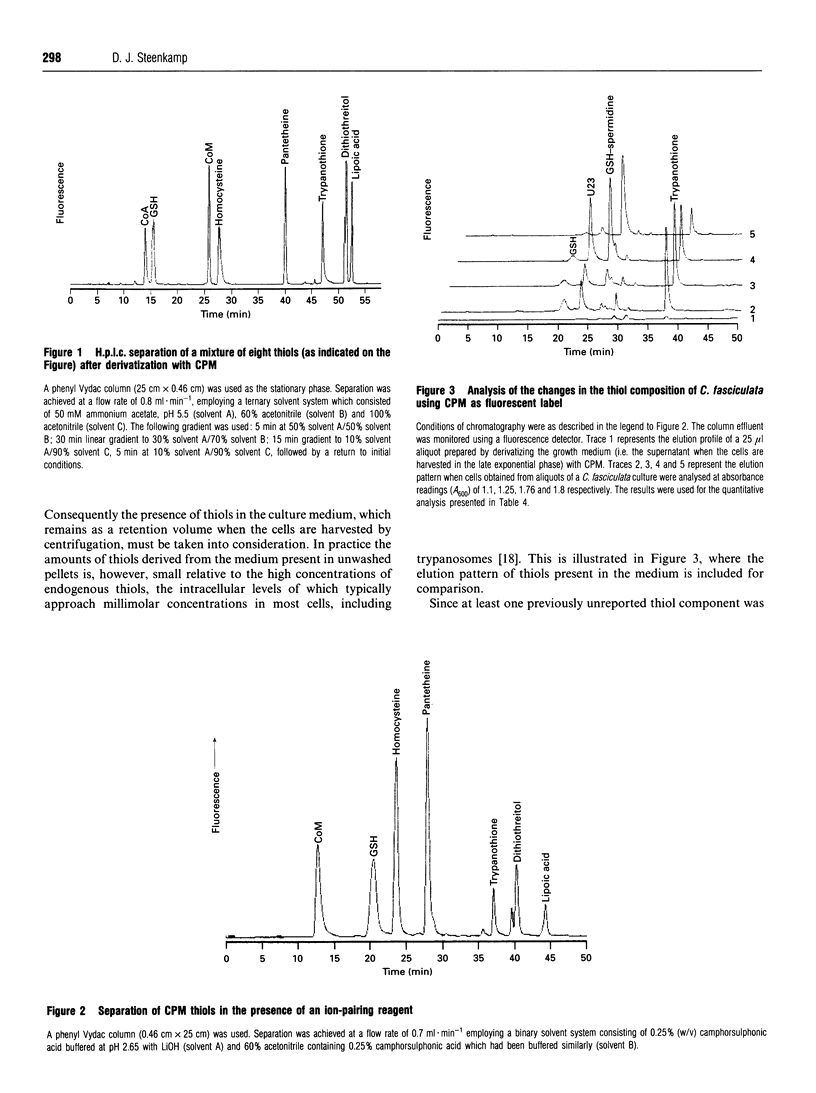
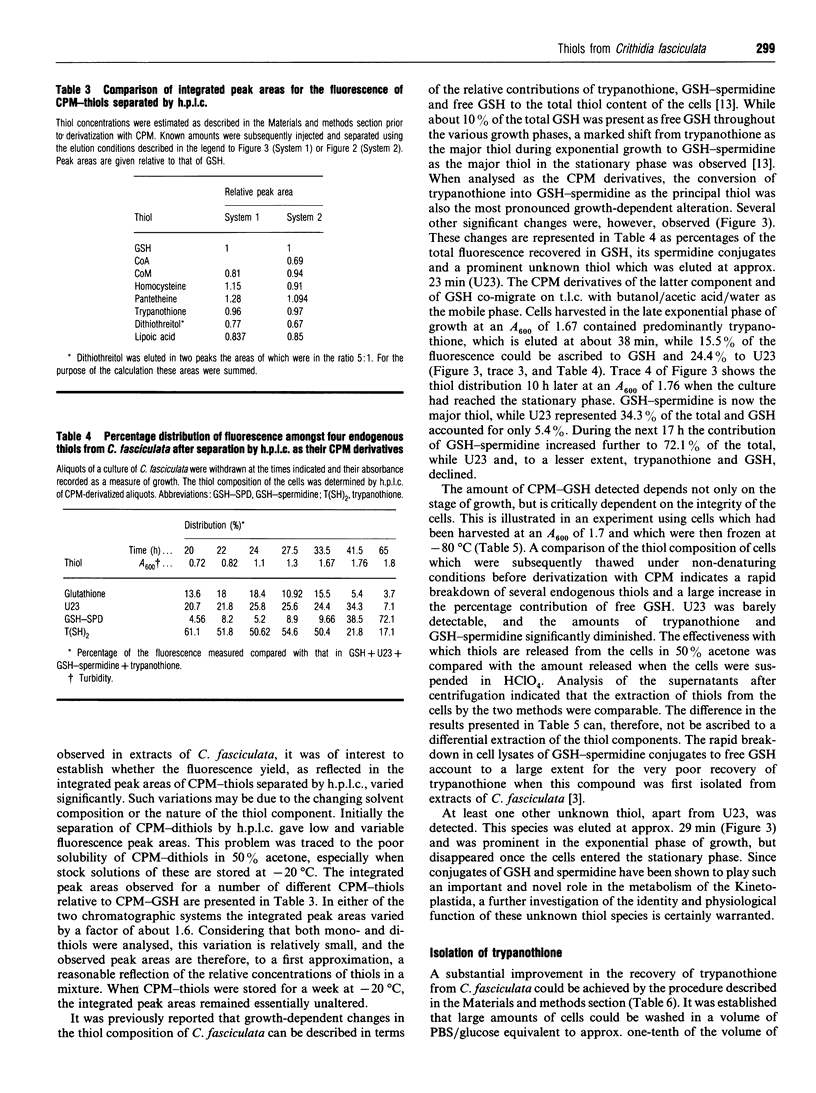
Methods for the qualitative and quantitative analysis of thiols by means of the fluorogenic reagent 7-diethylamino-3-(4'-maleimidylphenyl)-4-methylcoumarin are described, with particular reference to the trypanosomatid metabolites glutathionylspermidine (GSH-spermidine) and trypanothione. Second-order rate constants for the derivatization of seven different thiols under defined experimental conditions and at 21 degrees C varied between 619 +/- 34 and 10,560 +/- 236 M-1.s-1.T.l.c. of the thiols from Crithidia fasciculata was used to monitor the purification of trypanothione from this organism in three steps involving adsorption, ion-exchange and reversed-phase chromatography. The yield was approx. 50 mg of pure trypanothione from 100 g (wet wt.) of trypanosomatids. The method for the quantitative analysis of biological thiols is based on fluorometric detection after separation by reversed-phase or ion-paired chromatography on a phenyl-silica column. Analysis of the thiol composition of cell lysates prepared under nondenaturating conditions point to the rapid degradation of the GSH-spermidine conjugates. In addition to GSH, GSH-spermidine and trypanothione, at least one other prominent thiol was detected, and the contribution of this thiol to the total thiol content in the various growth phases of C. fasciculata was investigated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fairlamb A. H., Blackburn P., Ulrich P., Chait B. T., Cerami A. Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science. 1985 Mar 22;227(4693):1485–1487. doi: 10.1126/science.3883489. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Henderson G. B., Bacchi C. J., Cerami A. In vivo effects of difluoromethylornithine on trypanothione and polyamine levels in bloodstream forms of Trypanosoma brucei. Mol Biochem Parasitol. 1987 Jun;24(2):185–191. doi: 10.1016/0166-6851(87)90105-8. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Henderson G. B., Cerami A. The biosynthesis of trypanothione and N1-glutathionylspermidine in Crithidia fasciculata. Mol Biochem Parasitol. 1986 Dec;21(3):247–257. doi: 10.1016/0166-6851(86)90130-1. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H. Trypanothione metabolism and rational approaches to drug design. Biochem Soc Trans. 1990 Oct;18(5):717–720. doi: 10.1042/bst0180717. [DOI] [PubMed] [Google Scholar]
- Garcia R. A., Hirschberger L. L., Stipanuk M. H. Measurement of cyst(e)amine in physiological samples by high performance liquid chromatography. Anal Biochem. 1988 May 1;170(2):432–440. doi: 10.1016/0003-2697(88)90655-0. [DOI] [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr Determination of sulfhydryl groups with 2,2'- or 4,4'-dithiodipyridine. Arch Biochem Biophys. 1967 Mar;119(1):41–49. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Fairlamb A. H., Cerami A. Trypanothione dependent peroxide metabolism in Crithidia fasciculata and Trypanosoma brucei. Mol Biochem Parasitol. 1987 May;24(1):39–45. doi: 10.1016/0166-6851(87)90113-7. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Fairlamb A. H., Ulrich P., Cerami A. Substrate specificity of the flavoprotein trypanothione disulfide reductase from Crithidia fasciculata. Biochemistry. 1987 Jun 2;26(11):3023–3027. doi: 10.1021/bi00385a011. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Yamaguchi M., Novoa L., Fairlamb A. H., Cerami A. Biosynthesis of the trypanosomatid metabolite trypanothione: purification and characterization of trypanothione synthetase from Crithidia fasciculata. Biochemistry. 1990 Apr 24;29(16):3924–3929. doi: 10.1021/bi00468a019. [DOI] [PubMed] [Google Scholar]
- Krauth-Siegel R. L., Enders B., Henderson G. B., Fairlamb A. H., Schirmer R. H. Trypanothione reductase from Trypanosoma cruzi. Purification and characterization of the crystalline enzyme. Eur J Biochem. 1987 Apr 1;164(1):123–128. doi: 10.1111/j.1432-1033.1987.tb11002.x. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Shames S. L., Fairlamb A. H., Cerami A., Walsh C. T. Purification and characterization of trypanothione reductase from Crithidia fasciculata, a newly discovered member of the family of disulfide-containing flavoprotein reductases. Biochemistry. 1986 Jun 17;25(12):3519–3526. doi: 10.1021/bi00360a007. [DOI] [PubMed] [Google Scholar]
- Shim H., Fairlamb A. H. Levels of polyamines, glutathione and glutathione-spermidine conjugates during growth of the insect trypanosomatid Crithidia fasciculata. J Gen Microbiol. 1988 Mar;134(3):807–817. doi: 10.1099/00221287-134-3-807. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Isolation, characterization, and turnover of glutathionylspermidine from Escherichia coli. J Biol Chem. 1975 Apr 10;250(7):2648–2654. [PubMed] [Google Scholar]
- Tapuhi Y., Schmidt D. E., Lindner W., Karger B. L. Dansylation of amino acids for high-performance liquid chromatography analysis. Anal Biochem. 1981 Jul 15;115(1):123–129. doi: 10.1016/0003-2697(81)90534-0. [DOI] [PubMed] [Google Scholar]
- el-Waer A., Douglas K. T., Smith K., Fairlamb A. H. Synthesis of N-benzyloxycarbonyl-L-cysteinylglycine 3-dimethylaminopropylamide disulfide: a cheap and convenient new assay for trypanothione reductase. Anal Biochem. 1991 Oct;198(1):212–216. doi: 10.1016/0003-2697(91)90531-w. [DOI] [PubMed] [Google Scholar]


