Abstract
Nucleolytic resection of DNA ends is critical for homologous recombination, but its mechanism is not fully understood, particularly in mammalian meiosis. Here we examine roles of the conserved MRN complex (MRE11, RAD50, and NBS1) through genome-wide analysis of meiotic resection in mice with various MRN mutations, including several that cause chromosomal instability in humans. Meiotic DSBs form at elevated levels but remain unresected if Mre11 is conditionally deleted, thus MRN is required for both resection initiation and regulation of DSB numbers. Resection lengths are reduced to varying degrees in MRN hypomorphs or if MRE11 nuclease activity is attenuated in a conditional nuclease-dead Mre11 model. These findings unexpectedly establish that MRN is needed for longer-range extension of resection, not just resection initiation. Finally, resection defects are additively worsened by combining MRN and Exo1 mutations, and mice that are unable to initiate resection or have greatly curtailed resection lengths experience catastrophic spermatogenic failure. Our results elucidate multiple functions of MRN in meiotic recombination, uncover unanticipated relationships between short- and long-range resection, and establish the importance of resection for mammalian meiosis.
Keywords: Meiosis, DNA double-strand break resection, Mre11, Nbs1, Rad50, Spo11, spermatogenesis
INTRODUCTION
Homologous recombination during meiosis initiates with DNA double-strand breaks (DSBs) made by SPO11 protein, which remains covalently bound to DNA 5’ ends after strand breakage1,2. These DSBs must then be nucleolytically processed (resected) to generate the single-stranded DNA (ssDNA) needed for homology search and strand exchange (Figure 1A)3. Despite its central role in recombination, meiotic DSB resection remains poorly understood, particularly in mammals.
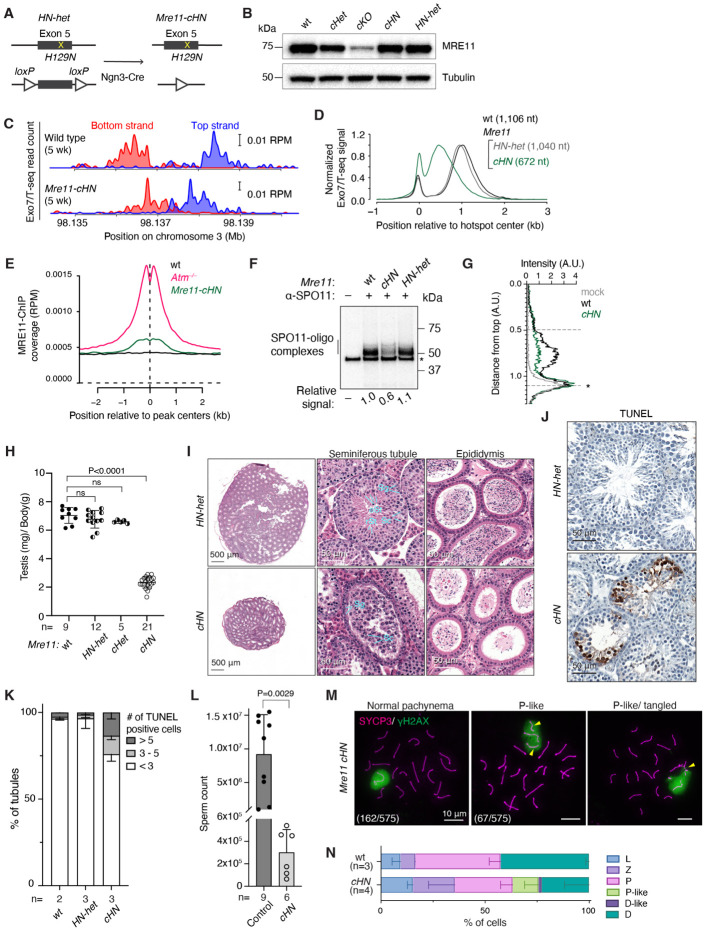
Figure 1. MRE11 is required for resection initiation.

(A) Early meiotic recombination steps and sequencing strategy. i, SPO11 (magenta ellipses) cuts dNa via a covalent protein–DNA intermediate. SPO11-bound strands are nicked (arrowheads) to give an entry point(s) for exonuclease(s). Bi-directional resection releases SPO11-oligo complexes and exposes long ssDNA tails. ii, Two-step resection model involving MRE11 (green Pacman) handing off to other exonucleolytic enzymes (black Pacman). iii, Sequencing adaptors are ligated to duplex ends after removal of ssDNA.
(B) Strategy for conditional deletion of Mre11.
(C) Reduced MRN protein levels in Mre11-cKO mice. Immunoblotting is shown of whole testis lysates from 5–7 wk old animals. Numbers below the MRE11 blot indicate amounts of a dilution series of wild-type (wt) lysate or band intensities for Mre11-cKO and Mre11-cHet relative to wild type and normalized to the loading control (tubulin).
(D) Representative PFA-fixed seminiferous tubule sections at 5–7 wk stained for MRE11 (brown color). In wild type, all cells except elongated spermatids stained positive for MRE11. In Mre11-cKO, tubules were categorized based on the presence of MRE11-positive spermatocytes. Arrowheads mark examples of MRE11-positive spermatogonia (red) or spermatocytes with (purple) or without (green) MRE11 staining. Scale bars, 50 μm.
(E) Quantification of seminiferous tubule classes illustrated in panel D. Number of animals tested: n=1 for wild type and n=2 for Mre11-cKO (mean ± range).
(F) Strand-specific S1-seq [reads per million mapped reads (RPM)] at a representative DSB hotspot. Signals from three (wild type) or two (Spo11−/− and Mre11-cKO) biological replicate libraries were averaged and plotted. The semi-synchronous first wave of spermatogenesis in juvenile mice enhances the signal:noise ratio in S1-seq libraries from wild type, but older mice (≥ 5 wk) are analyzed for Mre11-cKO because of their more penetrant phenotype (see Methods). Data are smoothed with a 151-bp Hann window. SPO11-oligo sequencing data here (top plot) and throughout are from Lange et al. (2016). The baseline of the y-axis for each plot is 0.
(G) Stereotyped distribution of resection endpoints around DSB hotspots. Heatmaps (data in 40-bp bins) show strand-specific reads around SPO11-dependent DSB hotspots from wild type [n = 13,960 from Lange et al. (2016)]. Each hotspot is shown as a horizontal line, strongest at the top. Sequencing signals were locally normalized by dividing by the total signal in a 4001-bp window around each hotspot’s center. Each hotspot thus has a total value of 1, to facilitate comparisons of spatial patterns between hotspots of different strengths.
The most detailed understanding of meiotic resection is currently for budding yeast, which uses a two-step DSB-processing mechanism (Figure 1A)3. In the first step, the conserved Mre11-Rad50-Xrs2 (MRX) complex together with Sae2 (homologous to MRE11-RAD50-NBS1 (MRN) and CtIP in mouse) nicks the Spo11-bound strands using the endonuclease activity of Mre11 and degrades ssDNA towards the DSB using the 3’→5’ exonuclease activity of Mre11. We will refer to this as resection initiation or short-range resection. In the second step, the more processive 5’→3’ exonuclease activity of Exo1 further degrades ssDNA away from the DSB. We will refer to this step as long-range resection, although it should be noted that this is considerably shorter than the long-range resection that can occur in non-meiotic contexts3.
The combined action of these nucleases generates long 3’-terminal ssDNA and releases Spo11 still covalently bound to short oligonucleotides (oligos)2,4-13. The average resection length in wild-type yeast is ~800 nt and this is reduced by more than half (to ~375 nt) in exo1 nuclease-deficient mutants11,12.
It is comparatively elusive how resection is executed in mammalian meiocytes. Similar to yeast, mouse DSB processing generates long 3’-terminal ssDNA (averaging ~1100 nt) and releases covalent SPO11-oligo complexes7,14,15. Unlike yeast, however, net resection length is decreased by only ~10% in mouse Exo1 mutants, suggesting that EXO1 plays only a modest role in resection or is redundant with another nuclease(s)14,15.
The contribution of MRN to resection has been difficult to address, in part because of experimental challenges due to the embryonic lethality of knockout mouse models lacking MRN subunits16-18. Conditional deletion of Nbs1 in germ cells resulted in reduced numbers of foci of RAD51 and other recombination proteins; this was interpreted to reflect a role for MRN in DSB processing, but resection per se was not directly assessed19. More recently, conditional Rad50 deletion was found to cause an increased occurrence of unresected DSBs and to reduce resection tract lengths20. However, how MRN promotes resection remains unclear. Finally, it also remains unclear how resection length ties in with successful execution of meiotic recombination and thus with fertility.
We address these questions here through genome-wide analysis of meiotic DSB resection in mouse spermatocytes conditionally depleted of MRE11 or carrying various MRN mutations, including several that model hypomorphic genetic variants found in human chromosomal instability syndromes21-28. Our findings indicate that MRN is essential for resection initiation in mammals, but also, surprisingly, for most long-range resection. Our findings further reveal how resection defects alter the binding of recombination factors to ssDNA and impinge on faithful execution of meiosis.
RESULTS
MRE11 is required to initiate resection
To circumvent the lethality of an Mre11 null mutation17, we used a conditional deletion strategy combining a floxed Mre11 allele29 with Ngn3-Cre30-32 (Figure 1B). Ngn3-Cre is expressed in spermatogonia during the mitotic divisions prior to meiotic entry, but not in spermatogonial stem cells (SSCs, Figure S1A)31,33. We will refer to Mre11flox/− Ngn3-Cre+ as Mre11-cKO (for conditional knockout), Mre11flox/+ Ngn3-Cre− or Mre11flox/flox Ngn3-Cre− as phenotypic wild type (wt), and Mre11flox/+ Ngn3-Cre+ and Mre11flox/− Ngn3-Cre− as cHet and Het, respectively.
MRE11 protein was reduced by about 20 fold in immunoblots of whole testis extracts from young adult Mre11-cKO males (5 wk old, Figure 1C). We used immunohistochemistry on seminiferous tubule sections to determine which cell types retained MRE11 protein (Figures 1D,E). Virtually all cells were positive for MRE11 in wild type, including the layers of spermatogenic cells and Sertoli cells within the tubules as well as interstitial somatic cells.
In Mre11-cKO mice, most tubules contained a basal layer(s) of spermatogonia and spermatocytes but lacked later-stage germ cells, and 16.1% of tubules lacked spermatocytes entirely (detailed further below) (Figures 1D,E). Spermatogonia and interstitial cells remained uniformly MRE11-positive as expected, but spermatocytes, when present, either lacked detectable MRE11 or were a mix of MRE11-positive and -negative in the same tubule. These findings indicate that the Mre11-flox allele is recombined efficiently but incompletely by Ngn3-Cre expression in spermatogonia, resulting in a substantial population of spermatocytes entering meiosis with no or low MRE11 protein. As described before29, RAD50 and NBS1 levels were also decreased when MRE11 was depleted (Figure 1C).
We visualized resection endpoints genome-wide using S1-sequencing (S1-seq), in which genomic DNA is digested with the ssDNA-specific nuclease S1 and the blunted DNA ends are captured and sequenced (Figure 1A)11,15,34. For some experiments, we used a combination of exonuclease VII and exonuclease T from Escherichia coli instead of nuclease S1 (Exo7/T-seq; based on END-seq14,35,36).
Most DSBs are formed in hotspots whose locations can be identified by sequencing of SPO11 oligos37,38 (Figure 1F, top). S1-seq and Exo7/T-seq yield Spo11-dependent reads distributed ~0.3–2 kb away from hotspot centers with defined polarity: top-strand reads from rightward-moving resection tracts and bottom-strand reads from leftward tracts15 (Figures 1A,F,G and S1B,C). Both methods also generate reads close to hotspot centers (Figures 1F,G and S1B,C). This central signal is thought to arise from recombination intermediates, possibly D loops14,15 (Figure S1D). Importantly, both S1-seq and Exo7/T-seq can also detect DSBs that have not been resected at all (Figure S1E)11,14,15,39,40.
S1-seq and Exo7/T-seq profiles at hotspots were dramatically altered in young adult Mre11-cKO mice (Figures 1F,G and S1B,C). Virtually all signals accumulated at hotspot centers in a pattern strikingly similar to SPO11 oligos in wild type, with a strong central cluster and weaker flanking clusters to either side (Figures 1F,G and S1B,C). These findings are markedly different from those in a recent Rad50 conditional knockout20. We conclude that DSBs remain unresected in the absence of MRE11.
MRE11 controls DSB numbers and restrains double cutting
To summarize global patterns and to facilitate comparisons between genotypes, we plotted genome-wide profiles around DSB hotspots by co-orienting and averaging the sequencing signal from top and bottom strand reads (Figure 2A). Compared to wild type, Mre11-cKO mice had elevated read counts at hotspots, with an increase well beyond the sample-to-sample variation in wild type (Figures 2A,B). In response to DSBs, MRN complexes activate ataxia-telangiectasia mutated (ATM) protein kinase (Tel1 in budding yeast)41-43. During meiosis, ATM/Tel1 regulates the number of DSBs via a negative feedback mechanism (reviewed in44) (Figure 2C). We therefore surmised that the increased sequencing read count in Mre11-cKO mice reflects, at least in part, an increase in the number of DSBs per cell because of a loss of MRN-dependent ATM activation.
Figure 2. MRE11 fosters DSB number control.
(A) Genome-average S1-seq and Exo7/T-seq at hotspots in 5-wk-old Mre11-cKO animals. The bottom-strand reads were flipped and combined with the top-strand reads and averaged (average of two biological replicates). Data are smoothed with a 151-bp Hann window. S1-seq in 14.5-dpp wild type is shown for comparison on the same scale (average of three biological replicates).
(B) Comparison of S1-seq signal strengths for Mre11-cKO (n = 2) versus wild type (n = 10 for juvenile, 1 for adult). Each point is the area under the genome-average curve (as in panel A) from −1500 to +2500 bp relative to the hotspot center for an individual S1-seq replicate. The P value is from a two-tailed Student’s t test.
(C) Role of MRE11 in ATM-mediated control of DSB formation and double cutting. Left, schematic illustrating ATM activation by MRE11 and subsequent negative feedback regulation of SPO11 activity. Right, schematic showing absence of resection and increase in SPO11 double cuts in the absence of MRE11.
(D) Top- and bottom-strand reads at hotspot centers are offset in Mre11-cKO, consistent with double cutting. The plot shows the genome-average strand-specific profile close to hotspot centers, smoothed with a 51-bp Hann window. The average SPO11-oligo profile is shown for comparison.
(E) Increased amounts of SPO11-oligo complexes with altered electrophoretic mobility in Mre11-cKO. A representative autoradiograph is shown of SPO11-oligo complexes immunoprecipitated from testis extracts from 5–6 wk old mice, radiolabeled with terminal transferase and [a-32P]-dCTP, and separated by SDS-PAGE. The signal intensity relative to wild type is indicated below. Asterisk: non-specific labeling artifact.
(F) SPO11 oligo lengths in Mre11-cKO. SPO11 oligos were purified from two mice of the indicated genotypes by immunoprecipitation and protease digestion, then radiolabeled with terminal transferase and [a-32P]-GTP and separated by denaturing urea PAGE. Both samples were run on the same gel, but the intensity of the Mre11-cKO signal was increased approximately threefold to facilitate comparison. Asterisks: non-specific species; nt: marker lengths in nucleotides. Lane traces (background-subtracted and normalized to the total lane signal) are shown to the right.
If so, we anticipated that Mre11-cKO mutants would also display another hallmark of ATM deficiency, namely, a high frequency of double cutting in which multiple SPO11 complexes cut the same DNA molecule in close proximity45-47. In support, the top-strand signal of the central S1-seq peak was shifted to the right of the SPO11-oligo peak, and the bottom-strand signal was shifted to the left (Figure 2D). This pattern is distinct from the central signal derived from recombination intermediates in wild type (Figure S2A), but is as expected if multiple DSBs are formed on the same DNA molecules within hotspots because only the outermost DSB ends will be represented in the final sequencing library (Figures 2C and S1E). Double cutting may also explain why, unlike for SPO11 oligos in wild type, the subsidiary peak to the right of the central peak is stronger on average than the left-side subsidiary peak for top-strand reads, and vice versa for bottom-strand reads (Figures 1G and 2D).
We further tested for double cutting by radiolabeling the 3′ ends of SPO11-oligo complexes immunoprecipitated from whole-testis extracts. This assay does not detect SPO11 if it is still bound to the high molecular weight DNA of unresected DSBs, but double cutting can generate SPO11-oligo complexes in the absence of MRE11 nuclease activity48, as has been shown in budding yeast45,47 (Figure 2C). We indeed observed SPO11-oligo complexes in Mre11-cKO mice, and these were at levels more than ten-fold greater on a per-testis basis than in wild-type or heterozygote controls (Figures 2E and S2B), comparable to Atm−/− mice48.
The major band of SPO11-oligo complexes from Mre11-cKO had slower mobility on SDS-PAGE than controls (Figure 2E). We therefore determined the size distribution of radiolabeled SPO11 oligos on denaturing PAGE (Figure 2F). Similar to SPO11 oligos purified from Atm−/− mice compared side by side, those from Mre11-cKO mice displayed a major species migrating slightly slower than the 34-nt marker plus a ladder of slower migrating bands with ~10-nt periodicity. This periodicity is a diagnostic feature of SPO11 double cuts and may reflect geometric constraints on the orientation of adjacent SPO11 dimers relative to the DNA45,47,49. Interestingly, however, compared with Atm−/−, the Mre11-cKO sample displayed substantially less labeled material migrating faster than the 34-nt marker (Figure 2F), which is the position where SPO11 oligos released by resection are expected to run48. This difference reflects that Atm−/− mice retain the ability to resect meiotic DSBs14,15 but Mre11-cKO mice do not.
We also examined resection patterns in juvenile (14.5 dpp) Mre11-cKO mice. Immunoblotting of whole-testis extracts showed that MRE11 protein was reduced, but to a lesser degree than in adults (~twofold lower than controls; Figure S2C). Similar to adults, these mice showed a greatly elevated central S1-seq signal at hotspots that had the spatial patterns diagnostic of unresected DSBs and double cutting (Figures S2D-F). Unlike adults, however, juveniles also had a substantial fraction of resected DSBs, although with a resection length distribution that was markedly shorter (modal length 897 nt) than in wild type (modal length 1015 nt) (Figure S2F). It is formally possible that spermatocytes in the initial wave of meiosis in juveniles have an additional (MRE11-independent) resection initiation mechanism that is not available in adults. We consider it more likely, however, that the weaker phenotype in juveniles reflects differences in the timing of Cre-mediated excision relative to DSB formation and/or differences in the kinetics of MRE11 protein rundown after excision.
Spermatogenesis failure in Mre11-deficient mice
Adult Mre11-cKO mice had significantly smaller testes than littermate wild-type or heterozygous controls (Figures 3A,B). In seminiferous tubule sections, layers of spermatogonia and spermatocytes were present but postmeiotic cells were largely absent, sections of epididymis appeared empty, and epididymal sperm counts were greatly reduced (Figures 3A,C). All Mre11-cKO tubules contained one or more layers of cells that stained positive for the mouse VASA homolog DDX4 (Figure S3A), which is expressed from spermatogonia to the round spermatid stage50. Tubules with apoptotic cells were also increased in frequency (Figures S3B,C). These Mre11-cKO phenotypes remained consistent to at least 16 wk of age (the oldest tested) (Figures S3D). These results, together with MRE11 staining (Figure 1D), support the interpretation that Mre11-cKO mice stably maintain an MRE11-positive SSC population that can initiate multiple waves of spermatogenesis, but most spermatocytes in each wave are then eliminated by apoptosis. We conclude that MRE11 protein is essential for male fertility in mice, as are NBS1 and RAD5019,20.
Figure 3. Spermatogenesis defects in Mre11-deficient mice.
(A) Bouin’s fixed testis and epididymis sections stained with hematoxylin and eosin (H&E). Sg: spermatogonia, Sc: spermatocyte, rSt: round spermatid, eSt: elongated spermatid.
(B) Ratios of testis weight (mg per pair) to body weight (g). Each point represents one animal; error bars indicate mean ± SD. The results of Student’s t tests are shown; ns, not significant (P > 0.05).
(C) Sperm counts (mean ± SD; P value from Student’s t test).
(D) Representative spreads of wild-type or Mre11-cKO spermatocytes showing normal SYCP3 staining at the indicated stages or abnormal (“Zygonema-like”) cells with thickened and/or tangled axes.
(E) Representative RPA2 staining on spermatocyte chromosome spreads. Examples are shown of Mre11-cKO cells with either low or high RPA2 focus counts.
(F) Spermatocyte stages based on SYCP3 staining (L: leptonema, Z: zygonema, Z-like: zygonema-like, P: pachynema, D: diplonema). Error bars indicate mean ± SD from three mice of each genotype.
(G) RPA2 focus numbers. Error bars represent mean ± SD for the indicated number of cells counted from 2 mice of each genotype. The stage of each cell was determined from the SYCP3 signal. The results of two-tailed Mann-Whitney U tests are shown.
To monitor chromosome dynamics and progression through prophase I, we immunostained spermatocyte chromosome spreads for the axis protein SYCP3, with or without costaining for the RPA2 subunit of the ssDNA-binding protein RPA. In wild type, SYCP3 forms short lines in leptonema as axes begin to form; the axes elongate and synapse with homologous partners to form stretches of synaptonemal complex (SC) in zygonema; SC spans the length of each pair of autosomes in pachynema; and then SC disassembles in diplonema (Figures 3D and S3E). RPA2 forms large numbers of foci on resected DSBs in leptonema and zygonema, then these foci decrease in number over the course of pachynema as DSB repair is completed (Figure 3E)51.
Spermatocytes in Mre11-cKO mice deviated substantially from the normal patterns. We observed cells with short SYCP3 axes and no SC, similar to normal leptonema but representing a higher fraction of total spermatocytes than in wild type (Figures 3D,F and S3E). Most cells had very few RPA2 foci (Figures 3E,G). We also observed cells with axes of various lengths along with SC, but most of these differed from normal zygotene cells in having only short stretches of staining for SC central region component SYCP1 and/or tangles of axes (Figure S3E). These “zygotene-like” cells were the most abundant class in Mre11-cKO (Figure 3F) and most were again highly depleted for RPA2 foci compared to wild type (Figures 3E,G). The deficit of RPA2 foci corroborates the resection defect seen in S1-seq. These results also show, not surprisingly, that unresected DSBs cannot support homologous pairing and synapsis.
In addition to these aberrant cells, there were also morphologically normal pachytene and diplotene cells, but much fewer than in wild type (Figures 3D,F and S3E). RPA2 focus numbers in the pachytene cells were indistinguishable from wild type (Figure 3G). We also observed a small fraction of leptotene and zygotene cells that had RPA2 focus numbers in the normal range for these stages (Figures 3E,G). We infer that these more normal-looking cells correspond to the MRE11-positive subpopulation of spermatocytes (Figure 1D), perhaps cells that escaped Cre-mediated excision. If so, these escapers appear to be too small a fraction of the total leptotene and zygotene cells (Figure 3F) to contribute an appreciable resection signal in population-average sequencing maps (Figures 1F,G and 2A).
Nuclease-dead MRE11 reveals an unexpected role in long-range resection
To test whether MRE11 nuclease activity is required for meiotic resection, we examined mice with the invariant active-site residue His-129 substituted to asparagine29. Human MRN complexes with this mutation lack nuclease activity in vitro52,53. Mre11-H129N is cell-lethal in chicken and human cells54 and is embryonically lethal in mice29, but the phenotype in mammalian meiosis has not been addressed. To circumvent the lethality, we generated Mre11H129N/flox mice carrying Ngn3-Cre (hereafter Mre11-cHN) (Figure 4A). Total MRE11 protein levels were normal in testis extracts from Mre11-cHN mice (Figure 4B), consistent with previous demonstration that the mutation does not destabilize the protein29.
Figure 4. Defects in long-range resection and spermatogenesis in mice expressing nuclease-dead MRE11.
(A) Conditional Mre11-H129N strategy combining Ngn3-Cre with compound heterozygous floxed and missense Mre11 exon 5 alleles.
(B) Total MRE11 protein levels are normal in Mre11-cHN mice. Immunoblots are shown of whole testis lysates from 5–7 wk old mice. In this experiment, the phenotypically wild-type control (wt) was Mre11wt/flox Ngn3-Cre− and the heterozygous HN strain (HN-Het) was Mre11H129N/flox Ngn3-Cre−.
(C) Exo7/T-seq at the same hotspot shown in Figure 1F.
(D) Genome-average Exo7/T-seq patterns at hotspots in 5-wk-old animals. The sequencing signal from top and bottom strand reads was co-oriented, averaged, and smoothed with a 151-bp Hann window, then internally normalized by setting the height of the resection peak to a value of 1. The normalization facilitates comparison of the shapes of the resection profiles separate from sample-to-sample variation in the signal:noise ratio of the sequencing read frequencies34. Non-normalized plots are shown in Figure S4C. Two biological replicates each for wild type and Mre11-cHN were averaged and plotted. The profile for HN-Het was from a single library.
(E) MRE11 accumulation at hotspots in Mre11-cHN. MRE11 ChIP-seq signal was averaged around hotspots for Mre11-cHN (n=3 biological replicates), wild type (n=2), and Atm−/− (n=1).
(F,G) Reduced amount of SPO11-oligo complexes in Mre11-cHN. An autoradiograph of radiolabeled complexes immunoprecipitated from testis extracts (F) and lane profiles (G) are shown.
(H) Reduced testis sizes. Error bars indicate mean ± SD. Results of Studen’s t tests are shown.
(I) Bouin’s fixed and H&E-stained seminiferous tubule and epididymis sections (7 wk old; cell type labels as in Figure 3A).
(J,K) Increased spermatocyte apoptosis in Mre11-cHN mice (7 wk). Representative TUNEL staining of seminiferous tubule sections (J) and quantification (K) are shown. Error bars in K indicate mean ± range. Wild type is reproduced from Figure S3C.
(L) Sperm counts (mean ± SD; P value from a Student’s t test). The control is a combination of wild type, HN-het, and cHet animals.
(M) Representative spreads of wild-type or Mre11-cHN spermatocytes showing either normally synapsed SYCP3 staining of autosomes and normal appearing γH2AX-positive sex body (left) or pachytene-like cells with unpaired or tangled autosomes (middle and right micrographs), which were associated with the γH2AX -positive domain (arrowheads). At lower left, the number of pachytene or pachytene-like cells observed and total number of SYCP3-positive cells counted.
(N) Spermatocyte stages based on SYCP3 and γH2AX staining. Error bars indicate mean ± SD.
Because the equivalent budding and fission yeast mutants form meiotic DSBs but cannot resect them6,55, we expected that Mre11-cHN mice would accumulate only unresected DSBs if the wild-type MRE11 protein expressed from the floxed allele were sufficiently depleted (Figure S4A, left). On the other hand, if some wild-type MRE11 protein persisted long enough to support resection initiation at a subset of DSBs in each cell, or at all DSBs in a subset of escapee cells, we instead expected to observe that some or all of the DSBs would be fully resected (Figure S4A, right). The latter prediction was premised on the idea that the long-range nuclease(s) should be able to complete resection normally as long as MRE11 endonuclease had incised the DNA.
Surprisingly, however, Exo7/T-seq patterns in young adult mice matched neither of these predictions when considering either individual hotspots (Figures 4C and S4B) or genome-average profiles (Figures 4D and S4C). We note two main findings. First, there was a modest increase in the relative amount of the sequencing signal at hotspot centers (Figure 4D), suggesting that some unresected DSBs are present. Consistent with this interpretation, we also observed a slight increase in the sequencing signal at hotspot centers on the nonhomologous parts of the X and Y chromosomes (Figure S4D). Central S1-seq or Exo7/T-seq signal from recombination intermediates does not appear at X and Y hotspots in wild type, but it can be detected when there are unresected DSBs, such as in Atm−/− mutants14,15 or Mre11-cKO (Figure S4E).
To further test for unresected DSBs, we performed chromatin immunoprecipitation (ChIP-seq) for MRE11, which was previously demonstrated to accumulate at hotspots when unresected DSBs are present in Atm−/− mutants, but not in wild type where all DSBs are resected14. Indeed, we observed a clear MRE11 ChIP-seq signal at hotspots in Mre11-cHN mice, albeit substantially less than in Atm−/− (Figures 4E and S4F,G). Taken together, these results suggest that a small fraction of DSBs remains unresected in Mre11-cHN mice. If so, however, this resection initiation defect is very weak compared to Atm−/− and especially Mre11-cKO, perhaps reflecting insufficient elimination of wild-type MRE11 protein in this conditional model (see Discussion).
The second and more surprising finding was that resection tracts were substantially shorter than normal in Mre11-cHN, averaging only 672 nt (Figure 4D). This defect was reproducible and stable, remaining the same with increasing age up to 11 wk (the oldest age tested; Figure S4H). We also observed a slight resection defect in mice heterozygous for Mre11-H129N (Mre11H129N/wt with Ngn3-Cre). Resection tracts in these mice averaged 1040 nt (94% of wild type) (Figure 4D), suggesting a weak dominant-negative effect of MRE11-H129N protein. Observing shorter average resection lengths reveals that MRE11 nuclease activity contributes to long-range resection, not just to resection initiation.
Unlike in Atm−/− and Mre11-cKO, the amount of SPO11-oligo complexes was not increased in Mre11-cHN (Figures 4F and S4I), consistent with MRE11-HN protein being competent to activate ATM/Tel1 in both yeast and mouse29,53,56,57. Instead, the yield on a per-testis basis was reduced by about half compared to wild type. The total Exo7/T-seq signal recovered at hotspots was also diminished in Mre11-cHN (Figure S4J). These decreases may reflect reduced DSB frequency and/or loss of signal because of spermatocyte apoptosis (described below). We also observed a shift in the migration of SPO11-oligo complexes on SDS-PAGE gels, with faster migrating species more depleted than slower migrating ones (Figure 4G). We posit that the presence of catalytically inactive MRE11 protein sometimes occludes potential nicking sites closer to SPO11, biasing the population of SPO11 oligos generated by the residual wild-type MRE11 protein to larger sizes than normal.
We were unable to assess a role for CtIP—a cofactor needed for MRN nuclease activity3 because conditional deletion of Ctip eliminated spermatogonia prior to meiotic entry (Figures S4K-N). We also tested Rad50S/S mice but found normal resection (Figure S4O), likely because this Rad50-K22M mutation was based on a relatively weak yeast allele because mimics of stronger alleles are cell-lethal23. Thus, available mouse mutants are unable to definitively test the requirement for MRN nuclease in resection initiation.
Spermatogenesis defects in mice with nuclease-dead MRE11
Mre11-cHN mice had pronounced defects in meiotic progression. Their testes were considerably smaller than those of control littermates (Figure 4H); seminiferous tubules contained spermatogonia and early stage spermatocytes but were depleted of spermatids and had an increased frequency of spermatocyte apoptosis (Figures 4I-K); and epididymal sperm counts were greatly diminished (Figures 4I,L). The rare residual sperm detected may have arisen from escapers in which Cre-mediated excision failed and/or from a subset of cells in which wild-type MRE11 protein persisted long enough after excision to support successful meiosis.
When prophase I stages were evaluated by staining spreads for SYCP3 and phosphorylated H2AX (γH2AX), we observed a subset of pachytene-like cells with unpaired or tangled chromosomes (Figures 4M,N), indicative of sporadic synaptic failure. We also observed an increase in the proportions of leptotene and zygotene cells relative to later stages (pachytene, pachytene-like, and diplotene) (Figure 4N). This increase may reflect a delay in completing synapsis, preferential apoptotic elimination of cells in later prophase I, or both.
Reduced resection lengths in MRN hypomorphic mutants
We further tested for MRN roles in meiotic resection using mice homozygous for hypomorphic mutations modeled on human disease alleles58 (Figure 5A). Mre11-ATLD1 is a nonsense mutation near the 3′ end of the coding sequence, recapitulating a mutation found in ataxia telangiectasia-like disorder21. Nbs1ΔB, which models Nijmegen breakage syndrome, produces an NBS1 protein lacking the N-terminal FHA and BRCT domains26. Mre11-ATLD1 and Nbs1ΔB cause intra-S and G2/M checkpoint defects, DNA damage sensitivity, chromosomal instability, and reduced ATM activity in cultured cells and they cause subfertility in mice, particularly in females21,26,59. The mutations also reduce protein stability and MRN nuclease activity in vitro60, but they have not previously been linked to overt resection defects.
Figure 5. Reduced resection length in MRN hypomorphs.
(A) Schematic of MRN hypomorphs. MRE11 R632* (R632 → stop) mimics human R633* identified in ATLD patients21. Nbs1ΔB produces an 80 kDa protein (red box), mimicking the 657del5 allele found in 95% of NBS patients26.
(B) S1-seq at the hotspot from Figure 1F, from 14.5-dpp mice. Wild type is reproduced from Figure 1F. Two biological replicates were averaged for each of the other genotypes.
(C) Heatmaps (data in 40-bp bins) of S1-seq reads around DSB hotspots.
(D) Genome-average S1-seq at hotspots from 14.5-dpp mice. Wild type is reproduced from Figure 2A. Data are smoothed with a 151-bp Hann window and normalized as described in Figure 4D.
(E–H) Minimal if any changes in RAD51 and DMC1 focus numbers during meiosis in Rad50-L1237F or Rad50-D69Y mice. Representative spreads stained for SYCP3 and DMC1 (E) and RAD51 (G) along with quantification (F, H) are shown. Each dot in the graphs is the focus count from a single nucleus; error bars indicate means ± SD. Results of unpaired t tests are shown for zygonema; differences at the other stages were not statistically significant (P > 0.05).
We also examined homozygous Rad50-D69Y and Rad50-L1237F mutants, which model recurrent mutations in human cancer affecting residues in the Walker A and B ATPase motifs, respectively61,62 (Figure 5A). Studies in S. cerevisiae and cultured mouse cells indicated that the human RAD50-L1240F mutant (L1237F in mouse) is a separation-of-function allele that is largely proficient in DNA repair, but defective in the activation of Tel1/ATM61. Similar outcomes were observed for modeling of human RAD50-D69Y in S. cerevisiae62.
Rad50-L1237F and Rad50-D69Y mice were derived by CRISPRmediated mutagenesis. MEFs from Rad50-D69Y mice displayed reduced ionizing radiation (IR)-provoked phosphorylation of KAP1 (an ATM target63) (Figure S5A); elevated mitotic indices after IR, consistent with defects in the ATM-dependent G2/M checkpoint (Figure S5B); and hypersensitivity to camptothecin when ATR was inhibited, as expected if compromised ATM activation cannot compensate for loss of ATR activity (Figure S5C). These findings confirm the separation of function predicted from prior studies in S. cerevisiae62, and verify that Rad50-D69Y, like Rad50-1237F61 is defective for ATM activation.
S1-seq maps from juvenile males demonstrated resection defects in all of these MRN mutants, demonstrating a striking allelic series(Figures 5B-D). These findings reinforce the conclusion that the MRN complex must be fully functional to support normal long-range resection, i.e., that MRN roles in DSB processing are not limited just to resection initiation. There was only a slight increase in central S1-seq signal at hotspot centers on the sex chromosomes in Nbs1ΔB, suggestive of a small number of unresected DSBs, but little if any evidence for this in the other mutants (Figure S5D).
Spermatogenesis in Nbs1ΔB and Mre11-ATLD1 mutant males was previously shown to be nearly normal, with only mildly delayed temporal progression and persistent RAD51 localization that was attributed, at least in part, to reduced functionality of these MRN hypomorphs in meiotic DSB repair21,59. The substantially shorter resection lengths we now observe in these mutants may contribute to this apparent DSB repair delay. Spermatogenesis also appeared to be largely normal in Rad50-L1237F and −D69Y mutants. Testis sizes and sperm counts were indistinguishable from littermate controls (Figure S5E-G); the frequency of spermatocyte apoptosis was not significantly increased (Figure S5H); the distribution of prophase I stages was not substantially altered (Figure S5I); and the appearance and disappearance of foci of strand exchange proteins occurred normally, with the exception that both mutants showed a statistically significant but quantitatively small reduction in the number of DMC1 foci at zygonema (Figures 5E-H). These findings show that meiotic recombination is robust in the face of even substantial deviations from normal resection lengths (e.g., up to the ~47% reduction in Mre11-ATLD1).
Side-by-side spatial organization of RAD51 and DMC1 is maintained in Nbs1ΔB mice
We tested whether the shortened resection lengths in Nbs1ΔB mice altered the occupancy and spatial disposition of ssDNA binding proteins RPA, DMC1, and RAD51 by performing ChIP followed by ssDNA sequencing (SSDS)64,65. In wild type, DMC1 tends to bind closer to the broken end, RAD51 binds nearer to the ssDNA/dsDNA boundary, and RPA can be found essentially anywhere along the ssDNA64 (Figure 6A). The SSDS signal for all three proteins was more compressed toward hotspot centers in the Nbs1ΔB mutant, as expected from the decreased length of ssDNA available, but the spatial pattern of DSB-proximal DMC1 and DSB-distal RAD51 was maintained (Figure 6B).
Figure 6. Side-by-side spatial organization of RAD51 and DMC1 is maintained despite shortened resection in Nbs1ΔB mice.
(A, B) Genome-wide profiles of DMC1, RAD51, and RPA2 ChIP followed by SSDS in wild type (A) or Nbs1ΔB (B). In the graphs above, top- and bottom-strand reads around hotspots were co-oriented, combined and averaged. These maps represent sequencing coverage rather than endpoint counts reported for S1- or Exo7/T-seq. Data are smoothed with a 151-bp Hann window. An estimated background was removed by subtracting the value of signal 2.5 kb away from the hotspot center, then profiles were normalized by setting the area under each curve (from −1.0 to 2.5 kb) to 1. Heatmaps below show SSDS signals in 40-bp bins, locally normalized by dividing each signal by the total signal in a 4001-bp window around each hotspot’s center. Each hotspot thus has a total value of 1, so that spatial patterns can be compared between hotspots of different strengths.
(C) Representative SIM images of spread spermatocytes from 14.5-dpp mice. Insets show zoomed views of the regions indicated by dashed boxes.
(D) DMC1 to RAD51 distances for axis-associated co-foci. Tukey box plots are shown for DMC1 foci that were £450 nm from an axis and £320 nm from the nearest RAD51 focus. The plus signs indicate means; horizontal lines are medians; box edges are interquartile range; whiskers indicate the most extreme data points which are ≤1.5 times the interquartile range from the box; individual points are outliers.
Previous studies in yeast and mice showed that foci of RAD51 and DMC1 often appear side by side64,66,67. We tested using structured illumination microscopy (SIM) whether this organization at individual DSB sites was retained in Nbs1ΔB mice, comparing homozygous mutants with heterozygous littermate controls (which have nearly normal resection lengths (Figure S6A)). Similar to previous results64, a majority of the foci of both DMC1 (93.8%) and RAD51 (79.4%) were located close to chromosome axes in Nbs1ΔB/+ heterozygotes (Figures 6C and S6B and Table S1). Moreover, the foci often occurred together in pairs: 80.7% of axis-associated DMC1 foci and 77.4% of of axis-associated RAD51 foci were within 320 nm of the other protein (Figures 6C and S6C and Table S1). The high degrees of both axis association and colocalization were retained in Nbs1ΔB/ΔB homozygotes (94.7% axis association for DMC1 and 86.3% for RAD51; 84.8% colocalization for DMC1 foci and 82.3% for RAD51) (Figures 6C and S6B,C and Table S1).
DMC1 and RAD51 foci were nearly always offset from one another, with a distance in the controls of 103.3 ± 67.5 nm (mean ± SD) between DMC1 foci and their RAD51 mates (Figure 6D and Table S1). This distance was decreased significantly in Nbs1ΔB mutants (P = 2.2 ′ 10−16, t test), but with a small effect size (96.8 nm ± 66.5).
Two conclusions emerge. First, the decreased distance between DMC1 and RAD51 foci when resection lengths are reduced supports the interpretation that paired foci represent binding of both proteins to the same ssDNA tail and not to separate DSB ends, similar to the situation inferred from yeast and mouse studies66,67. Second, the effect of the Nbs1ΔB mutation on interfocus distances (less than 7% decrease on average) was much smaller than the effect on both resection length (43% decrease, Figure 5D) and ChIP profiles (Figure 6B). This difference between cytology and population-average molecular assays shows that ssDNA length is not the most important determinant of the threedimensional spatial organization of DSB repair foci. This conclusion in turn fits with the idea that individual DMC1 and RAD51 filaments are fairly short, estimated at ~100 nt in yeast and ~400 nt in mouse66,67. Because filaments are only long enough to occupy a small fraction of the available ssDNA, even a substantial reduction in resection lengths should not cause a proportionate decrease in the size of cytologically observable foci.
Additive contributions of EXO1 and the NBS1 N terminus to long-range resection
In vitro, yeast MRX promotes Exo1 activity by providing nicks for Exo1 to act on and by fostering Exo1 binding to DNA ends68-71. To query the interplay between MRN and EXO1 in meiotic resection, we generated double mutant mice homozygous for both Nbs1ΔB and Exo1 mutations. An Exo1 null is synthetically lethal with Nbs1ΔB72, so we used the nuclease-deficient Exo1-D173A (DA) allele73, reasoning that the milder somatic phenotype of Exo1DA74 might allow us to obtain double mutants. Indeed, Nbs1ΔB Exo1DA doubly homozygous mice were viable, albeit recovered at a sub-mendelian frequency (Figure S7A) and exhibiting lower body weights (Figure S7B).
Nbs1ΔB Exo1DA double mutants showed a 111-nt decrease in the average resection length compared to Nbs1ΔB alone(Figures 7A,B and S7C). Since this is similar to the 126-nt average decrease in Exo1DA compared to wild type, EXO1 appears to work additively with MRN. We also conclude that the Nbs1ΔB resection defect is not attributable to a defect in EXO1 activity.
Figure 7. Nbs1ΔB and Exo1DA mutations affect resection additively.
(A) S1-seq at the hotspot from Figure 1F. Because of the smaller size of Exo1DA Nbs1ΔB pups, we collected samples at 16.5 dpp for this experiment. Wild type (14.5 dpp) is reproduced from Figure 1F; two biological replicates were averaged for each of the other genotypes.
(B) Genome-average S1-seq patterns at hotspots, smoothed with a 151-bp Hann window. Wild type and Nbs1ΔB (14.5dpp) are reproduced from Figure 5D. Mean resection lengths are indicated.
(C) Reduced central S1-seq signal (presumed recombination intermediates) in Nbs1ΔB mutants. The plot shows the relative amount of central signal calculated as the area under the curve (AUC) for the central peak (−300 to +100 bp relative to hotspot centers) divided by the AUC for the resection peak (+100 to +2500 bp). For wild type, in each of three independent time courses, samples were collected from a single litter daily at approximately the same time each day.
(D) Homolog pairing and/or synapsis defects. Representative spreads of Nbs1ΔB Exo1DA spermatocytes are shown with either normal-appearing SYCP3 and γH2AX staining or with unsynapsed or tangled chromosomes within the γH2AX-stained domain (yellow arrowheads). Blue arrowheads point to examples of γH2AX flares on synapsed autosomes.
(E) Spermatocyte stages based on SYCP3 and γH2AX staining. Error bars indicate mean ± SD of the indicated number of animals.
(F) Percentage of pachytene or pachytene-like cells with no, 1–3, or >3 γH2AX flares. Error bars indicate mean ± SD of the indicated number of animals.
(G) Frequencies of tubules with the indicated number of TUNEL-positive cells. Error bars indicate mean ± range for the indicated number of animals.
(H) Reduced testis size in Nbs1ΔB Exo1DA double mutants (5–11 wk old). Error bars indicate mean ± SD. P values are from Student’s t tests.
(I) Sperm counts (mean ± SD). Results of Student’s t tests are shown.
Relative to the S1-seq signal from resection endpoints, the central signal from recombination intermediates appeared lower in Nbs1ΔB Exo1DA than in wild type or the single mutants (Figure 7B). To evaluate this, we measured total read count (area under the curve) separately for the central peak and for the resection endpoint peak and calculated the ratio between these values (Figure 7C). In wild type, the central peak was reproducibly ~40% of the level of the resected DSBs from 13.5 to 16.5 dpp. This ratio was lower at 12.5 dpp (~33%) in two of three experiments (Figure 7C). Since this earlier age is likely to be more enriched for earlier prophase I spermatocytes, the lower relative amount of central signal may reflect timing differences between DSB formation (appearance of the resected DSB signal) and formation of the recombination intermediates detected by S1-seq.
In Nbs1ΔB mice, the relative amount of central signal was substantially lower at 14.5 dpp (27.5% of the DSB signal; mean of n = 2) and it increased but remained lower than wild type at 16.5 dpp (34.7%; Figure 7C). The central signal at 16.5 dpp was even lower in Nbs1ΔB Exo1DA (28.8%) but the Exo1DA mutation by itself had no effect. These decreased yields of the central signal in Nbs1ΔB single or double mutants may indicate that fewer of the relevant recombination intermediates are formed, that they turn over more rapidly, and/or that they exhibit structural features that make them more difficult to detect by S1-seq. Regardless of the source of the changes, however, it appears that Nbs1ΔB mutants experience alterations in interhomolog recombination that are exacerbated when combined with Exo1DA.
Reinforcing this view, Nbs1ΔB Exo1DA double mutants displayed an elevated frequency of pachytene-like cells with synaptic failures (tangled and/or unpaired chromosomes) and a higher proportion of zygotene cells than normal, possibly indicative of delays in completing synapsis (Figures 7D,E). Pachytene and pachytene-like cells from the double mutant also showed an increased frequency of γH2AX flares on synapsed chromosomes, indicative of delays in DSB repair (Figures 7D,F). Seminiferous tubule sections showed few if any elongating spermatids in Nbs1ΔB Exo1DA double mutants (Figure S7D) and an elevated frequency of apoptotic spermatocytes (Figures 7G and S7E). As a consequence of this germ cell loss, the mice had substantially reduced testis sizes (Figure 7H) and were essentially devoid of epididymal sperm (Figures 7I and S7D). Single Nbs1ΔB or Exo1DA mutants showed few of these defects, consistent with previous studies59,74, although apoptotic tubules were observed at modestly elevated frequencies and testis sizes were reduced (Figures 7G,H).
DISCUSSION
Unlike the paradigmatic budding yeast pathway for meiotic DSB resection11,12, mice rely less on EXO114,15 and we now show that multiple MRN mutations confer drastic resection defects (see also20). Thus, while the canonical two-step handoff from MRN(X) to EXO1 occurs in both yeast and mammals, we find that mouse MRN is essential not just for resection initiation, but also unexpectedly for most long-range resection as well. Since Exo1DA combined additively with MRN deficiency, EXO1 appears to “polish” resection tracts that are generated primarily through MRN.
ATM deficiency decreases resection lengths for a subset of DSBs14,15. Thus, because the MRN hypomorphs compromise ATM activation21,26,75, their resection defects could be an indirect consequence of diminished ATM signaling. However, Mre11-cHN mice appear to have normal ATM activity29,53,56,57 (Figure 4F), suggesting that their defective resection is tied more directly to attenuated MRN activity per se. By extension, the resection defects in MRN hypomorphic adults and Mre11-cKO juveniles may be explained in a similar manner. We cannot exclude that reduced ATM activity also contributes, but Mre11-ATLD1 and Nbs1ΔB mutants only modestly attenuate meiotic ATM activation as judged by their retention of most ATM-dependent DSB control76, so this explanation would require that resection is particularly sensitive to small decreases in ATM activation. Moreover, the resection-promoting function of ATM itself remains unknown, so the opposite causality is also plausible: i.e., resection defects in Atm mutants might hypothetically be an indirect consequence of losing ATM-dependent regulation of MRN.
We consider two plausible ways in which MRN might promote long-range resection aside from ATM activation. First, it might directly resect DNA via iterative nicking that spreads progressively from the DSB end. Although yeast MRX and human MRN preferentially cleave the 5’-terminal DNA strand ~15–45 nt away from a blocked DNA end in vitro77-80, MRN can both oligomerize and diffuse on DNA81,82. Moreover, MRX can resect unrepairable DSBs in vivo more than one kilobase in yeast lacking long-range resection enzymes83, and iterative nicking was proposed to explain both short-range resection in vegetative yeast cells84 and the ~300 nt average resection in meiotic cells lacking Exo1 activity11.
A second, non-exclusive possibility is that MRN recruits or activates another nuclease(s). MRN enhances DNA2 nuclease in vitro68,85-88, although it remains unclear if the same is true in vivo. CtIP also promotes DNA2 activity both in vitro and in vivo89,90. Budding yeast Dna2 is unlikely to be important for meiotic resection because its partner helicase Sgs1 is dispensable10-12. However, the possibly mammal-specific interaction between CtIP and DNA289,90 may make DNA2 a candidate for long-range resection in mouse meiosis15.
In contrast to its unanticipated requirement in long-range resection, MRN involvement in resection initiation was expected. However, the specific genetic dependencies were intriguing. For example, we were surprised to observe only minor defects in resection initiation in Mre11-cHN mice. It is formally possible that MRE11 nuclease is dispensable for clipping SPO11 from DSBs even though MRE11 protein itself is required. However, we consider it more likely that this result instead reflects continuing presence of sufficient wild-type MRE11. For example, dimerization with continuously expressed MRE11-H129N might slow the degradation of wild-type protein compared to what happens in Mre11-cKO. In this scenario, mutant/wild-type heterodimers are still active for strand cleavage since they retain a functional nuclease active site. Alternatively, MRE11-H129N might amplify activity of a small amount of residual wild-type protein in a structural role via heterodimer formation or oligomerization on DNA. It is also possible that binding of inactive MRN complexes to DSBs allows another nuclease(s) to be recruited to initiate resection in an unconventional manner.
The Mre11-cKO mutant is the first example in mammals of a yeast rad50S-like meiotic phenotype, namely, accumulation of completely unresected DSBs6,91-93. This finding definitively establishes that MRN is essential for resection initiation. The elevated DSB sequencing signal and double cutting in this mutant also confirms and extends the previous conclusion, based on modest elevation of SPO11-oligo complexes in Mre11-ATLD1 and Nbs1ΔB, that MRN promotes ATM activation in the context of controlling SPO11 activity76. While this manuscript was in preparation, conditional deletion of Rad50 (with Stra8-Cre) was also found to cause an increased frequency of unresected DSBs, but to a much more modest extent than we observed with Mre11-cKO and without an apparent increase in DSB levels or evidence of double-cutting20. Because resection was not directly assessed for Nbs1 conditional deletion (with Vasa-Cre), it is unclear whether NBS1 is required for resection initiation19. The differences between the conditional mutants may reflect differences in timing and efficiency of gene deletion and protein disappearance rather than different requirements for the individual MRN subunits.
Our findings may also indicate that mouse MRE11 is dispensable for DSB formation, unlike in S. cerevisiae and Caenorhabditis elegans, but similar to Schizosaccharomyces pombe, Drosophila melanogaster, and Arabidopsis thaliana [reviewed in94]. However, this interpretation should be viewed cautiously because Mre11-cKO spermatocytes may retain trace amounts of MRE11 protein that are sufficient for DSB formation but not resection. In this vein, we note that Mre11 and Ctip null mutations are both cell-lethal, whereas conditional deletion of Mre11 but not of Ctip was tolerated by spermatogonia and premeiotic spermatocytes. This is consistent with both proteins being essential for mitotic germ cell divisions, but MRE11 protein persisting longer than CtIP after Ngn3-Cre-mediated excision.
In addition to defining the genetic control of DSB resection, our findings offer insight into the minimal resection length needed for successful downstream steps. The extensive pairing and synapsis defects in Mre11-cKO mice met the expectation that recombination cannot proceed without ssDNA generated by resection. However, our findings showed that meiosis is remarkably robust as long as some resection occurs. Recombination, chromosome pairing, synapsis, and chromosome segregation all occurred efficiently with slightly more than half the normal average resection length (Mre11-ATLD1). Severe problems were seen only in the Nbs1ΔB Exo1DA double mutant, with its only modest further decrement in resection length. But even in this mutant, although most cells failed to complete meiosis, most chromosomes paired and synapsed well, indicating that most DSBs were successfully engaging in recombination.
It is unclear why recombination begins to fail in the Nbs1ΔB Exo1DA double mutant but not appreciably in the Nbs1ΔB or Mre11-ATLD1 single mutants. The minimal length of ssDNA needed to support homology searching and strand exchange in mammalian meiosis is unknown, but vegetative yeast cells can execute recombination with less than 40 bp of homology, and normal recombination efficiency appears to need only ~100–250 bp95-99. Moreover, RAD51 and DMC1 filaments are thought to occupy only a few hundred nucleotides66,67. Although the distribution of resection tract lengths is highly stereotyped across all mouse hotspots, there is substantial variation from DSB to DSB, and perhaps between the two sides of a single DSB. It has been suggested for yeast that normal resection lengths ensure that all DSBs are resected enough to support efficient, accurate recombination12. It is therefore possible that Nbs1ΔB Exo1DA double mutants become vulnerable to recombination defects because a large enough subset of their DSBs fall below the minimum length needed for strand exchange per se or for proper choice of homologous recombination partner100.
MATERIALS AND METHODS
Mice
Experiments conformed to the US Office of Laboratory Animal Welfare regulatory standards and were approved by the Memorial Sloan Kettering Cancer Center Institutional Animal Care and Use Committee. Mice were maintained on regular rodent chow with continuous access to food and water until euthanasia by CO2 asphyxiation prior to tissue harvest. Previously described Mre11-flox and Mre11-H129N29 and Ctip-flox101,102 alleles were crossed with Ngn3-Cre30 to create male germline-specific mutations.
To generate Mre11-cKO experimental mice, Mre11flox/flox (or Mre11wt/flox based on availability) mice were crossed with Mre11+/− Ngn3-Cre+ mice. The Mre11-deletion allele (Mre11−) was derived by Cre-mediated recombination of the Mre11-flox allele. To generate Mre11-cHN experimental mice, Mre11H129N/+ Ngn3-Cre+ mice were crossed with Mre11flox/flox mice. To generate Ctip-cKO mice, Ctipflox/flox mice were crossed with Ctip+/− Ngn3-Cre+ mice. The Ctip deletion allele (Ctip−) was derived by Cre-mediated recombination of Ctip-flox.
Although Ngn3-Cre is also expressed in embryonic pancreas and endocrine cells30, we did not observe any gross defects of mutant animals created with Ngn3-Cre up through the ages of testis sample collection. Previously described Atm (Barlow et al. 1996), Spo11103, Rad50S23, Mre11-ATLD121, Nbs1ΔB26, and Exo1DA (Zhao et al. 2018) mutations were maintained on a congenic C57BL/6J strain background. Nbs1ΔB Exo1DA animals were generated by crossing Nbs1ΔB mice with Exo1DA mice. To enhance the survival rate of double homozygous male mice for experimental purposes, female pups were euthanized by hypothermia between 4–6-dpp in accordance with the approved animal protocol.
To generate targeted Rad50 mutations, a guide RNA cassette with sequence (5’-TACATTTGTTCATGATCCCA(AGG)) and single-stranded donor DNA (5’- TTATAAAAATTTAATTCTTAAAATACAAAACTTTACAGACACCATTACCTTGGGATaATGAACAAATGTATTTCCTTTGGTTCCAGGAGGGAAATCTCCAGTACAA) harboring desired missense mutations for D69>Y, or a guide RNA cassette with sequence (5’-TCTCGGTCGAGATTTGTTGT(CGG)) and single-stranded donor DNA (5’- GGAAACCTTCTGTCTGAACTGTGGCATCCTTGCCTTGGATGAGCCGACAACAAATtttGACCGAGAAAACATTGAGTCTCTTGCACATGCTTTGGTTGAGTAAGTA) harboring desired missense mutations for L1237>F were microinjected into pronuclei of zygotes. Founder mice were crossed to C57BL/6J mice purchased from Jackson Laboratories to obtain germline transmission, then heterozygous animals were backcrossed to C57BL/6J for at least three generations.
S1-seq and Exo7/T-seq
Choice of sequencing method and animal ages:
S1-seq was originally applied to juvenile testis samples because these samples lack post-meiotic cells and are thus enriched for spermatocytes that contain DSBs and recombination intermediates15. We continued to use juvenile samples for most experiments here, but found that Cre-mediated excision of the Mre11-flox construct was more penetrant in adult samples. Initial attempts to perform S1-seq on adult samples gave poor signal strength from SPO11-generated DSBs at hotspots because of the presence of S1-sensitive triplex secondary structures in the DNA104. We therefore initially used Exo7/T-seq for analysis of resection in Mre11-cKO and Mre11-cHN mice, because exonuclease VII and exonuclease T require DNA ends and thus do not give sequencing reads from many of the non-DSB structures that S1-seq can pick up. We later found that increasing the pH during the nuclease S1 digestion step greatly improved the specificity for SPO11-initiated events by avoiding the formation of triplex structures34, so we repeated the analysis of Mre11-cKO using S1-seq on adult samples. For the wild-type time courses (Figure 7C), each time point was processed up to the agarose plug step, then S1-seq maps were prepared in a single batch after collecting the last time point.
Library preparation and sequencing:
Libraries were prepared as described elsewhere34, based on S1-seq15 and END-seq14,105. Briefly, testes from mice at the indicated ages were decapsulated and digested with collagenase type IV (Worthington) and Dispase II (Sigma). The resulting seminiferous tubule preparations were then further treated with TrypLE Express enzyme (GIBCO) and DNase I (Sigma) for 15 min at 35 °C. TrypLE enzyme was inactivated with 5% FBS and tubules were further dissociated by repeated pipetting. Cells were then passed through a 70-μm cell strainer (BD Falcon).
One to two million cells from juvenile testes or two to three million cells from adult testes were embedded in plugs of 1% low-melting-point agarose (Lonza). After a brief incubation at 4 °C until the agarose became solid, plugs were incubated with proteinase K (Roche) in lysis buffer (0.5 M EDTA at pH 8.0, 1% N-lauroylsarcosine sodium salt) at 50 °C over two nights. Plugs were washed five times for 20 min with TE (10 mM Tris-HCl at pH 7.5, 1 mM EDTA at pH 8.0), and then incubated with 100 μg/ml RNase A (Thermo) for 3 hr at 37 °C. Plugs were then washed five times with TE and stored in TE at 4 °C until usage.
All library types were prepared in the same way aside from the ssDNA digestion step. For S1-seq, plugs were equilibrated with S1 buffer (50 mM sodium acetate, 280 mM NaCl, 4.5 mM ZnSO4, pH 4.7 at 25 °C), then treated with 9 U of nuclease S1 (Promega) for 20 min at 37 °C. For Exo7/T-seq, plugs were instead equilibrated with exonuclease VII buffer (50 mM Tris-HCl, 50 mM sodium phosphate, 8 mM EDTA, 10 mM 2-mercaptoethanol, pH 8 at 25 °C), then treated with 50 U of exonuclease VII (NEB) for 60 min at 37 °C. Plugs were then equilibrated in NEBuffer 4 and treated with 75 U of exonuclease T (NEB) for 90 min at 24 °C.
Plugs were next equilibrated with T4 polymerase buffer (T4 ligase buffer (NEB) supplemented with 100 μg/ml BSA and 100 μM dNTPs (Roche)) then incubated with 30 U T4 DNA polymerase (NEB) at 12 °C for 30 min. Plugs were then washed in TE and equilibrated in T4 ligase buffer on ice. Biotinylated P5 adaptors were ligated to the blunted ends with 2000 U T4 DNA Ligase (NEB) at 16 °C for 20 hr. After ligation, plugs were soaked in TE overnight at 4 °C to diffuse excess unligated adaptors out of the plugs. The plugs were then equilibrated in 1× β-agarase I buffer (10 mM Bis-Tris-HCl, 1 mM EDTA, pH 6.5), incubated at 70 °C for 15 min to melt the agarose with brief vortexing every 5 min, mixed well, cooled to 42 °C, and digested with 2 μl of β-agarase I (NEB) at 42 °C for 90 min. DNA was sheared to fragment sizes ranging 200–500 bp with a Covaris system (E220 Focused-ultrasonicator, microtube-500) using the following parameters: delay 300 s then three cycles of [peak power 175 W, duty factor 20%, cycles/burst 200, duration 30 s, and delay 90 s]. The DNA was precipitated with ethanol, then dissolved in TE. Unligated adaptors were removed with SPRIselect beads (Beckman Coulter). Fragments containing the biotinylated adaptor were further purified with Dynabeads M-280 streptavidin (Thermo) and DNA ends were repaired using the End-it DNA End-repair kit (Lucigen). P7 adaptors were ligated to DNA fragments, then PCR was done directly on the bead-immobilized fragments. PCR products were purified with AMPure XP beads (Beckman Coulter) to remove primer dimers and unligated adaptors.
After Qubit (Thermo) quantification and quality control by Agilent BioAnalyzer, libraries were pooled and sequenced on the Illumina NextSeq, HiSeq, or NovaSeq platforms in the Integrated Genomics Operation (IGO) at Memorial Sloan Kettering Cancer Center. A spike-in of bacteriophage FX174 DNA was added to increase diversity when necessary and for quality control purposes. We obtained paired-end reads of 50 bp.
Preprocessing and mapping:
Base calls were performed using bcl-convert software v.3.9.3 to 3.10.5 or bcl2fastq software v.2.20. Reads were trimmed and filtered by Trim Galore version 0.6.6 with the arguments --paired --length 15 (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/). Sequence reads were mapped onto the mouse reference genome (mm10) by bowtie2 version 2.3.5.1106 with the arguments -N 1 -X 1000. Duplicated reads were removed by Picard (https://broadinstitute.github.io/picard/). Uniquely and properly mapped reads (MAPQ ≥ 20) were extracted by samtools with the argument -q 20 (http://www.htslib.org/). Reads were assigned to the nucleotide immediately next to the biotinylated adaptor. Mapping statistics are in Supplemental Table S2.
Bioinformatic analysis and plotting:
Maps were analyzed using R version 4.0.3 (http://www.r-project.org). To generate genome-average profiles around hotspots (e.g., Figure 4D), an estimated background was removed by subtracting from all values the value of signal 2,500 bp away from the hotspot center. The signal was then normalized to the peak height of resection endpoints, i.e, the maximum value among positions from 100 to 2,500 bp. Negative values were set as zero for plotting purposes. Where appropriate, data were smoothed with a Hann function.
To calculate the mean resection length, S1-seq signal was averaged across hotspots and an estimated background was removed by subtracting from all values the value of signal 2.5 kb away from the hot spot center. The signal close to and further away from the hot spot center was excluded by setting values of positions <100 bp and >2.5 kb to zero. Fractions of total signal were calculated every 100 bp and the mean resection length was calculated. The modal resection length in Figure S2F was determined by the peak position of the genome-average profile between 100 bp and 2,500 bp from hotspot centers.
The color scale of heatmaps were defined after local normalization of sequencing signal at each hotspot. Signals in 40-bp bins were divided by the total signal in a 4,001-bp window around each hotspot center, so that each row has a total value of 1 regardless of the strengths of hotspots and color reflects the local spatial pattern. Normalized signals were classified into 10 groups by deciles and color-coded.
MRE11 ChIP-seq
MRE11 ChIP was performed following the previously published DISCOVER-seq protocol107. In brief, fresh testes of 5–7 wk old wild-type, Atm−/−, or Mre11-cHN mice were dissociated into single cells and resuspended in DMEM. PFA (16% stock, Pierce) was added to a final concentration of 1% and incubated for 15 min with gentle agitation, then the crosslinking was stopped by adding 2.5 M glycine to a final concentration of 125 mM, followed by 3 min incubation on ice. The nuclei were prepared as described107 and up to 10 million nuclei were resuspended in 500 μl lysis buffer (0.5% N-lauroylsarcosine, 0.1% sodium deoxycholate, 10 mM Tris-HCl pH 8.0, 100 mM NaCl, 1 mM EDTA) supplemented with protease inhibitor (Roche), and sonicated to fragment sizes ranging 200–300 bp with a Covaris system (E220 Focused-ultrasonicator, microtube-500) using the following parameters: delay 300 s then six cycles of [peak power 75 W, duty factor 10%, cycles/burst 200, duration 150 s, and delay 60 s]. Sheared nuclei were centrifuged at 20,000 g for 10 min at 4 °C to remove debris and Triton-X (1% (vol/vol) final concentration) and lysis buffer were added to make the total volume 3 ml.
For each immunoprecipitation, 10 μl of anti-MRE11 (Novus Biologicals NB100-142) was pre-mixed with 100 μl protein A Dynabeads, then added to chromatin lysates and incubated overnight at 4 °C with end-over-end rotation. Immunoprecipitated DNA was eluted and crosslinks were reversed as described107. ChIP sequencing libraries were prepared from cleaned DNA as described for SSDS libraries108 without the boiling step after the end-repair and dA-tailing steps. After ligation with sequencing adaptors, the library was amplified by 15 cycles of PCR. The amplified library was purified and sequenced on the Illumina NovaSeq platform in the IGO. We obtained paired-end reads of 100 bp.
Base calls were performed using bcl-convert software v.3.9.3 to 3.10.5 or bcl2fastq software v.2.20. Sequencing reads were processed and mapped as described for resection libraries and uniquely and properly mapped reads were counted, and maps were further analyzed using R version 4.0.3. Mapping statistics are in Supplemental Table S2.
Single-stranded DNA sequencing (SSDS) library preparation and data analysis
ChIP followed by SSDS was performed as previously described64,108. In brief, a decapsulated, fresh or fresh-frozen testis of 8-12 wk old wild-type or Nbs1ΔB mice was placed in room temperature 1% PFA (16% stock, Pierce) in PBS and incubated for 15 min with gentle agitation. The crosslinking was stopped by adding 2.5 M glycine to a final concentration of 125 mM, followed by 5 min incubation at room temperature. Fixed cells were immediately homogenized in a Dounce homogenizer with 20 strokes of the tight-fitting pestle, then passed through a 70 μm cell strainer. The nuclei were prepared as described108, resuspended in 900 μl shearing buffer (0.1% SDS, 10 mM Tris-HCl pH 8.0, 1 mM EDTA) supplemented with protease inhibitor (Roche), split into two ~500 μl aliquots and sonicated to fragment sizes ranging 500–1000 bp with a Covaris system (E220 Focused-ultrasonicator, microtube-500) using the following parameters: delay 300 s then three cycles of [peak power 75 W, duty factor 10%, cycles/burst 200, duration 150 s, and delay 60 s]. Sheared nuclei were centrifuged at 12,000 g for 10 min at 4 °C to remove debris. The sonicated chromatin was then transferred to a 3 ml Slide-A-Lyzer G2 dialysis cassette (Pierce) and dialyzed with ChIP buffer (16.7 mM Tris-HCl, pH 8.0, 167 mM NaCl, 1.2 mM EDTA, 0.01% SDS, 1.1% Triton X-100) for 5 hr at 4 °C with constant but slow stirring. For each immunoprecipitation, 20 μl of anti-DMC1 (Abcam, ab11054), 10 μl of anti-RAD51 (Novus Biologicals, NB100-148), or 10 μl of anti-RPA2 (Abcam, ab76420) was added to chromatin lysates and incubated overnight at 4 °C with end-over-end rotation.
As described previously108, protein G or A Dynabeads were added to the chromatin and antibody mixture and incubated 2 hr at 4 °C, then immunoprecipitated DNA was washed and eluted. To reverse DNA-protein crosslinks, 12 μl of 5 M NaCl was added to 300 μl of eluted sample and incubated overnight at 65 °C. DNA was cleaned with MinElute PCR Cleanup Kit (Qiagen) after proteinase K (Thermo) treatment. Following end repair and dA-tailing, DNA was incubated at 95 °C for 3 min and cooled to room temperature to enrich for single-stranded DNA. After ligation with sequencing adaptors, SSDS library was amplified with 12 cycles of PCR. The amplified library was purified and sequenced on the Illumina NovaSeq platform in the IGO. We obtained paired-end reads of 100 bp.
Base calls were performed using bcl-convert software v.3.9.3 to 3.10.5 or bcl2fastq software v.2.20. We then ran a previously described bioinformatic pipeline on the resulting reads for identification of single-stranded sequences108. Sequence reads at SPO11-dependent hotspots were analyzed using R version 4.0.3.
Sperm counts
Sperm isolation was performed as described109. Briefly, both caudal and caput epididymis were dissected from >5 wk old mice, with all excess fat and tissue trimmed away, and placed on top of filter mesh of 5 ml tubes with cell-strainer caps (BD) filled completely with PBS. After 5 min, the cap was carefully lifted out of the PBS to break the fluid surface tension, releasing plumes of sperm into the tube. This was repeated until sperm were no longer released after lifting out the cap. The cap (and tissue) was removed, and the tube sealed with parafilm. The sperm were pelleted in a swinging bucket rotor for 2 min at 4,000 g. The supernatant was aspirated down to ~1 ml, and then gently resuspended by quick vortex pulses and incubated for 1 min at 60 °C. Inactivated sperm were mixed with Trypan Bule solution and placed on a hematocytometer for counting.
Spermatocyte chromosome spreads and immunostaining
Spreads were prepared as described with minor modifications110. When preparing a sequencing library and spreads from testes collected at the same time, testis cells were dissociated following the procedures in library preparation until passage through the 70 μm cell strainer (see above), then continued as described below. Briefly, decapsulated testis samples were placed in 2 ml TIM buffer (104 mM NaCl, 45 mM KCl, 1.2 mM MgSO4, 0.6 mM KH2PO4, 0.1% glucose, 6 mM sodium lactate, 1 mM sodium pyruvate, pH 7.3), with 200 μl collagenase (20 mg/ml), then shaken at 350 rpm for 15 min at 32 °C. After washing, tubules were resuspended in 2 ml TIM buffer with 200 μl trypsin solution (7 mg/ml) and 20 μl DNase I (400 μg/ml) and shaken for 15 min at 350 rpm at 32 °C. To stop trypsin digestion, 500 μl of FBS was added. Resuspended cells were filtered through a 70 μm cell strainer and TIM buffer was added to 15 ml. After three washes with DNase I, cells were resuspended in 10 ml of TIM buffer and distributed in 1 ml aliquots to Eppendorf tubes, centrifuged, and the supernatant was removed. The cell pellet was resuspended in 80 μl pre-warmed 0.1 M sucrose and incubated for 10 min. From the cell suspension, 40 μl was added to a Superfrost glass slide covered with 130 μl 1% PFA (with 0.1% Triton X-100, pH 9.2) in a humid chamber. Slides were kept in the closed humid chamber for at least 2 hr at 4 °C, then air dried and rinsed with 0.4% Photo-Flo (Kodak). After air drying, slides were wrapped in aluminum foil and stored at −80 °C.
For immunofluorescence, slides were blocked for 10 min with dilution buffer (0.2% BSA, 0.2% fish skin gelatin, 0.05% Tween-20 in 1× PBS) at room temperature. Using a humid chamber, slides were incubated with 100 μl primary antibody solution overnight at 4 °C. After washing, secondary antibody was added for 1 hr at 37 °C. After washing, slides were mounted with Vectashield containing DAPI (H-1000, Vector Laboratories). Primary and secondary antibodies are listed in Supplemental Table S3.
Histology
Testes and epididymides dissected from adult mice were fixed in Bouin’s fixative for 4 to 5 hr at room temperature, or in 4% PFA overnight at 4 °C. Bouin’s fixed testes were washed in 15 ml milli-Q water on a horizontal shaker for 1 hr at room temperature, followed by five 1-hr washes in 15 ml of 70% ethanol on a roller at 4 °C. PFA-fixed tissues were washed four times for 5 min in 15 ml milli-Q water at room temperature. Fixed tissues were stored in 70% ethanol before embedding in paraffin and sectioning at 5 μm. The tissue sections were deparaffinized with EZPrep buffer (Ventana Medical Systems). Antigen retrieval was performed with CC1 buffer (Ventana Medical Systems). Sections were blocked for 30 min with Background Buster solution (Innovex), and then avidin-biotin blocked for 8 min (Ventana Medical Systems). Hematoxylin and eosin (H&E) staining and immunohistochemical TUNEL assays were performed by the MSK Molecular Cytology Core Facility using the Autostainer XL (Leica Microsystems, Wetzlar, Germany) automated stainer for H&E or PAS with hematoxylin counterstain, and using the Discovery XT processor (Ventana Medical Systems, Oro Valley, Arizona) for TUNEL. Testis sections were incubated with anti-DDX4 or anti-MRE11 for 5 hr, followed by 60 min incubation with biotinylated goat anti-rabbit (Vector Labs) at 1:200 dilution (Supplemental Table S3). Detection was performed with DAB detection kit (Ventana Medical Systems) according to manufacturer’s instructions. Slides were counterstained with hematoxylin and coverslips were mounted with Permount (Fisher Scientific).
Image acquisition and analysis
Images of spread spermatocytes were acquired on a Zeiss Axio Observer Z1 Marianas Workstation, equipped with an ORCA-Flash 4.0 camera, illuminated by an X-Cite 120 PC-Q light source, with 100× 1.4 NA oil immersion objective. Marianas Slidebook (Intelligent Imaging Innovations, Denver Colorado) software was used for acquisition. Whole histology slides were scanned and digitized with the Panoramic Flash Slide Scanner (3DHistech, Budapest, Hungary) with a 20× 0.8 NA objective (Carl Zeiss, Jena, Germany). High-resolution histology images were acquired with a Zeiss Axio Imager microscope using a 63× 1.4 NA oil immersion objective (Carl Zeiss, Jena, Germany). All images were processed in Fiji111. To quantify foci of RPA, DMC1, or RAD51, staging of spermatocytes was assessed by SYCP3 staining (% of axes synapsed) and only foci colocalizing with chromosome axis (SYCP3-signal) were manually counted. Statistical analysis was done with Graphpad Prism 9.
Structured illumination microscopy
Spermatocytes from juvenile Nbs1ΔB homozygous and heterozygous littermates (13–15 dpp) were prepared for surface spreading and subsequent immunofluorescence as described previously64,112. Briefly, testes were dissected and placed in 1× PBS, pH 7.4 at room temperature before removal of the tunica albuginea. Seminiferous tubules were incubated in hypotonic extraction buffer (30 mM Tris-HCl pH 8.2, 50 mM sucrose, 17 mM trisodium citrate dihydrate, 5 mM EDTA, 2.5 mM DTT, 1 mM PMSF) for 20 min at room temperature. A homogeneous cell suspension was made in 100 mM sucrose and fixed on slides covered with a 1% PFA solution (pH 9.2) containing 0.15% Triton X-100 for at least 3 hr in a humid chamber. Slides were either used for immunofluorescence staining immediately or stored at −80 °C. For immunofluorescence, slides were blocked for 30 min at room temperature in PBST (1x phosphate-buffered saline (PBS) containing 0.1% Triton X-100) containing 3% nonfat milk. Slides were incubated with primary antibodies overnight in a humid chamber at 37 °C. Slides were washed 3 × 10 min in PBST, then incubated with secondary antibody for 1 hr at 37 °C in a humid chamber. Both primary and secondary antibodies were diluted in PBST containing 3% nonfat milk. After secondary antibody incubation, slides were washed 3 × 10 min in the dark with PBST and the slides were mounted with VECTASHIELD mounting medium containing DAPI (H-1000, Vector Laboratories).
Structured illumination microscopy (3D-SIM) was performed at the Bio-Imaging Resource Center in Rockefeller University using an OMX Blaze 3D-SIM super-resolution microscope (Applied Precision), equipped with 405 nm, 488 nm and 568 nm lasers, and 100× 1.40 NA UPLSAPO oil objective (Olympus). Image stacks of several μm thickness were taken with 0.125 μm optical section spacing and were reconstructed in Deltavision softWoRx 7.0.0 software with a Wiener filter of 0.002 using wavelength specific experimentally determined OTF functions. Maximum intensity projection images were acquired in Deltavision softWoRx 7.0.0 software. Data presented in this study were pooled from two independent staining experiments. To ensure correct alignment of fluorescent channels in all SIM experiments, multi-color fluorescent beads (TetraSpeck microspheres, 100 nm, Life Technologies, T7279) were imaged and used to correct shifts from passage of different fluorescence wave lengths through the optical path, as previously described64. SIM image analyses were as previously described64.
Immunoblotting
About one million dissociated testis cells were boiled with NuPAGE LDS Sample Buffer (Life Technologies) and separated on 3%–8% Tris-acetate NuPAGE precast gels (Life Technologies) at 150 V for 70 min. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes by wet transfer method in Tris-glycine-20% methanol, at 120 V for 40 min at 4°C. Membranes were blocked with 5% non-fat milk in 1′ Tris buffered saline, 0.1% Tween (TBS-T) for 30 min at room temperature on an orbital shaker. Blocked membranes were incubated with primary antibodies overnight at 4°C. Membranes were washed with TBS-T for 30 min at room temperature, then incubated with HRP-conjugated secondary antibodies for 1 hr at room temperature. Membranes were washed with TBS-T for 30 min and the signal was developed by ECL Prime (GE Healthcare). Primary and secondary antibodies are listed in Supplemental Table S3.
SPO11-oligo complexes and denaturing PAGE
We purified SPO11-oligo complexes from testes of adults (> 5 wk old) as previously described110. Briefly, testes were decapsulated, flash frozen in liquid nitrogen and stored at −80 °C. Testes were homogenized and extracts were cleared by ultracentrifugation. SPO11 was then immunoprecipitated with mouse monoclonal anti-SPO11-antibody 180 and protein A-agarose beads. SPO11 was eluted in SDS-containing buffer then subjected to a second round of immunoprecipitation with the same antibody. The material from the second immunoprecipitation was radiolabeled with terminal deoxynucleotidyl transferase and [α-32P]-CTP then eluted with Laemmli sample buffer and separated by SDS-PAGE. Proteins were transferred to PVDF membrane. Alternatively, the size distribution of SPO11 oligos purified from two adult Atm-KO or Mre11-cKO mice was determined by protease digestion followed by radiolabeling with [α-32P]-GTP followed by electrophoresis on a 15% denaturing polyacrylamide gel, which was then dried on filter paper as previously described113. Radio-labelled species were detected with Typhoon phosphor imager after 48 hr exposure with Fuji phosphor screens, then quantified in Fiji111.
Cell culture
Mouse embryonic fibroblasts (MEFs) were generated and cultured as described23. Mitotic index was quantified using early passage primary MEFs by measuring Ser10 phosphorylation of histone H3 by flow cytometry 1 hr after 3 Gy of IR exposure. Cells were treated with 10 μM of ATM inhibitor (KU55933, 11855, Sigma) 30 min before IR exposure as an ATM deficient control. Two independent MEF cell lines for either wild type or Rad50-D69Y were tested. For the colony formation assay, cells were immortalized as described23, then plated with different doses of CPT for 24 hr and washed and cultured in drug-free media. For ATR inhibition, cells were treated with 50 nM of ATR inhibitor (VE822, S7102, Selleckchem) 2 hr before CPT treatment. After 10 days of growth, colonies were visualized by crystal violet stain (0.5% crystal violet, 25% methanol).
Supplementary Material
ACKNOWLEDGMENTS
This article is subject to the Open Access to Publications policy of the Howard Hughes Medical Institute (HHMI). HHMI lab heads have previously granted a nonexclusive CC BY 4.0 license to the public and a sublicensable license to HHMI in their research articles. Pursuant to those licenses, the author-accepted manuscript of this article can be made freely available under a CC BY 4.0 license immediately upon publication. We thank W. Edelmann (Albert Einstein College of Medicine, New York, NY), R. Baer (Columbia University, New York, NY), Francesca Cole (MD Anderson Cancer Center, Houston, TX) and D. O. Ferguson (University of Michigan School of Medicine, Ann Arbor, MI) for generously sharing mouse lines. We thank A. Lukaszewicz (Univ. Michigan) and M. Jasin (MSK) for discussion. We thank the MSK Molecular Cytology core facility (N. Fan and M. Pulina) for histology; the MSK Integrated Genomics Operation (IGO) for sequencing; and the MSK Mouse Genetics Colony Management Group for assistance with mouse husbandry. MSK core facilities are supported by National Cancer Institute cancer center support grant P30 CA08748. The IGO was further funded by the Cycle for Survival and the Marie-Josée and Henry R. Kravis Center for Molecular Oncology. We thank M. Arter, E. Suranyi, H. Murakami, A. Shabro, V. Macera, and other members of the Keeney laboratory for discussions and experimental advice. This work was supported by NIH grants R35 GM118092 (to S.Keeney), R01 GM59413 (to J.H.J.P.), R35 GM136278 (to J.H.J.P.), and the Brain Pool Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2022H1D3A2A01096332 to S. Kim).
Data Availability
Raw and processed sequencing S1-, Exo7/T-seq, MRE11-ChIP, and SSDS data have been deposited in the Gene Expression Omnibus (GEO) repository under accession numbers GSE266258, GSE266151, and GSE266271. We used additional S1-seq and Exo7/T-seq data from GEO accession numbers GSE265863 and GSE22945034,114. We used SPO11-oligo data from GEO accession numbers GSE84689 and Atm−/− MRE11 ChIP-seq data from GEO accession GSE13891514,37.
REFERENCES
- 1.Bergerat A., de Massy B., Gadelle D., Varoutas P.C., Nicolas A., and Forterre P. (1997). An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature 386, 414–417. 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- 2.Keeney S., Giroux C.N., and Kleckner N. (1997). Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88, 375–384. 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 3.Cejka P., and Symington L.S. (2021). DNA End Resection: Mechanism and Control. Annu Rev Genet 55, 285–307. 10.1146/annurev-genet-071719-020312. [DOI] [PubMed] [Google Scholar]
- 4.Hartsuiker E., Mizuno K., Molnar M., Kohli J., Ohta K., and Carr A.M. (2009). Ctp1CtIP and Rad32Mre11 nuclease activity are required for Rec12Spo11 removal, but Rec12Spo11 removal is dispensable for other MRN-dependent meiotic functions. Mol Cell Biol 29, 1671–1681. 10.1128/MCB.01182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milman N., Higuchi E., and Smith G.R. (2009). Meiotic DNA double-strand break repair requires two nucleases, MRN and Ctp1, to produce a single size class of Rec12 (Spo11)-oligonucleotide complexes. Mol Cell Biol 29, 5998–6005. MCB.01127-09 [pii] 10.1128/MCB.01127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreau S., Ferguson J.R., and Symington L.S. (1999). The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol Cell Biol 19, 556–566. 10.1128/mcb.19.1.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neale M.J., Pan J., and Keeney S. (2005). Endonucleolytic processing of covalent protein-linked DNA double-strand breaks. Nature 436, 1053–1057. 10.1038/nature03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannavo E., and Cejka P. (2014). Sae2 promotes dsDNA endonuclease activity within Mre11-Rad50-Xrs2 to resect DNA breaks. Nature 514, 122–125. 10.1038/nature13771. [DOI] [PubMed] [Google Scholar]
- 9.Garcia V., Phelps S.E., Gray S., and Neale M.J. (2011). Bidirectional resection of DNA double-strand breaks by Mre11 and Exo1. Nature 479, 241–244. 10.1038/nature10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keelagher R.E., Cotton V.E., Goldman A.S., and Borts R.H. (2011). Separable roles for Exonuclease I in meiotic DNA double-strand break repair. DNA Repair (Amst) 10, 126–137. 10.1016/j.dnarep.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Mimitou E.P., Yamada S., and Keeney S. (2017). A global view of meiotic double-strand break end resection. Science 355, 40–45. 10.1126/science.aak9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zakharyevich K., Ma Y., Tang S., Hwang P.Y., Boiteux S., and Hunter N. (2010). Temporally and biochemically distinct activities of Exo1 during meiosis: double-strand break resection and resolution of double Holliday junctions. Mol Cell 40, 1001–1015. 10.1016/j.molcel.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshpande R.A., Lee J.H., Arora S., and Paull T.T. (2016). Nbs1 Converts the Human Mre11/Rad50 Nuclease Complex into an Endo/Exonuclease Machine Specific for Protein-DNA Adducts. Mol Cell 64, 593–606. 10.1016/j.molcel.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Paiano J., Wu W., Yamada S., Sciascia N., Callen E., Paola Cotrim A., Deshpande R.A., Maman Y., Day A., Paull T.T., and Nussenzweig A. (2020). ATM and PRDM9 regulate SPO11-bound recombination intermediates during meiosis. Nat Commun 11, 857. 10.1038/s41467-020-14654-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada S., Hinch A.G., Kamido H., Zhang Y., Edelmann W., and Keeney S. (2020). Molecular structures and mechanisms of DNA break processing in mouse meiosis. Genes Dev 34, 806–818. 10.1101/gad.336032.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo G., Yao M.S., Bender C.F., Mills M., Bladl A.R., Bradley A., and Petrini J.H. (1999). Disruption of mRad50 causes embryonic stem cell lethality, abnormal embryonic development, and sensitivity to ionizing radiation. Proc Natl Acad Sci U S A 96, 7376–7381. 10.1073/pnas.96.13.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao Y., and Weaver D.T. (1997). Conditional gene targeted deletion by Cre recombinase demonstrates the requirement for the double-strand break repair Mre11 protein in murine embryonic stem cells. Nucleic Acids Res 25, 2985–2991. 10.1093/nar/25.15.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J., Petersen S., Tessarollo L., and Nussenzweig A. (2001). Targeted disruption of the Nijmegen breakage syndrome gene NBS1 leads to early embryonic lethality in mice. Curr Biol 11, 105–109. 10.1016/s0960-9822(01)00019-7. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B., Tang Z., Li L., and Lu L.Y. (2020). NBS1 is required for SPO11-linked DNA double-strand break repair in male meiosis. Cell Death Differ 27, 2176–2190. 10.1038/s41418-020-0493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Lin Z., Yan J., Zhang X., and Tong M.H. (2024). A Rad50-null mutation in mouse germ cells causes reduced DSB formation, abnormal DSB end resection and complete loss of germ cells. Development 151. 10.1242/dev.202312. [DOI] [PubMed] [Google Scholar]
- 21.Theunissen J.W., Kaplan M.I., Hunt P.A., Williams B.R., Ferguson D.O., Alt F.W., and Petrini J.H. (2003). Checkpoint failure and chromosomal in-stability without lymphomagenesis in Mre11(ATLD1/ATLD1) mice. Mol Cell 12, 1511–1523. 10.1016/s1097-2765(03)00455-6. [DOI] [PubMed] [Google Scholar]
- 22.Yu Z., Vogel G., Coulombe Y., Dubeau D., Spehalski E., Hebert J., Fer-guson D.O., Masson J.Y., and Richard S. (2012). The MRE11 GAR motif regulates DNA double-strand break processing and ATR activation. Cell Res 22, 305–320. 10.1038/cr.2011.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bender C.F., Sikes M.L., Sullivan R., Huye L.E., Le Beau M.M., Roth D.B., Mirzoeva O.K., Oltz E.M., and Petrini J.H. (2002). Cancer predispo-sition and hematopoietic failure in Rad50(S/S) mice. Genes Dev 16, 2237–2251. 10.1101/gad.1007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roset R., Inagaki A., Hohl M., Brenet F., Lafrance-Vanasse J., Lange J., Scandura J.M., Tainer J.A., Keeney S., and Petrini J.H. (2014). The Rad50 hook domain regulates DNA damage signaling and tumorigenesis. Genes Dev 28, 451–462. 10.1101/gad.236745.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang J., Bronson R.T., and Xu Y. (2002). Targeted disruption of NBS1 reveals its roles in mouse development and DNA repair. EMBO J 21, 1447–1455. 10.1093/emboj/21.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams B.R., Mirzoeva O.K., Morgan W.F., Lin J., Dunnick W., and Petrini J.H. (2002). A murine model of Nijmegen breakage syndrome. Curr Biol 12, 648–653. 10.1016/s0960-9822(02)00763-7. [DOI] [PubMed] [Google Scholar]
- 27.Difilippantonio S., Celeste A., Fernandez-Capetillo O., Chen H.T., Reina San Martin B., Van Laethem F., Yang Y.P., Petukhova G.V., Eckhaus M., Feigenbaum L., et al. (2005). Role of Nbs1 in the activation of the Atm kinase revealed in humanized mouse models. Nat Cell Biol 7, 675–685. 10.1038/ncb1270. [DOI] [PubMed] [Google Scholar]
- 28.Stracker T.H., Morales M., Couto S.S., Hussein H., and Petrini J.H. (2007). The carboxy terminus of NBS1 is required for induction of apoptosis by the MRE11 complex. Nature 447, 218–221. 10.1038/nature05740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buis J., Wu Y., Deng Y., Leddon J., Westfield G., Eckersdorff M., Sekiguchi J.M., Chang S., and Ferguson D.O. (2008). Mre11 nuclease activity has essential roles in DNA repair and genomic stability distinct from ATM activation. Cell 135, 85–96. 10.1016/j.cell.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schonhoff S.E., Giel-Moloney M., and Leiter A.B. (2004). Neurogen-in 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol 270, 443–454. 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida S., Takakura A., Ohbo K., Abe K., Wakabayashi J., Yamamoto M., Suda T., and Nabeshima Y. (2004). Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol 269, 447–458. 10.1016/j.ydbio.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 32.Zheng K., and Wang P.J. (2012). Blockade of pachytene piRNA biogenesis reveals a novel requirement for maintaining post-meiotic germline genome integrity. PLoS Genet 8, e1003038. 10.1371/journal.pgen.1003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida S., Sukeno M., Nakagawa T., Ohbo K., Nagamatsu G., Suda T., and Nabeshima Y. (2006). The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 133, 1495–1505. 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- 34.Kim S., Yamada S., Maekawa K., and Keeney S. (2024). Optimized methods for mapping DNA double-strand-break ends and resection tracts and application to meiotic recombination in mouse spermatocytes. bioRxiv, 2024.2008.2010.606181. 10.1101/2024.08.10.606181. [DOI] [Google Scholar]
- 35.Canela A., Maman Y., Jung S., Wong N., Callen E., Day A., Kieffer-Kwon K.R., Pekowska A., Zhang H., Rao S.S.P., et al. (2017). Genome Organization Drives Chromosome Fragility. Cell 170, 507–521 e518. 10.1016/j.cell.2017.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canela A., Sridharan S., Sciascia N., Tubbs A., Meltzer P., Sleckman B.P., and Nussenzweig A. (2016). DNA Breaks and End Resection Measured Genome-wide by End Sequencing. Mol Cell 63, 898–911. 10.1016/j.molcel.2016.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lange J., Yamada S., Tischfield S.E., Pan J., Kim S., Zhu X., Socci N.D., Jasin M., and Keeney S. (2016). The Landscape of Mouse Meiotic Double-Strand Break Formation, Processing, and Repair. Cell 167, 695–708 e616. 10.1016/j.cell.2016.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smagulova F., Gregoretti I.V., Brick K., Khil P., Camerini-Otero R.D., and Petukhova G.V. (2011). Genome-wide analysis reveals novel molecular features of mouse recombination hotspots. Nature 472, 375–378. 10.1038/nature09869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claeys Bouuaert C., Tischfield S.E., Pu S., Mimitou E.P., Arias-Palomo E., Berger J.M., and Keeney S. (2021). Structural and functional characterization of the Spo11 core complex. Nat Struct Mol Biol 28, 92–102. 10.1038/s41594-020-00534-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mimitou E.P., and Keeney S. (2018). S1-seq Assay for Mapping Processed DNA Ends. Methods Enzymol 601, 309–330. 10.1016/bs.mie.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart G.S., Maser R.S., Stankovic T., Bressan D.A., Kaplan M.I., Jas-pers N.G., Raams A., Byrd P. J., Petrini J.H., and Taylor A.M. (1999). The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell 99, 577–587. 10.1016/s0092-8674(00)81547-0. [DOI] [PubMed] [Google Scholar]
- 42.Taylor A.M., Groom A., and Byrd P.J. (2004). Ataxia-telangiectasia-like disorder (ATLD)-its clinical presentation and molecular basis. DNA Repair (Amst) 3, 1219–1225. 10.1016/j.dnarep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Uziel T., Lerenthal Y., Moyal L., Andegeko Y., Mittelman L., and Shiloh Y. (2003). Requirement of the MRN complex for ATM activation by DNA damage. EMBO J 22, 5612–5621. 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lukaszewicz A., Lange J., Keeney S., and Jasin M. (2018). Control of meiotic double-strand-break formation by ATM: local and global views. Cell Cycle 17, 1155–1172. 10.1080/15384101.2018.1464847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson D., Crawford M., Cooper T., Claeys Bouuaert C., Keeney S., Llorente B., Garcia V, and Neale M.J. (2021). Concerted cutting by Spo11 illuminates meiotic DNA break mechanics. Nature 594, 572–576. 10.1038/s41586-021-03389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lukaszewicz A., Lange J., Keeney S., and Jasin M. (2021). De novo deletions and duplications at recombination hotspots in mouse germlines. Cell 184, 5970–5984 e5918. 10.1016/j.cell.2021.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prieler S., Chen D., Huang L., Mayrhofer E., Zsoter S., Vesely M., Mbogning J., and Klein F. (2021). Spo11 generates gaps through concerted cuts at sites of topological stress. Nature 594, 577–582. 10.1038/s41586-021-03632-x. [DOI] [PubMed] [Google Scholar]
- 48.Lange J., Pan J., Cole F., Thelen M.P., Jasin M., and Keeney S. (2011). ATM controls meiotic double-strand-break formation. Nature 479, 237–240. 10.1038/nature10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Y., Wang J., Liu K., Zheng Z., Arter M., Bouuaert C.C., Pu S., Patel D.J., and Keeney S. (2023). Cryo-EM structure of the Spo11 core complex bound to DNA. bioRxiv. 10.1101/2023.10.31.564985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toyooka Y., Tsunekawa N., Takahashi Y., Matsui Y., Satoh M., and Noce T. (2000). Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech Dev 93, 139–149. 10.1016/s0925-4773(00)00283-5. [DOI] [PubMed] [Google Scholar]
- 51.Moens P.B., Kolas N.K., Tarsounas M., Marcon E., Cohen P.E., and Spyropoulos B. (2002). The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA-DNA interactions without reciprocal recombination. J Cell Sci 115, 1611–1622. 10.1242/jcs.115.8.1611. [DOI] [PubMed] [Google Scholar]
- 52.Arthur L.M., Gustausson K., Hopfner K.P., Carson C.T., Stracker T.H., Karcher A., Felton D., Weitzman M.D., Tainer J., and Carney J.P. (2004). Structural and functional analysis of Mre11-3. Nucleic Acids Res 32, 1886–1893. 10.1093/nar/gkh343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee J.H., Mand M.R., Deshpande R.A., Kinoshita E., Yang S.H., Wyman C., and Paull T.T. (2013). Ataxia telangiectasia-mutated (ATM) kinase activity is regulated by ATP-driven conformational changes in the Mre11/Rad50/Nbs1 (MRN) complex. J Biol Chem 288, 12840–12851. 10.1074/jbc.M113.460378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoa N.N., Shimizu T., Zhou Z.W., Wang Z.Q., Deshpande R.A., Paull T.T., Akter S., Tsuda M., Furuta R., Tsutsui K., et al. (2016). Mre11 Is Essential for the Removal of Lethal Topoisomerase 2 Covalent Cleavage Complexes. Mol Cell 64, 580–592. 10.1016/j.molcel.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 55.Farah J.A., Cromie G., Steiner W.W., and Smith G.R. (2005). A novel recombination pathway initiated by the Mre11/Rad50/Nbs1 complex eliminates palindromes during meiosis in Schizosaccharomyces pombe. Genetics 169, 1261–1274. 10.1534/genetics.104.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Limbo O., Porter-Goff M.E., Rhind N., and Russell P. (2011). Mre11 nuclease activity and Ctp1 regulate Chk1 activation by Rad3ATR and Tel-1ATM checkpoint kinases at double-strand breaks. Mol Cell Biol 31, 573–583. 10.1128/MCB.00994-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clerici M., Mantiero D., Lucchini G., and Longhese M.P. (2006). The Saccharomyces cerevisiae Sae2 protein negatively regulates DNA damage checkpoint signalling. EMBO Rep 7, 212–218. 10.1038/sj.embor.7400593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stracker T.H., and Petrini J.H. (2011). The MRE11 complex: starting from the ends. Nat Rev Mol Cell Biol 12, 90–103. nrm3047 [pii] 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cherry S.M., Adelman C.A., Theunissen J.W., Hassold T.J., Hunt P.A., and Petrini J.H. (2007). The Mre11 complex influences DNA repair, synapsis, and crossing over in murine meiosis. Curr Biol 17, 373–378. 10.1016/j.cub.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J.H., Ghirlando R., Bhaskara V., Hoffmeyer M.R., Gu J., and Paull T.T. (2003). Regulation of Mre11/Rad50 by Nbs1: effects on nucleotide-dependent DNA binding and association with ataxia-telangiectasia-like disorder mutant complexes. J Biol Chem 278, 45171–45181. 10.1074/jbc.M308705200. [DOI] [PubMed] [Google Scholar]
- 61.Al-Ahmadie H., Iyer G., Hohl M., Asthana S., Inagaki A., Schultz N., Hanrahan A.J., Scott S.N., Brannon A.R., McDermott G.C., et al. (2014). Synthetic lethality in ATM-deficient RAD50-mutant tumors underlies outlier response to cancer therapy. Cancer Discov 4, 1014–1021. 10.1158/2159-8290.CD-14-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hohl M., Mojumdar A., Hailemariam S., Kuryavyi V., Ghisays F., Sorenson K., Chang M., Taylor B.S., Patel D.J., Burgers P.M., et al. (2020). Modeling cancer genomic data in yeast reveals selection against ATM function during tumorigenesis. PLoS Genet 16, e1008422. 10.1371/journal.pgen.1008422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ziv Y., Bielopolski D., Galanty Y., Lukas C., Taya Y., Schultz D.C., Lukas J., Bekker-Jensen S., Bartek J., and Shiloh Y. (2006). Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nat Cell Biol 8, 870–876. 10.1038/ncb1446. [DOI] [PubMed] [Google Scholar]
- 64.Hinch A.G., Becker P.W., Li T., Moralli D., Zhang G., Bycroft C., Green C., Keeney S., Shi Q., Davies B., and Donnelly P. (2020). The Configuration of RPA, RAD51, and DMC1 Binding in Meiosis Reveals the Nature of Critical Recombination Intermediates. Mol Cell 79, 689–701 e610. 10.1016/j.molcel.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khil P.P., Smagulova F., Brick K.M., Camerini-Otero R.D., and Petukhova G.V. (2012). Sensitive mapping of recombination hotspots using sequencing-based detection of ssDNA. Genome Res 22, 957–965. 10.1101/gr.130583.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown M.S., Grubb J., Zhang A., Rust M.J., and Bishop D.K. (2015). Small Rad51 and Dmc1 Complexes Often Co-occupy Both Ends of a Meiotic DNA Double Strand Break. PLoS Genet 11, e1005653. 10.1371/journal.pgen.1005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slotman J.A., Paul M.W., Carofiglio F., de Gruiter H.M., Vergroesen T., Koornneef L., van Cappellen W.A., Houtsmuller A.B., and Baarends W.M. (2020). Super-resolution imaging of RAD51 and DMC1 in DNA repair foci reveals dynamic distribution patterns in meiotic prophase. PLoS Genet 16, e1008595. 10.1371/journal.pgen.1008595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicolette M.L., Lee K., Guo Z., Rani M., Chow J.M., Lee S.E., and Paull T.T. (2010). Mre11-Rad50-Xrs2 and Sae2 promote 5’ strand resection of DNA double-strand breaks. Nat Struct Mol Biol 17, 1478–1485. 10.1038/nsmb.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang W., Daley J.M., Kwon Y., Xue X., Krasner D.S., Miller A.S., Nguyen K.A., Williamson E.A., Shim E.Y., Lee S.E., et al. (2018). A DNA nick at Ku-blocked double-strand break ends serves as an entry site for exonuclease 1 (Exo1) or Sgs1-Dna2 in long-range DNA end resection. J Biol Chem 293, 17061–17069. 10.1074/jbc.RA118.004769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gobbini E., Cassani C., Vertemara J., Wang W., Mambretti F., Casari E., Sung P., Tisi R., Zampella G., and Longhese M.P. (2018). The MRX complex regulates Exo1 resection activity by altering DNA end structure. EMBO J 37. 10.15252/embj.201798588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shim E.Y., Chung W.H., Nicolette M.L., Zhang Y., Davis M., Zhu Z., Paull T.T., Ira G., and Lee S.E. (2010). Saccharomyces cerevisiae Mre11/Rad50/Xrs2 and Ku proteins regulate association of Exo1 and Dna2 with DNA breaks. EMBO J 29, 3370–3380. 10.1038/emboj.2010.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rein K., Yanez D.A., Terre B., Palenzuela L., Aivio S., Wei K., Edelmann W., Stark J.M., and Stracker T.H. (2015). EXO1 is critical for embryogenesis and the DNA damage response in mice with a hypomorphic Nbs1 allele. Nucleic Acids Res 43, 7371–7387. 10.1093/nar/gkv691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao X., Zhang Y., Wilkins K., Edelmann W., and Usdin K. (2018). MutLgamma promotes repeat expansion in a Fragile X mouse model while EXO1 is protective. PLoS Genet 14, e1007719. 10.1371/journal.pgen.1007719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang S., Lee K., Gray S., Zhang Y., Tang C., Morrish R.B., Tosti E., van Oers J., Amin M.R., Cohen P.E., et al. (2022). Role of EXO1 nuclease activity in genome maintenance, the immune response and tumor suppression in Exo1D173A mice. Nucleic Acids Res 50, 8093–8106. 10.1093/nar/gkac616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morales M., Theunissen J.W., Kim C.F., Kitagawa R., Kastan M.B., and Petrini J.H. (2005). The Rad50S allele promotes ATM-dependent DNA damage responses and suppresses ATM deficiency: implications for the Mre11 complex as a DNA damage sensor. Genes Dev 19, 3043–3054. 10.1101/gad.1373705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pacheco S., Marcet-Ortega M., Lange J., Jasin M., Keeney S., and Roig I. (2015). The ATM signaling cascade promotes recombination-dependent pachytene arrest in mouse spermatocytes. PLoS Genet 11, e1005017. 10.1371/journal.pgen.1005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anand R., Ranjha L., Cannavo E., and Cejka P. (2016). Phosphorylated CtIP Functions as a Co-factor of the MRE11-RAD50-NBS1 Endonuclease in DNA End Resection. Mol Cell 64, 940–950. 10.1016/j.molcel.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 78.Deshpande R.A., Myler L.R., Soniat M.M., Makharashvili N., Lee L., Lees-Miller S.P., Finkelstein I.J., and Paull T.T. (2020). DNA-dependent protein kinase promotes DNA end processing by MRN and CtIP. Sci Adv 6, eaay0922. 10.1126/sciadv.aay0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reginato G., Cannavo E., and Cejka P. (2017). Physiological protein blocks direct the Mre11-Rad50-Xrs2 and Sae2 nuclease complex to initiate DNA end resection. Genes Dev 31, 2325–2330. 10.1101/gad.308254.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sartori A.A., Lukas C., Coates J., Mistrik M., Fu S., Bartek J., Baer R., Lukas J., and Jackson S.P. (2007). Human CtIP promotes DNA end resection. Nature 450, 509–514. 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Myler L.R., Gallardo I.F., Soniat M.M., Deshpande R.A., Gonzalez X.B., Kim Y., Paull T.T., and Finkelstein I.J. (2017). Single-Molecule Imaging Reveals How Mre11-Rad50-Nbs1 Initiates DNA Break Repair. Mol Cell 67, 891–898 e894. 10.1016/j.molcel.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kissling V.M., Reginato G., Bianco E., Kasaciunaite K., Tilma J., Cereghetti G., Schindler N., Lee S.S., Guerois R., Luke B., et al. (2022). Mre11-Rad50 oligomerization promotes DNA double-strand break repair. Nat Commun 13, 2374. 10.1038/s41467-022-29841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gnugge R., Reginato G., Cejka P., and Symington L.S. (2023). Sequence and chromatin features guide DNA double-strand break resection initiation. Mol Cell 83, 1237–1250 e1215. 10.1016/j.molcel.2023.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cannavo E., Reginato G., and Cejka P. (2019). Stepwise 5’ DNA end-spe-cific resection of DNA breaks by the Mre11-Rad50-Xrs2 and Sae2 nuclease ensemble. Proc Natl Acad Sci U S A 116, 5505–5513. 10.1073/pnas.1820157116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cannavo E., Cejka P., and Kowalczykowski S.C. (2013). Relationship of DNA degradation by Saccharomyces cerevisiae exonuclease 1 and its stimulation by RPA and Mre11-Rad50-Xrs2 to DNA end resection. Proc Natl Acad Sci U S A 110, E1661–1668. 10.1073/pnas.1305166110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cejka P., Cannavo E., Polaczek P., Masuda-Sasa T., Pokharel S., Campbell J.L., and Kowalczykowski S.C. (2010). DNA end resection by Dna2-Sgs1-RPA and its stimulation by Top3-Rmi1 and Mre11-Rad50-Xrs2. Nature 467, 112–116. 10.1038/nature09355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nimonkar A.V., Genschel J., Kinoshita E., Polaczek P., Campbell J.L., Wyman C., Modrich P., and Kowalczykowski S.C. (2011). BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev 25, 350–362. 10.1101/gad.2003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niu H., Chung W.H., Zhu Z., Kwon Y., Zhao W., Chi P., Prakash R., Seong C., Liu D., Lu L., et al. (2010). Mechanism of the ATP-dependent DNA end-resection machinery from Saccharomyces cerevisiae. Nature 467, 108–111. 10.1038/nature09318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ceppi I., Cannavo E., Bret H., Camarillo R., Vivalda F., Thakur R.S., Romero-Franco A., Sartori A.A., Huertas P., Guerois R., and Cejka P. (2023). PLK1 regulates CtIP and DNA2 interplay in long-range DNA end resection. Genes Dev 37, 119–135. 10.1101/gad.349981.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ceppi I., Howard S.M., Kasaciunaite K., Pinto C., Anand R., Seidel R., and Cejka P. (2020). CtIP promotes the motor activity of DNA2 to accelerate long-range DNA end resection. Proc Natl Acad Sci U S A 117, 8859–8869. 10.1073/pnas.2001165117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alani E., Padmore R., and Kleckner N. (1990). Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell 61, 419–436. 10.1016/0092-8674(90)90524-i. [DOI] [PubMed] [Google Scholar]
- 92.McKee A.H., and Kleckner N. (1997). A general method for identifying recessive diploid-specific mutations in Saccharomyces cerevisiae, its application to the isolation of mutants blocked at intermediate stages of meiotic prophase and characterization of a new gene SAE2. Genetics 146, 797–816. 10.1093/genetics/146.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prinz S., Amon A., and Klein F. (1997). Isolation of COM1, a new gene required to complete meiotic double-strand break-induced recombination in Saccharomyces cerevisiae. Genetics 146, 781–795. 10.1093/genet-ics/146.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lam I., and Keeney S. (2014). Mechanism and regulation of meiotic recombination initiation. Cold Spring Harb Perspect Biol 7, a016634. 10.1101/cshperspect.a016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hua S.B., Qiu M., Chan E., Zhu L., and Luo Y. (1997). Minimum length of sequence homology required for in vivo cloning by homologous recombination in yeast. Plasmid 38, 91–96. 10.1006/plas.1997.1305. [DOI] [PubMed] [Google Scholar]
- 96.Inbar O., Liefshitz B., Bitan G., and Kupiec M. (2000). The relationship between homology length and crossing over during the repair of a broken chromosome. J Biol Chem 275, 30833–30838. 10.1074/jbc.C000133200. [DOI] [PubMed] [Google Scholar]
- 97.Ira G., and Haber J.E. (2002). Characterization of RAD51-independent break-induced replication that acts preferentially with short homologous sequences. Mol Cell Biol 22, 6384–6392. 10.1128/MCB.22.18.6384-6392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jinks-Robertson S., Michelitch M., and Ramcharan S. (1993). Substrate length requirements for efficient mitotic recombination in Saccharomyces cerevisiae. Mol Cell Biol 13, 3937–3950. 10.1128/mcb.13.7.3937-3950.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manivasakam P., Weber S.C., McElver J., and Schiestl R.H. (1995). Micro-homology mediated PCR targeting in Saccharomyces cerevisiae. Nucleic Acids Res 23, 2799–2800. 10.1093/nar/23.14.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang P.C., Hong S., Mimitou E.P., Kim K.P., Murakami H., and Keeney S. (2024). Meiotic DNA break resection and recombination rely on chromatin remodeler Fun30. bioRxiv. 10.1101/2024.04.17.589955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bothmer A., Rommel P.C., Gazumyan A., Polato F., Reczek C.R., Muellenbeck M.F., Schaetzlein S., Edelmann W., Chen P.L., Brosh R.M. Jr., et al. (2013). Mechanism of DNA resection during intrachromosomal recombination and immunoglobulin class switching. J Exp Med 210, 115–123. 10.1084/jem.20121975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reczek C.R., Shakya R., Miteva Y., Szabolcs M., Ludwig T., and Baer R. (2016). The DNA resection protein CtIP promotes mammary tumorigenesis. Oncotarget 7, 32172–32183. 10.18632/oncotarget.8605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baudat F., Manova K., Yuen J.P., Jasin M., and Keeney S. (2000). Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell 6, 989–998. 10.1016/s1097-2765(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 104.Maekawa K., Yamada S., Sharma R., Chaudhuri J., and Keeney S. (2022). Triple-helix potential of the mouse genome. Proc Natl Acad Sci U S A 119, e2203967119. 10.1073/pnas.2203967119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Canela A., Maman Y., Huang S.N., Wutz G., Tang W., Zagnoli-Vieira G., Callen E., Wong N., Day A., Peters J.M., et al. (2019). Topoisomerase II-Induced Chromosome Breakage and Translocation Is Determined by Chromosome Architecture and Transcriptional Activity. Mol Cell 75, 252–266 e258. 10.1016/j.molcel.2019.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Langmead B., Trapnell C., Pop M., and Salzberg S.L. (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human ge-nome. Genome Biol 10, R25. gb-2009-10-3-r25 [pii] 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wienert B., Wyman S.K., Yeh C.D., Conklin B.R., and Corn J.E. (2020). CRISPR off-target detection with DISCOVER-seq. Nat Protoc 15, 1775–1799. 10.1038/s41596-020-0309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brick K., Pratto F., Sun C.Y., Camerini-Otero R.D., and Petukhova G. (2018) Analysis of Meiotic Double-Strand Break Initiation in Mammals. Methods Enzymol 601, 391–418. 10.1016/bs.mie.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peterson S.E., Keeney S., and Jasin M. (2020). Mechanistic Insight into Crossing over during Mouse Meiosis. Mol Cell 78, 1252–1263 e1253. 10.1016/j.molcel.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boekhout M., Karasu M.E., Wang J., Acquaviva L., Pratto F., Brick K., Eng D.Y., Xu J., Camerini-Otero R.D., Patel D.J., and Keeney S. (2019). REC114 Partner ANKRD31 Controls Number, Timing, and Location of Meiotic DNA Breaks. Mol Cell 74, 1053–1068 e1058. 10.1016/j.mol-cel.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peters A.H., Plug A.W., van Vugt M.J., and de Boer P. (1997). A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res 5, 66–68. 10.1023/a:1018445520117. [DOI] [PubMed] [Google Scholar]
- 113.Lam I., Mohibullah N., and Keeney S. (2017). Sequencing Spo11 Oligonucleotides for Mapping Meiotic DNA Double-Strand Breaks in Yeast. Methods Mol Biol 1471, 51–98. 10.1007/978-1-4939-6340-9_3. [DOI] [PubMed] [Google Scholar]
- 114.Xu J., Li T., Kim S., Boekhout M., and Keeney S. (2023). Essential roles of the ANKRD31-REC114 interaction in meiotic recombination and mouse spermatogenesis. Proc Natl Acad Sci U S A 120, e2310951120. 10.1073/pnas.2310951120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw and processed sequencing S1-, Exo7/T-seq, MRE11-ChIP, and SSDS data have been deposited in the Gene Expression Omnibus (GEO) repository under accession numbers GSE266258, GSE266151, and GSE266271. We used additional S1-seq and Exo7/T-seq data from GEO accession numbers GSE265863 and GSE22945034,114. We used SPO11-oligo data from GEO accession numbers GSE84689 and Atm−/− MRE11 ChIP-seq data from GEO accession GSE13891514,37.