Abstract
Peripuberty is a significant period of neurodevelopment with long-lasting effects on the brain and behavior. Blocking type 1 corticotropin-releasing factor receptors (CRFR1) in neonatal and peripubertal rats attenuates detrimental effects of early-life stress on neural plasticity, behavior, and stress hormone action, long after exposure to the drug has ended. CRFR1 antagonism can also impact neural and behavioral development in the absence of stressful stimuli, suggesting sustained alterations under baseline conditions. To investigate this further, we administered a CRFR1 antagonist (CRFR1a), R121919, to young adolescent male and female rats across 4 days. Following each treatment, rats were tested for locomotion, social behavior, mechanical allodynia, or PPI of the acoustic startle reflex. Acute CRFR1 blockade immediately reduced PPI in peripubertal males, but not females. In adulthood, each assay was repeated without CRFR1a exposure to test for long-term effects of the adolescent treatment, with males continuing to experience deficits in PPI, while females displayed altered locomotion, PPI, and social behavior. The amygdala was collected to measure long-term effects on gene expression in pathways related to neural plasticity and neurodevelopmental disorders. Relative expression of cannabinoid type 1 receptors (CB1R), which mediate sensorimotor and HPA function, was also measured. In the adult amygdala, peripubertal CRFR1a induced alterations in pathways related to neural plasticity and stress in males and lower expression of CB1R protein in females. Understanding how acute exposure to neuropharmacological agents can have sustained impacts on brain and behavior, in the absence of further exposures, has important clinical implications for adolescent psychiatric treatment protocols.
Keywords: adolescence, puberty, corticotropin releasing factor, neuroplasticity, sex differences, amygdala, transcriptomics
Introduction
Corticotropin-releasing factor (CRF) is a major regulator of the stress response through its actions on the hypothalamic-pituitary-adrenal (HPA) axis and its associated receptors (CRFR) type 1 and 2 (Binder and Nemeroff, 2010). CRF works in brain regions such as the amygdala and hippocampus to alter neural plasticity, learning and memory, and future responses to stressors (Vandael et al., 2021). Stress exposure during sensitive periods of early development can have long-lasting effects, including the upregulation of associated CRF receptors in the brain (Veenit et al., 2014; Núñez Estevez et al., 2020; Zhao et al., 2021). Other effects include an increased risk of developing cognitive deficits and psychopathologies, such as anxiety disorder and schizophrenia in humans (Carr et al., 2013; Smith and Pollak, 2020) and associated symptoms in other animals (Fenoglio et al., 2005; Wang et al., 2012; Toth et al., 2015; Veenit et al., 2014; Hu et al., 2020; Núñez Estevez et al., 2020; Zhao et al., 2021). Treatment with CRFR1 antagonists (CRFR1a) at sensitive periods, such as during neonatal development or in peripuberty, can reduce neural and behavioral impairments in animal models of early life stress (Ivy et al., 2010; Short et al., 2020; Veenit et al., 2014). In these studies, a week-long exposure to the drug attenuated hippocampal deficits in long-term potentiation (LTP), prevented hippocampal increases in CRF, and mitigated associated changes in social, affective, and cognitive functioning in male rodents. Notably, these rescue effects were observed months after treatment exposure. Moreover, peripubertal CRFR1 blockade alone, in the absence of a negative experience also affected the stress response in later life (Veenit et al., 2014). This highlights that CRFR1 deficiency, and not only upregulation, can shape behavioral and cognitive functioning. Together, these data are suggestive of sustained changes in neural- and behavioral plasticity following alterations to CRFR1 functioning that occur during discrete developmental periods such as puberty.
The short- and long-term behavioral changes brought on by exposure to new experiences and environments are the result of neuroplasticity, which occurs when the brain changes how it functions in response to these novel experiences. Stress-related brain systems are particularly sensitive to these changes in synaptic function and plasticity (Chen et al., 2012; Dos-Santos et al., 2023). For example, CRF acting in the amygdala has been shown to enhance excitability of CRFR1 neurons and trigger changes in neural signaling, leading to long-lasting changes in sensorimotor gating, anxiety-like behavior, and fear discrimination (Rajbhandari et al., 2015; Rainnie et al., 2004; Sanford et al., 2017). Neural and behavioral alterations are facilitated by expression changes in genes related to plasticity, such as those encoding for Eph receptor A4 and adenosine receptor A2a (Epha4 Deininger et al., 2008; Adora2a; Simões et al., 2016), as well as genes associated with neurodevelopmental disorders (glial fibrillary acidic protein, gfap; teashirt zinc finger homeobox, Tshz3; Herrero et al., 2020; Caubit et al., 2016) and stress (CRF receptors Crhr1 and Crhr2). Furthermore, CRF has been shown to directly regulate synaptic function and subsequent behavioral outcomes through activation of cannabinoid receptors (Jamieson et al., 2022; Ruat et al., 2021). These stress-sensitive pathways are particularly malleable during sensitive periods of development such as early adolescence (Filetti et al., 2023).
In the absence of stressors, studies in adult rodents have found that neither acute nor chronic blockade of CRFR1 affected circulating levels of corticosterone or adrenocorticotropic hormone (ACTH; Keck et al., 2001; Gutman et al., 2011). However, while acute CRFR1a administration had no effect on sensorimotor gating as measured by the extent of pre-pulse inhibition (PPI) of the acoustic startle reflex (Sutherland and Conti, 2011), chronic infusions of CRFR1a have been shown to reduce anxiety-like behaviors in defensive withdrawal tasks in control animals that were not exposed to stressful stimuli (Aborelius et al., 2000; Gutman et al., 2011). Gutman and colleagues (2011) also observed that the density of CRF mRNA in the central nucleus of the amygdala (CeA) decreased after chronic blockade of CRFR1, which was likely tied to reductions in anxiety-like behavior (McCall et al., 2015). Taken together, these data suggest that CRFR1a may modulate some behaviors under baseline conditions when a stressful stimulus is not present. However, these studies only evaluated adult male rats, which does not account for known sex differences in CRF and stress circuit functioning (Bangasser et al., 2019; Salvatore et al., 2018), nor do they consider the influences of various developmental periods. Stress, or lack thereof, has been shown to influence the brain more prominently during early life and during peripuberty, compared to adulthood (Kirby et al., 2013; Lee and Jung, 2024). More research is needed to understand how acute and chronic blockade of CRFR1 at discrete time points across development might affect behavioral function in both male and female animals.
Although CRFR1a prevents adverse effects associated with a stressful experience (Ivy et al., 2010; Short et al., 2020; Veenit et al., 2014), it is also important to understand how altered functioning of CRF receptors may affect neural plasticity and behavior in the absence of explicit stressful stimuli. Clinically, individuals can receive neuropharmacological interventions during acute, chronic, or even under low or “regular” stress conditions. Moreover, general increases in pediatric prescribing rates have prompted policies and practices to help prevent the inappropriate use of medications during childhood and adolescence (Kelleher et al., 2020). Understanding the mechanisms of how exposure to psychoactive agents at defined developmental periods can continue to influence brain function and behavior, under different environmental conditions and without further medication exposure, is crucial for improving treatment protocols in adolescent psychiatry. This is relevant for CRFR1 antagonists where there has been a sustained interest for their clinical potential amongst the scientific community (Alizamini et al., 2023; Künzel et al., 2003; Lv et al., 2022; Schreiber et al., 2018; Zorilla & Koob, 2010). To this end, we acutely administered a CRFR1a, without an accompanying stressor, to male and female rats across 4 days during early adolescence. One hour after each drug treatment, rats were tested for locomotor behavior, social preference, social discrimination, mechanical allodynia, or PPI of the acoustic startle reflex. These behavioral tests were repeated in adulthood, but without CRFR1a treatment, and amygdala samples were collected to assess whether blockade of CRFR1 during early adolescence imparts long-term changes in genes related to neural plasticity and stress responses. Lastly, because alterations in cannabinoid type 1 receptor (CB1R) expression are associated with impaired sensorimotor gating (Ortega-Álvaro et al, 2015), as well as altered HPA responses and anxiety (Bourque et al., 2008), expression of CB1R in the amygdala was also quantified.
Experimental Procedures
Animals and Experimental Overview
Male and female Sprague Dawley rats were purchased from Charles River Laboratories (Males: Wilmington, MA; Females: Raleigh, NC) and housed with ad libitum access to food and water on a light/dark cycle (0700–1900 light) at 20°C. After habituating to the facility, animals were bred (1 male to 2 females) and pregnancy confirmed by the visualization of spermatozoa in vaginal samples, a sustained diestrus phase, and constant body weight gain. In line with the 3Rs of animal research’s principle of Reduction (NC3Rs, n.d), the experimental subjects from this study were siblings retained at weaning from a previous maternal immune activation study (Martz et al., 2024) whose mothers were control dams treated with saline on gestational day 15. All animal procedures were approved by the Massachusetts College of Pharmacy and Health Sciences (MCPHS) Institutional Animal Care and Use Committee and were carried out in compliance with the Association for Assessment and Accreditation of Laboratory Animal Care. The experimental procedures and timeline are presented in Fig. 1A.
Figure 1.
Experimental Timeline
Day of birth was designated as postnatal day (P)1 and offspring were culled to 10 pups per litter (5 males and 5 females, wherever possible), staying with their mothers until weaning on P21. At that point, offspring were placed into clean cages in same-sex pairs where they were maintained throughout the rest of the study. Offspring were treated with four daily subcutaneous injections of either the selective CRFR1a R121919 (CRFR1a; 10mg/kg, MCE MedChemExpress, Monmouth, NJ) or pyrogen free saline (vehicle control), 1-hour prior to behavioral testing. Specifically, on P30, animals received either CRFR1a or vehicle exposure and were evaluated on their locomotor activity followed by a social preference test. On P31 animals were evaluated on their social discrimination ability. Prepulse inhibition (PPI) and von Frey tests were counterbalanced between P32 and P33. We selected a dose of 10 mg/kg because others have reported behavioral effects with R121919 administration at this dose, administration route, and behavioral testing timeframe (Wood et al., 2012; Wood et al., 2013). Selective receptor occupancy for CRFR1a in the brain has been confirmed using peripherally administered doses between 2.5 mg/kg to 20 mg/kg, 1-hour after R121919 treatment (Heinrichs et al., 2002). To test the potential for CRFR1a to induce enduring neuroplasticity, offspring were again evaluated on these behavioral metrics at >P90 one week prior to brain tissue collection by P100. Each measure included only one male and one female from each litter (see Table 1).
Table 1.
Total number of animals and litters included on each measure.
| Treatment | Number of Offspringa Included in Adolescent Behavior Analyses | Number of Offspringa Included in Adult Behavior Analyses | Number of Offspringa Included in Adult Western Blot Analyses | Number of Offspringa Included in Adult RNASeq Analyses |
|---|---|---|---|---|
| Saline | Male: 8 Female: 8 |
Male: 8 Female: 8 |
Male: 5 Female: 5 |
Male: 3 Female: 3 |
| CRFR1a | Male: 8 Female: 8 |
Male: 8 Female: 8 |
Male: 5 Female: 5 |
Male: 3 Female: 3 |
CRFR1a: corticotropin-releasing factor receptor 1 antagonist
Only one male and one female offspring were evaluated from each litter.
Offspring Behavioral Analyses
Locomotor activity (P30 & >P90) was evaluated using automated behavioral monitoring software (Any-maze, Wood Dale, IL) to record distance travelled (m; Yan & Kentner, 2017; Connors et al., 2014; Núñez Estevez et al., 2020). This was followed by a five-minute social preference test (P30 & >P90) which consisted of two cleaned wire containment cups placed on each end of an arena. One cup held a novel untreated rat of the same sex, age, size, and strain and the other cup held a novel object. The location of novel rats (n = 10) and objects was interchanged between trials. Rats were scored as actively investigating when their nose was directed within 2 cm of a containment cup, or it was touching the cup. A social preference index was calculated by the equation ([time spent with the rat] / [time spent with the inanimate object + time spent with the rat]) − 0.5 ; Scarborough et al., 2020) and the number of visits to the novel rat was recorded. Twenty-four hours later (P31 & >P91), animals were placed back into the arena for an evaluation of social discrimination. This test was run in an identical manner to the social preference test except both cups held a novel untreated rat of the same sex, age, size, and strain. In one cup was a novel rat, while the second cup held the familiar rat the experimental animal had been introduced to the day before. A social discrimination index was similarly calculated by the equation ([time spent with the novel rat] / [time spent with the familiar + time spent with the novel rat]) − 0.5).
PPI and von Frey tests were counterbalanced in terms of their presentation test day (e.g., P32/P33 or >P92/P93). For PPI of the acoustic startle reflex, animals were placed into acoustic startle chambers (San Diego Instruments, San Diego, CA, USA) and allowed to habituate for 300 sec prior to the test session. A 65 dB background noise was present throughout the habituation period and test session, even when there was no presentation of a stimulus during a trial. During stimulus trials, rats were exposed to a 40 ms pulse of 120 dB white noise with or without the presentation of a prepulse. Prepulse intensity consisted of one of three intensity types: 8, 12, or 16 dB greater than the background noise. No-stimulus, pulse-alone, and prepulse-plus-pulse trials were pseudorandomly presented 10 times, and the average trial interval was 15 ± 5 seconds. The % PPI for each prepulse intensity was calculated using the following formula: 1-(mean reactivity on prepulse-plus-pulse trials / mean reactivity on pulse alone trials) × 1/100 (Giovanoli et al., 2013).
With respect to the von Frey test, animals were habituated inside an acrylic cage with a wire grid floor for 30-minutes. Mechanical allodynia was evaluated using a pressure-meter which consisted of a hand-held force transducer fitted with a 0.7mm2 polypropylene tip (electronic von Frey anesthesiometer, IITC, Inc, Life Science Instruments, Woodland Hills, CA, USA). The tip was applied to the animal’s left hind paw with an increasing pressure until a flexion reflex occurred. The threshold of the applied weight (grams) was automatically recorded by the electronic pressure-meter when the animal withdrew its paw. The average of four test trials was calculated as the mechanical allodynia threshold (Yan & Kentner, 2017).
Western Blot Analyses
On approximately P100, a week after the adult behavioral tests, offspring were anesthetized with a mixture of ketamine/xylazine (150 mg/kg /50 mg/kg, i.p.). After intracardial perfusion with PBS, brains were removed, placed over ice and the amygdala dissected. Samples were frozen on dry ice and stored at −80°C until further processing. To measure cannabinoid receptor 1 (CB1R) expression in adult brain using western blotting, 30μg of protein was loaded into each well of MiniProtean® gels (Bio Rad Laboratories, Cat. #4568104). Gels were transferred onto nitrocellulose membranes (Bio Rad Laboratories, Cat. #1620147) and blocked in 5% nonfat milk with TBS + 0.05% Tween 20 (TBST) for 1 hour at room temperature (CB1). Membranes were washed with TBST 3×10 minutes and incubated in a 1:1000 dilution of primary antibody in TBS (Abcam Cat. #ab259323) solution overnight at 4°C. Following this, membranes were washed with TBST 3×5 minutes and incubated in an HRP-conjugated secondary antibody in a 1:1000 dilution (Abcam Cat. #ab131366), made in 1% nonfat milk with TBS, for 1 hour at room temperature. Membranes were then washed and exposed to chemiluminescent substrate for 5 minutes (Thermo Fisher Scientific, Cat. #34580) and then scanned with a LI-COR C-DiGit Scanner (Model # 3600). Membranes were then stripped (Thermo Fisher, Cat. # 21062) for 15 minutes at 37°C, blocked in 5% nonfat milk with TBST for 1 hour at room temperature, washed, and incubated in beta actin primary antibody (1:1000, Thermo Fisher Scientific, Cat. #MA515739) for 1 hour at room temperature. Membranes were again exposed to the chemiluminescence substrate for 5 minutes and imaged. Densitometry measures were used to obtain a ratio of Antibody target/β-actin to quantify differences between groups.
RNA Sequencing
Following RNA-isolation (see Martz et al., 2024), amygdala samples (n = 3 per group) were more broadly analyzed using RNA Sequencing (RNA-seq) by Azenta Life Sciences (South Plainfield, NJ, USA) as described previously (Martz et al., 2024). An Agilent TapeStation (Agilent Technologies, Palo Alto, CA, USA) system was used to run the library and an Illumina NovaSeq 6000 (Illumina, San Diego, CA, USA) was used to sequence the samples according to the manufacturer’s instructions using a 2×150bp Paired End (PE) configuration (see Martz et al., 2024). The CLC Genomics Workbench v.23.0.2 software (QIAGEN Digital Insights, Aarhus, Denmark) was used for differential expression (DE) analysis and genes with an absolute fold change (FC) > 1.3, a Benjamini–Hochberg corrected p-value < 0.05, and false discovery (FDR) value < 0.05, were considered significantly DE. Volcano plots were constructed using the log2fold change and −log10 p-value in GraphPad Prism v.9.5.1. Heatmaps were generated using the free browser program Morpheus (https://software.broadinstitute.org/morpheus). Rank-rank hypergeometric overlap (RRHO) was used as a threshold-free approach to determine the extent of concordance/discordance between transcriptomic datasets (Cahill et al., 2018).
Statistical Analyses
Statistical Software for the Social Sciences (SPSS) was used to perform statistical analyses. Because the behavioral and adult tissue datasets were not powered to evaluate sex-differences directly, male, and female animals were evaluated separately (Ordoñes Sanchez et al., 2021). One-way ANOVAs were applied for behavioral and western blot data as appropriate, except for the body weight and PPI data which we evaluated using repeated measures ANOVA. In the latter case, repeated measures ANOVA was performed to compare differences in %PPI across each trial type. Body weight was used as a covariate for the evaluation of mechanical allodynia in the von Frey test (Yan & Kentner, 2017). The partial eta-squared (np2) is reported as an index of effect size for the ANOVAs (Miles & Shevlin, 2001). All data are expressed as mean ± SEM.
RNA sequencing data were analyzed as previously described (Martz et al., 2024). Briefly, DESeq2 was employed to identify differentially expressed (DE) genes based on a p < 0.05, Benjamini–Hochberg false discovery rate corrected (FDR) and fold change (FC) > 1.3 and RRHO2 was used as a threshold-free approach to determine the extent of concordance/discordance between transcriptomic datasets (Cahill et al., 2018). Heatmaps were generated using the MultiExperiment Viewer (National Library of Medicine, USA) and gene ontology was determined using the Database for Annotation, Visualization and Integrated Discovery functional annotation cluster tool (https://david.ncifcrf.gov/).
Results
CRFR1 antagonism has acute and sex-dependent effects on adolescent behavior
Repeated subchronic administration of CRFR1a, R121919 did not affect body weight in either male or female adolescent rats (p>0.05, Supplemental Fig. 1A,B). Similarly, acute CRFR1 antagonism did not impact adolescent locomotor activity, social preference, the number of visits made to a social conspecific (p>0.05, Fig. 2A,B,C), nor was social discrimination or mechanical allodynia affected (p>0.05, Supplemental Fig. 2A,B). Systemic blockade of CRFR1 did however interrupt percent inhibition of the acoustic startle reflex, or %PPI, which was used to assess sensorimotor gating. A significant main effect of CRFR1a showed attenuated %PPI response across the 77 dB and 81 dB prepulse intensities in male adolescents (77 dB: F(1, 14) = 10.174, p = 0.007, np2 = 0.439; 81 dB: F(1, 14) = 8.080, p = 0.014, np2 = 0.383, Fig. 2D). Mean %PPI was calculated by collapsing %PPI across all dB intensities. There was a main effect of CRFR1 antagonism, where mean %PPI was reduced in males exposed to R121919 (F(1, 14) = 7.962, p = 0.014, np2 = 0.363, Fig. 2E).
Figure 2. Adolescent behavioral functioning is altered in a sex-specific manner following acute CRFR1 antagonism.
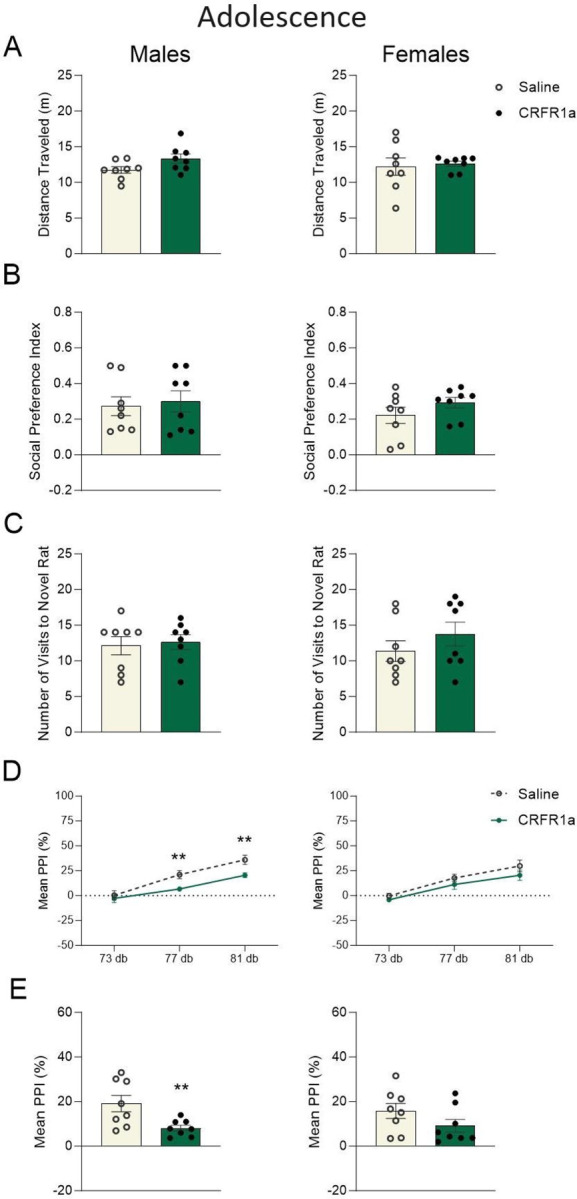
Graphs display male (left) and female (right) data for adolescent A) total distance traveled (m), B) social preference index, C) total number of visits to a novel rat in the social preference test, D) percent prepulse inhibition (PPI%) of the acoustic startle reflex across each trial type, and (E) mean %PPI collapsed across all trial types. Data are expressed as mean ±SEM. *p < 0.05, **p < 0.01, Saline versus adolescent CRFR1 antagonism, n = 8.
CRFR1 antagonism during peripuberty has sustained and sex-dependent effects on behavioral plasticity
One-way ANOVA revealed that acute peripubertal CRFR1a exposure was associated with increased locomotor activity in female adult rats (F(1, 14) = 10.675, p = 0.006, np2 = 0.443, Fig. 3A). While the social preference index was not affected (p>0.05, Fig. 3B), adult females exposed to pubertal CRFR1a displayed a higher number of visits to their novel conspecifics (F(1, 14) = 4.173, p = 0.048, np2 = 0.252, Fig. 3C). This effect appeared to be specific to the social component of the test as female animals did not differ in the number of visits to the novel object (p>0.05; Female Saline: 4.13±0.40 versus Female CRHR1a: 4.50±0.73; Male Saline: 7.38±1.10 versus Male CRFR1a: 6.00±0.60). Moreover, adolescent CRFR1a was associated with modest, yet persistent reductions in the acoustic startle response of adult male and female rats, despite there being no active drug exposure at the time of testing (Males (81 dB): F(1, 14) = 5.510, p = 0.034, np2 = 0.282; Females (73 dB): F(1, 14) = 7.028, p = 0.019, np2 = 0.334, Fig. 3D). %Mean PPI (p > 0.05, Fig. 3E), social discrimination, and mechanical allodynia were not affected in adulthood of either males or females (p > 0.05, Supplemental Fig. 2C,D).
Figure 3. Adolescent exposure to CRFR1 antagonism induced prolonged and sex-specific effects on behavioral plasticity.
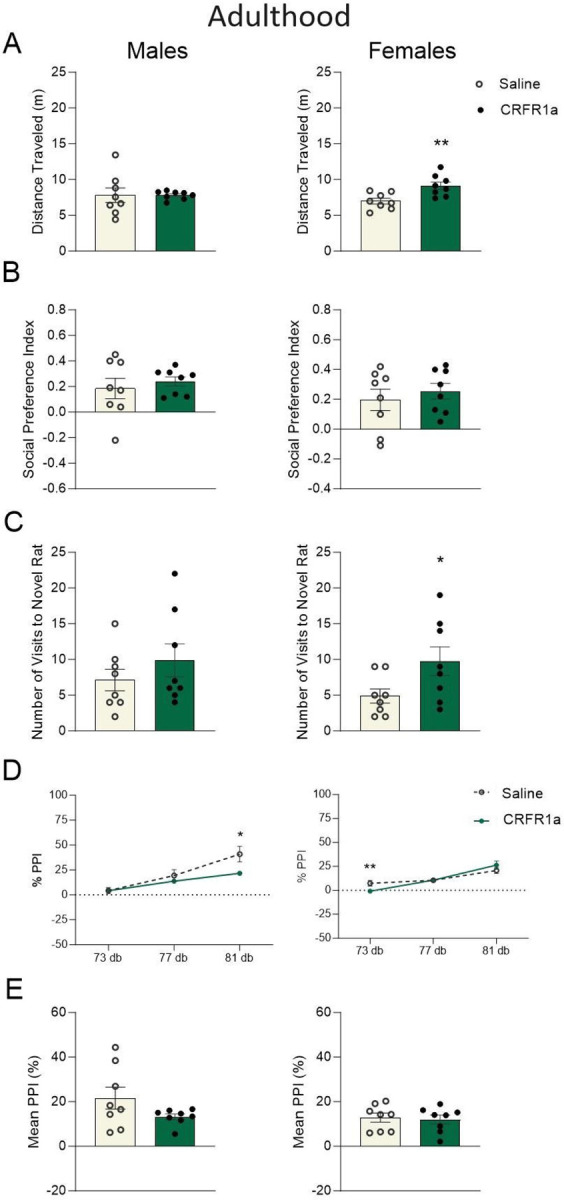
Graphs display male (left) and female (right) data for adult A) total distance traveled (m), B) social preference index, C) total number of visits to a novel rat in the social preference test, D) percent prepulse inhibition (PPI%) of the acoustic startle reflex across each trial type, and (E) mean %PPI collapsed across all trial types. Data are expressed as mean ±SEM. *p < 0.05, **p < 0.01, Saline versus adolescent CRFR1 antagonism, n = 8.
Peripubertal CRFR1 antagonism imparted lasting effects on adult neurophysiology and neuroplasticity markers
CB1 receptor expression was decreased in the amygdala of female animals exposed to CRFR1 antagonism during puberty (F(1, 14) = 7.981, p = 0.022, np2 = 0.499, Fig. 4A), but was unchanged in males (p > 0.05, Fig. 4A).
Figure 4. Adolescent CRFR1 antagonism reduced cannabinoid receptor 1 expression in a sex-dependent manner.
Graphs display male (left) and female (right) data for adult A) cannabinoid receptor 1 (CB1) protein expression in the amygdala. Data are expressed as mean ±SEM. *p < 0.05, **p < 0.01, Saline versus adolescent CRFR1 antagonism, n = 8.
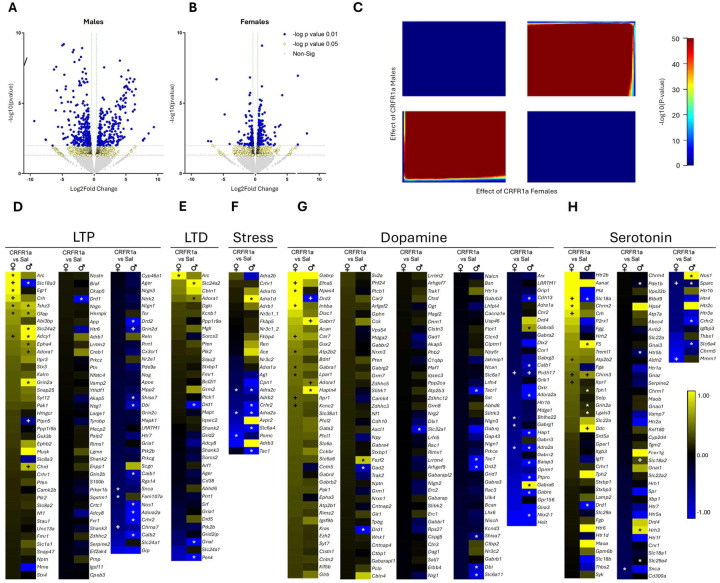
RNA sequencing was performed on amygdala tissue of adult male and female animals to evaluate the long-term transcriptional effects of peripubertal CRFR1a exposure. In males, 219 genes were significantly DE by CRFR1a (q<0.05 versus Saline; Fig. 5A) and 31 genes were significantly DE by CRFR1a exposure in females (q<0.05; Fig. 5B). Threshold-free RRHO analysis showed substantial overlap in gene expression profiles across males and females as a function of CRFR1a exposure (Fig. 5C). Supplemental Fig. 3 shows the top pathways affected by peripubertal CRHR1a and include pathways related to behavior and cognition, GABAergic synapses, and neuron projection development in males and cell junction organization, regulation of nervous system development, and locomotor behavior in females. Supplemental Results Tables 1, 2, and 3 show the sex specificity of these effects. Notably, the modulation of chemical synaptic transmission was affected by CRHR1a in both sexes. Heatmap clustering identified alterations in genes belonging to long-term potentiation (LTP; Fig. 5D) and long-term depression (LTD; Fig. 5E), in addition to stress (Fig. 5F), dopamine (Fig. 5G), and serotonin (Fig. 5H) pathways in peripubertal CRFR1a exposed males and females. Pathways related to glucocorticoid binding, oxytocin, cannabinoids, glutamate/GABA, parvalbumin, and microglia were also identified (Supplemental Fig. 4A–F). For more information on heatmap clustering, see Supplemental Results Table 1. Threshold-free RRHO analysis showed a high degree of overlap between the effect of sex in controls and the effect of sex in CRFR1a exposed rats (Supplemental Fig. 5).
Figure 5. Transcriptomic analyses of amygdala tissue samples collected from adult male and female animals following peripubertal exposure to CRFR1a.
Volcano plots for differentially expressed (DE) transcripts in A) male (1064 genes) and B) female (31 genes) rats, with −log10 pvalue on the y-axis and log2 FC on the x-axis. Each dot represents a gene, with colors indicating the magnitude of significance (yellow = p < 0.05, FC > 1.3; blue = p < 0.01, FC > 1.3). Threshold-free RRHO analysis indicates that the transcriptional alterations that occur as a function of C) peripubertal CRFR1a exposure largely overlap between males and females. Heatmaps of D) long-term potentiation (LTP), E) long-term depression (LTD), F) stress, G) dopamine, and H) serotonin related genes, with effects represented as FC between Saline and CRFR1a exposed males, and between Saline and CRFR1a exposed females. *Genes that reached the Benjamini-Hochberg corrected effect of 20%. +Genes with p < 0.05 and FC > 1.3.
Discussion
In the present study, we replicate and extend on evidence demonstrating that temporary blockade of CRFR1 during the peripubertal period induces long-term programming effects on adult behavior (Vennit et al., 2014). Our own findings suggest that this behavioral programming can occur in both male and female rats and is likely due to persistent changes in gene pathways associated with neural plasticity and stress. Injections of the CRFR1a, R121919, immediately reduced PPI of the acoustic startle reflex in adolescent males, but not females, suggesting that adolescent male rats may be more sensitive to the effects of this drug. In adulthood, CRFR1a exposed males continued to show deficits in PPI, while alterations in locomotion, PPI, and social behavior emerged in their female CRFR1a counterparts. Although the behavioral programming effects of CRFR1 antagonism were more evident in adult females, transcriptomic data revealed that males experienced greater changes in gene expression in the amygdala. In contrast, adult CRFR1a females had lower relative expression of CB1R protein in the amygdala compared to control animals, while no differences were present in males. This indicates that changes to the endocannabinoid system may contribute to some of the behavioral changes observed in females. Overall, these findings identify peripuberty as a sensitive period for disruption of CRFR1 signaling, even in the absence of a stressor.
Although this work did not investigate the effects of CRFR1 blockade in the context of stress, adolescent administration of R121919 had long-term effects on the expression of genes in the amygdala related to stress and anxiety in both males and females. CRFR2, encoded by the gene Crhr2, performs functions distinct from those facilitated by CRFR1, but the two receptors work in concert to orchestrate stress responses (Kishimoto et al., 2020; Vasconcelos et al., 2020). In our males, temporary blockade of CRFR1 during the peripubertal period caused long-term downregulation of Crhr2 in adulthood, indicating that stress signaling in the amygdala was permanently affected. In addition, expression of the genes encoding for Protachykinin-1 (Tac1) and solute carrier family 6 member 4 (Slc6a4) were also downregulated in males, but not females. Slc6a4 knockout mice demonstrated increased anxiety-like behavior and potentiated HPA stress responses (Holmes, 2008), while upregulation of the Tac1 gene in the amygdala has been shown to occur in response to activation of glucocorticoid receptors and is thought to induce anxiety-like behaviors (Hay et al., 2014). In our females, the gene encoding for proopiomelanocortin (Pomc), the protein responsible for producing ACTH and therefore corticosterone, was downregulated in CRFR1a females but not in CRFR1a males. In the amygdala, expression of proopiomelanocortin mRNA has been shown to increase following a foot shock stressor in rats (Yamano et al., 2004). In sum, dysregulation of these genes in our CRFR1a-treated rats may be indicative of permanent changes to stress systems in the brain, particularly among males. Therefore, dysregulation of these genes in our CRFR1a males may be indicative of permanent changes to stress systems in the brain.
In addition to its role in stress signaling, the amygdala is an important modulator of behaviors involving social tasks and sensorimotor gating (Ko, 2017; Wan and Swerdlow, 1997; Cano et al., 2021). Furthermore, CRFR1 is densely expressed in the amygdala (Dedic et al., 2018; Wolfe et al., 2019) and has been implicated in the modulation of social behavior and PPI in rodents (Hostetler and Ryabinin, 2013; Risbrough et al., 2004; Groenink et al., 2008; Sutherland and Conti, 2011). In a social preference task, adult female mice treated with CRFR1a 1 hour before testing spent less time with a novel mouse compared to a novel object. In contrast, CRFR1a males had a stronger preference for their novel conspecific (Piccin and Contarino, 2020). In our study, CRFR1a administered to peripubertal rats 1 hour before a social preference task had no immediate effects in either males or females. It is unclear if the discrepancy in our findings compared to those of Piccin and Contarino (2020) is due to the difference in species, CRFR1a drug, the disparate ages of the animals, or a combination of these factors. When our animals were tested in adulthood, although there was still no effect on social preference, adult CRFR1a females surprisingly made a greater number of visits to the novel rat than did saline controls. Gene expression data from the amygdala revealed that three genes previously implicated in sociability: dopamine receptor D2 (Drd2), oxytocin (Oxt), and calbindin 1 (Calb1; Harris et al., 2016; Froemke and Young, 2021; Ike et al., 2023), were downregulated in our adult CRFR1a males, but not CRFR1a females. This suggests that male rats may have experienced some neural compensation, protecting them against CRFR1a-induced changes in social behavior. Moreover, Harris and colleagues (2016) found that the gene encoding for CRFR1 (Crhr1) was downregulated in the amygdala of male, but not female, Calb1 knockout mice, further indicating that calbindin and CRFR1 have interdependent patterns of expression in males. Taken together, sex differences in amygdalar function may underlie the discrepancies in social behavior following adolescent CRFR1a exposure in male and female rats.
With respect to sensorimotor gating, 1) adult male rats that received infusions of CRF or 2) adult male mice that were bred to overexpress CRF, each exhibited reductions in PPI that were rescued by administration of CRFR1 antagonism (Risbrough et al., 2004; Groenink et al., 2008). Conversely, Sutherland and Conti (2011) found that PPI was not altered by CRFR1a in adult male rats that had experienced restraint stress, suggesting that the effects of CRFR1a on PPI may vary based on stress experience and physiological state. Here we show that peripubertal administration of a CRFR1a, under basal conditions, reduced PPI both acutely and chronically for male rats, but only in adulthood for female rats. Divergent sensorimotor gating behaviors have been associated with neurodevelopmental disorders such as autism and schizophrenia (Perry et al., 2007; San-Martin et al., 2020), and we found that expression of genes associated with these disorders (glial fibrillary acidic protein, Gfap; teashirt zinc finger homeobox, Tshz3; cadherin-13, Cdh13; cholinergic receptor muscarinic 2, Chrm2; Herrero et al., 2020; Caubit et al., 2016; Sohal and Rubenstein, 2019; Boiko et al., 2020) was altered in both male and female amygdalae. In males and females exposed to CRFR1a, Gfap and Tshz3 increased expression, while Chrm2 was upregulated in females only. Clinical data has also implicated Cdh13 in Attention Deficit/Hyperactivity Disorder (ADHD; Mavroconstanti et al., 2013). Interestingly, Cdh13 was downregulated in our CRFR1a males, but not in the CRFR1a females that displayed increased locomotion in the open field test. Notably, Cdh13 has also been identified as an important regulator of the inhibitory/excitatory balance between glutamate and GABA neurons (Mossink et al., 2022), a relationship that is often disrupted in individuals with neurodevelopmental disorders (Gao and Penzes, 2015). Among the males that received the CRFR1a, expression of four other genes related to GABAergic signaling (Gabra6, Gabra5, Gabre, and Gad2) was dysregulated, while expression of only one GABA-related gene (Gabrg1) was altered among CRFR1a females. GABAergic neurons act in the amygdala to inhibit inappropriate behavioral responses, so disruptions in this system could lead to detrimental changes in behavior (Jie et al., 2018). In sum, the sex differences in expression of genes related to neurodevelopmental disorders and excitatory/inhibitory balance may have contributed to the disparity in behavioral deficits and age of onset between males and females exposed to the CRFR1a.
Changes in neural plasticity induced by CRFR1a have previously been shown, but in the context of subsequent exposure to stress (Ivy et al., 2010; Short et al., 2020). Here, we also find that many of the genes influenced by CRFR1 exposure relate to synaptic plasticity and metaplasticity, which, as a family, underwent the most pronounced changes in expression. In our CRFR1a males, there were changes in the expression of several genes related to plasticity, including Eph receptor A4 (Epha4; Deininger et al., 2008), shisa family member 7 (Shisa7; Schmitz et al., 2017), nitric oxide synthase 1 (Nos1; Hardingham et al., 2013), phosphodiesterase (Pdelb; McQuown et al., 2019), galectin 3 (Lgals3; Chen et al., 2017), and adenosine receptor A2a (Adora2a; Simões et al., 2016). Adenosine receptor A2a is a receptor shown to be involved in synaptic plasticity of excitatory inputs to the amygdala (Simões et al., 2016; Harris et al., 2018). In our study, amygdalar expression of Adora2 was downregulated in males as a function of peripubertal CRHR1a; interestingly, Adora2 has previously been shown to disrupt fear acquisition and fear memory in male mice (Simões et al., 2016). Likewise, Epha4 has been shown to facilitate LTP in the amygdala (Deininger et al., 2008), but Epha4 was dysregulated in the males exposed to the CRFR1a. In females compared to males, changes in gene expression were present, but largely among a disparate set of genes related to plasticity. Altered expression in females was observed in the plasticity-associated genes encoding for Wnt family member 7B (Wnt7b; McLeod and Salinas, 2018), alpha-synuclein (Snca; Cheng et al., 2011), Neuronal PAS domain-containing protein 4 (Npas4; Fu et al., 2020), early growth response 1 (Egr1; Duclot and Kabbaj, 2017), and activity-regulated cytoskeleton-associated protein (Arc; Korb and Finkbeiner, 2011). Within the amygdala, Arc-expressing neurons have been found to facilitate fear memory formation in mice through synaptic modifications (Gouty-Colomer et al., 2016). Although we did not assess fear behavior or learning and memory, it is likely that peripubertal blockade of CRFR1 would influence adult fear and memory formation/consolidation, based on the genes affected in both males and females. Another potential influence on neural and behavioral plasticity comes from the activity of CB1R (Chevaleyre and Castillo, 2004), of which relative protein expression in the amygdala was decreased in CFRF1a females compared to controls, but not in males. Postsynaptic release of an endocannabinoid can inhibit activation of a presynaptic GABAergic cell through activation of CB1R, which inhibits long term depression (LTD) and subsequently facilitates LTP (Chevaleyre and Castillo, 2004; Monday et al., 2020). Decreased levels of CB1R protein in females may be indicative of less inhibition of inhibitory neurotransmission (LTD), and therefore less priming for future LTP induction.
In summary, this work identifies peripuberty as a malleable period of development, during which time the brain is sensitive to discrete and temporary changes in CRF signaling activity, even in the absence of a stressor. Blockade of CRFR1 for 4 days during early adolescence had long-lasting effects on synaptic plasticity and other neural domains, including dysregulation of genes implicated in neurodevelopmental disorders and stress, in both males and females. While many studies have shown that too much stress during adolescence negatively impacts neurodevelopment (Carr et al., 2013), it is also known that too little stress can have negative effects (Kirby et al., 2013). Here we see that an acute influence on central stress systems during early adolescence has long-lasting and sex-specific effects on neural and behavioral outcomes. These findings have important implications for the clinical use of psychoactive treatments during the peripubertal period, given their potential for far-reaching and sustained impacts on brain development and behavior.
Supplementary Material
Graphs display male (left) and female (right) data for adult A) adolescent and B) adult body weights (g). Data are expressed as mean ±SEM. *p < 0.05, **p < 0.01, Saline versus adolescent CRFR1 antagonism, n = 8.
Graphs display male (left) and female (right) data for adolescent (top panel) and adult (bottom panel) A,C) social discrimination and B,D) mechanical allodynia threshold (g) on the von Frey test. Data are expressed as mean ±SEM. *p < 0.05, **p < 0.01, Saline versus adolescent CRFR1 antagonism, n = 8.
A) Top pathways represented by transcripts differentially expressed in females (A) and males (B) as a function of CRFR1a.
Heatmaps of A) glucocorticoid binding-, B) oxytocin-, C) cannabinoid-, D) glutamate (Glu)/GABA -, E) parvalbumin-, and F) microglia-related genes, with effects represented as fold change (FC) between CRFR1a and Saline exposed males, and between CRFR1a and Saline exposed females. *Genes that reached the Benjamini-Hochberg corrected effect of 20%. +Genes with p<0.05 and FC>1.3.
Supplemental Figure 5. Threshold-free RRHO analysis showed a high degree of overlap between the effect of sex in controls and the effect of sex in CRFR1a exposed rats.
Supplemental Results Table 1. Gene Expression in Saline vs. CRFR1a Amygdala
Supplemental Results Table 2. Females CRFR1a vs Sal
Supplemental Results Table 3. Males CRFR1a vs Sal
Funding and Disclosures
This project was funded by NIMH under Award Numbers R15MH114035 (to ACK), R01MH120066 (to MLS), and the Massachusetts College of Pharmacy and Health Sciences (MCPHS) Center for Research and Discovery (T.J.L and S.K), and MCPHS Summer Undergraduate Research Fellow (SURF) program (T.J.L and S.K). The authors wish to thank Ms. Holly DeRosa and Ada Cheng for their technical assistance in earlier phases of this project. The authors would also like to thank the MCPHS Schools of Pharmacy and Arts & Sciences for their continual support, and Azenta Life Sciences where the RNA-seq was performed. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the financial supporters.
Funding Statement
This project was funded by NIMH under Award Numbers R15MH114035 (to ACK), R01MH120066 (to MLS), and the Massachusetts College of Pharmacy and Health Sciences (MCPHS) Center for Research and Discovery (T.J.L and S.K), and MCPHS Summer Undergraduate Research Fellow (SURF) program (T.J.L and S.K). The authors wish to thank Ms. Holly DeRosa and Ada Cheng for their technical assistance in earlier phases of this project. The authors would also like to thank the MCPHS Schools of Pharmacy and Arts & Sciences for their continual support, and Azenta Life Sciences where the RNA-seq was performed. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the financial supporters.
Footnotes
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alizamini MM, Fattahi M, Sayehmiri F, Haghparast A, Liang J. Regulatory Role of PFC Corticotropin-Releasing Factor System in Stress-Associated Depression Disorders: A Systematic Review. Cell Mol Neurobiol. 2023. Jul;43(5):1785–1797. doi: 10.1007/s10571-022-01289-2. Epub 2022 Oct 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arborelius L, Skelton KH, Thrivikraman KV, Plotsky PM, Schulz DW, Owens MJ. Chronic administration of the selective corticotropin-releasing factor 1 receptor antagonist CP-154,526: behavioral, endocrine and neurochemical effects in the rat. J Pharmacol Exp Ther. 2000. Aug;294(2):588–97. [PubMed] [Google Scholar]
- Bangasser DA, Eck SR, Ordoñes Sanchez E. Sex differences in stress reactivity in arousal and attention systems. Neuropsychopharmacology. 2019. Jan;44(1):129–139. doi: 10.1038/s41386-018-0137-2. Epub 2018 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2010. Jun;15(6):574–88. doi: 10.1038/mp.2009.141. Epub 2009 Dec 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodi CM, Vassoler FM, Byrnes EM. Adolescent experience affects postnatal ultrasonic vocalizations and gene expression in future offspring. Dev Psychobiol. 2016. Sep;58(6):714–23. doi: 10.1002/dev.21411. Epub 2016 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiko AS, Ivanova SA, Pozhidaev IV, Freidin MB, Osmanova DZ, Fedorenko OY, Semke AV, Bokhan NA, Wilffert B, Loonen AJM. Pharmacogenetics of tardive dyskinesia in schizophrenia: The role of CHRM1 and CHRM2 muscarinic receptors. World J Biol Psychiatry. 2020. Jan;21(1):72–77. doi: 10.1080/15622975.2018.1548780. Epub 2019 Jan 9. [DOI] [PubMed] [Google Scholar]
- Bourque SL, Iqbal U, Reynolds JN, Adams MA, Nakatsu K. Perinatal iron deficiency affects locomotor behavior and water maze performance in adult male and female rats. J Nutr. 2008. May;138(5):931–7. doi: 10.1093/jn/138.5.931. [DOI] [PubMed] [Google Scholar]
- Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology. 2014. Jul;155(7):2635–46. doi: 10.1210/en.2014-1040. Epub 2014 May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano JC, Huang W, Fénelon K. The amygdala modulates prepulse inhibition of the auditory startle reflex through excitatory inputs to the caudal pontine reticular nucleus. BMC Biol. 2021. Jun 3;19(1):116. doi: 10.1186/s12915-021-01050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CP, Martins CM, Stingel AM, Lemgruber VB, Juruena MF. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J Nerv Ment Dis. 2013. Dec;201(12):1007–20. doi: 10.1097/NMD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Caubit X, Gubellini P, Andrieux J, Roubertoux PL, Metwaly M, Jacq B, Fatmi A, Had-Aissouni L, Kwan KY, Salin P, Carlier M, Liedén A, Rudd E, Shinawi M, Vincent-Delorme C, Cuisset JM, Lemaitre MP, Abderrehamane F, Duban B, Lemaitre JF, Woolf AS, Bockenhauer D, Severac D, Dubois E, Zhu Y, Sestan N, Garratt AN, Lydia Kerkerian-Le Goff, Fasano L. TSHZ3 deletion causes an autism syndrome and defects in cortical projection neurons. Nat Genet. 2016. Nov;48(11):1359–1369. doi: 10.1038/ng.3681. Epub 2016 Sep 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill KM, Huo Z, Tseng GC, Logan RW, Seney ML. Improved identification of concordant and discordant gene expression signatures using an updated rank-rank hypergeometric overlap approach. Sci Rep. 2018. Jun 25;8(1):9588. doi: 10.1038/s41598-018-27903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Andres A. L., Frotscher M., & Baram T. Z. (2012). Tuning synaptic transmission in the hippocampus by stress: the CRH system. Frontiers in cellular neuroscience, 6, 13. 10.3389/fncel.2012.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Ma YL, Lin CH, Cheng SJ, Hsu WL, Lee EH. Galectin-3 Negatively Regulates Hippocampus-Dependent Memory Formation through Inhibition of Integrin Signaling and Galectin-3 Phosphorylation. Front Mol Neurosci. 2017. Jul 11;10:217. doi: 10.3389/fnmol.2017.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Vivacqua G, Yu S. The role of α-synuclein in neurotransmission and synaptic plasticity. J Chem Neuroanat. 2011. Dec;42(4):242–8. doi: 10.1016/j.jchemneu.2010.12.001. Epub 2010 Dec 16. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004. Sep 16;43(6):871–81. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Connors EJ, Shaik AN, Migliore MM, Kentner AC. Environmental enrichment mitigates the sex-specific effects of gestational inflammation on social engagement and the hypothalamic pituitary adrenal axis-feedback system. Brain Behav Immun. 2014. Nov;42:178–90. doi: 10.1016/j.bbi.2014.06.020. Epub 2014 Jul 7. [DOI] [PubMed] [Google Scholar]
- Dedic N, Chen A, Deussing JM. The CRF Family of Neuropeptides and their Receptors - Mediators of the Central Stress Response. Curr Mol Pharmacol. 2018;11(1):4–31. doi: 10.2174/1874467210666170302104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger K, Eder M, Kramer ER, Zieglgänsberger W, Dodt HU, Dornmair K, Colicelli J, Klein R. The Rab5 guanylate exchange factor Rin1 regulates endocytosis of the EphA4 receptor in mature excitatory neurons. Proc Natl Acad Sci U S A. 2008. Aug 26;105(34):12539–44. doi: 10.1073/pnas.0801174105. Epub 2008 Aug 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos-Santos RC, Sweeten BLW, Stelly CE, Tasker JG. The Neuroendocrine Impact of Acute Stress on Synaptic Plasticity. Endocrinology. 2023. Sep 23;164(11):bqad149. doi: 10.1210/endocr/bqad149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclot F, Kabbaj M. The Role of Early Growth Response 1 (EGR1) in Brain Plasticity and Neuropsychiatric Disorders. Front Behav Neurosci. 2017. Mar 6;11:35. doi: 10.3389/fnbeh.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Stone BA, Kapadia BJ, Baram TZ. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology. 2005. Sep;146(9):4090–6. doi: 10.1210/en.2004-1285. Epub 2005 Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filetti C, Kane-Grade F, Gunnar M. The Development of Stress Reactivity and Regulation in Children and Adolescents. Curr Neuropharmacol. 2024;22(3):395–419. doi: 10.2174/1570159X21666230808120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Young LJ. Oxytocin, Neural Plasticity, and Social Behavior. Annu Rev Neurosci. 2021. Jul 8;44:359–381. doi: 10.1146/annurev-neuro-102320-102847. Epub 2021 Apr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Guo O, Zhen Z, Zhen J. Essential Functions of the Transcription Factor Npas4 in Neural Circuit Development, Plasticity, and Diseases. Front Neurosci. 2020. Dec 1;14:603373. doi: 10.3389/fnins.2020.603373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med. 2015;15(2):146–67. doi: 10.2174/1566524015666150303003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, Riva MA, Mortensen PB, Feldon J, Schedlowski M, Meyer U. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013. Mar 1;339(6123):1095–9. doi: 10.1126/science.1228261. Erratum in: Science. 2014 Apr 11;344(6180):151. Erratum in: Science. 2014 Mar 7;343(6175):1077. Feldon, Joram [added]. [DOI] [PubMed] [Google Scholar]
- Green SB, Salkind NJ (2005). Using SPSS for Windows and Macintosh Analyzing and Understanding Data. (4th Edition), Pearson, New Jersey. [Google Scholar]
- Gouty-Colomer LA, Hosseini B, Marcelo IM, Schreiber J, Slump DE, Yamaguchi S, Houweling AR, Jaarsma D, Elgersma Y, Kushner SA. Arc expression identifies the lateral amygdala fear memory trace. Mol Psychiatry. 2016. Mar;21(3):364–75. doi: 10.1038/mp.2015.18. Epub 2015 Mar 24. Erratum in: Mol Psychiatry. 2016 Aug;21(8):1153. doi: 10.1038/mp.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenink L, Dirks A, Verdouw PM, de Graaff M, Peeters BW, Millan MJ, Olivier B. CRF1 not glucocorticoid receptors mediate prepulse inhibition deficits in mice overexpressing CRF. Biol Psychiatry. 2008. Feb 15;63(4):360–8. doi: 10.1016/j.biopsych.2007.06.002. Epub 2007 Aug 23. [DOI] [PubMed] [Google Scholar]
- Gutman DA, Owens MJ, Thrivikraman KV, Nemeroff CB. Persistent anxiolytic affects after chronic administration of the CRF receptor antagonist R121919 in rats. Neuropharmacology. 2011. Jun;60(7–8):1135–41. doi: 10.1016/j.neuropharm.2010.10.004. Epub 2010 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham N, Dachtler J, Fox K. The role of nitric oxide in pre-synaptic plasticity and homeostasis. Front Cell Neurosci. 2013. Oct 31;7:190. doi: 10.3389/fncel.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EP, Abel JM, Tejada LD, Rissman EF. Calbindin Knockout Alters Sex-Specific Regulation of Behavior and Gene Expression in Amygdala and Prefrontal Cortex. Endocrinology. 2016. May;157(5):1967–79. doi: 10.1210/en.2016-1055. Epub 2016 Mar 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NA, Isaac AT, Günther A, Merkel K, Melchior J, Xu M, Eguakun E, Perez R, Nabit BP, Flavin S, Gilsbach R, Shonesy B, Hein L, Abel T, Baumann A, Matthews R, Centanni SW, Winder DG. Dorsal BNST α2A-Adrenergic Receptors Produce HCN-Dependent Excitatory Actions That Initiate Anxiogenic Behaviors. J Neurosci. 2018. Oct 17;38(42):8922–8942. doi: 10.1523/JNEUROSCI.0963-18.2018. Epub 2018 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay CW, Shanley L, Davidson S, Cowie P, Lear M, McGuffin P, Riedel G, McEwan IJ, MacKenzie A. Functional effects of polymorphisms on glucocorticoid receptor modulation of human anxiogenic substance-P gene promoter activity in primary amygdala neurones. Psychoneuroendocrinology. 2014. Sep;47(100):43–55. doi: 10.1016/j.psyneuen.2014.04.017. Epub 2014 May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S, Kelly O, Anisman H. Murine tumor necrosis factor-alpha sensitizes plasma corticosterone activity and the manifestation of shock: modulation by histamine. J Neuroimmunol. 2002. Oct;131(1–2):60–9. doi: 10.1016/s0165-5728(02)00259-x. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology. 2002. Aug;27(2):194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Herrero MJ, Velmeshev D, Hernandez-Pineda D, Sethi S, Sorrells S, Banerjee P, Sullivan C, Gupta AR, Kriegstein AR, Corbin JG. Identification of amygdala-expressed genes associated with autism spectrum disorder. Mol Autism. 2020. May 27;11(1):39. doi: 10.1186/s13229-020-00346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008. Sep;32(7):1293–314. doi: 10.1016/j.neubiorev.2008.03.006. Epub 2008 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler CM, Ryabinin AE. The CRF system and social behavior: a review. Front Neurosci. 2013. May 31;7:92. doi: 10.3389/fnins.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Maita I, Phan ML, Gu E, Kwok C, Dieterich A, Gergues MM, Yohn CN, Wang Y, Zhou JN, Qi XR, Swaab DF, Pang ZP, Lucassen PJ, Roepke TA, Samuels BA. Early-life stress alters affective behaviors in adult mice through persistent activation of CRH-BDNF signaling in the oval bed nucleus of the stria terminalis. Transl Psychiatry. 2020. Nov 11;10(1):396. doi: 10.1038/s41398-020-01070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike KGO, Lamers SJC, Kaim S, de Boer SF, Buwalda B, Billeter JC, Kas MJH. The human neuropsychiatric risk gene Drd2 is necessary for social functioning across evolutionary distant species. Mol Psychiatry. 2024. Feb;29(2):518–528. doi: 10.1038/s41380-023-02345-z. Epub 2023 Dec 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, Gall CM, Lynch G, Baram TZ. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci. 2010. Sep 29;30(39):13005–15. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson BB, Kim JS, Iremonger KJ. Cannabinoid and vanilloid pathways mediate opposing forms of synaptic plasticity in corticotropin-releasing hormone neurons. J Neuroendocrinol. 2022. Apr;34(4):e13084. doi: 10.1111/jne.13084. Epub 2022 Jan 17. [DOI] [PubMed] [Google Scholar]
- Jensen Peña C, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS One. 2012;7(6):e39791. doi: 10.1371/journal.pone.0039791. Epub 2012 Jun 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie F, Yin G, Yang W, Yang M, Gao S, Lv J, Li B. Stress in Regulation of GABA Amygdala System and Relevance to Neuropsychiatric Diseases. Front Neurosci. 2018. Aug 14;12:562. doi: 10.3389/fnins.2018.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ME, Welt T, Wigger A, Renner U, Engelmann M, Holsboer F, Landgraf R. The anxiolytic effect of the CRH(1) receptor antagonist R121919 depends on innate emotionality in rats. Eur J Neurosci. 2001. Jan;13(2):373–80. doi: 10.1046/j.0953-816x.2000.01383.x. [DOI] [PubMed] [Google Scholar]
- Kelleher KJ, Rubin D, Hoagwood K. Policy and Practice Innovations to Improve Prescribing of Psychoactive Medications for Children. Psychiatr Serv. 2020. Jul 1;71(7):706–712. doi: 10.1176/appi.ps.201900417. Epub 2020 Mar 19. [DOI] [PubMed] [Google Scholar]
- Kentner AC, Bilbo SD, Brown AS, Hsiao EY, McAllister AK, Meyer U, Pearce BD, Pletnikov MV, Yolken RH, Bauman MD. Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology. 2019. Jan;44(2):245–258. doi: 10.1038/s41386-018-0185-7. Epub 2018 Aug 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner AC, James JS, Miguelez M, Bielajew C. Investigating the hedonic effects of interferon-alpha on female rats using brain-stimulation reward. Behav Brain Res. 2007. Feb 12;177(1):90–9. doi: 10.1016/j.bbr.2006.10.033. Epub 2006 Nov 28. [DOI] [PubMed] [Google Scholar]
- Kentner AC, Scalia S, Shin J, Migliore MM, Rondón-Ortiz AN. Targeted sensory enrichment interventions protect against behavioral and neuroendocrine consequences of early life stress. Psychoneuroendocrinology. 2018. Dec;98:74–85. doi: 10.1016/j.psyneuen.2018.07.029. Epub 2018 Jul 30. [DOI] [PubMed] [Google Scholar]
- Kirby ED, Muroy SE, Sun WG, Covarrubias D, Leong MJ, Barchas LA, Kaufer D. Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. Elife. 2013. Apr 16;2:e00362. doi: 10.7554/eLife.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000. Apr;24(4):415–9. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- Ko J. Neuroanatomical Substrates of Rodent Social Behavior: The Medial Prefrontal Cortex and Its Projection Patterns. Front Neural Circuits. 2017. Jun 13;11:41. doi: 10.3389/fncir.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb E, Finkbeiner S. Arc in synaptic plasticity: from gene to behavior. Trends Neurosci. 2011. Nov;34(11):591–8. doi: 10.1016/j.tins.2011.08.007. Epub 2011 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzel HE, Zobel AW, Nickel T, Ackl N, Uhr M, Sonntag A, Ising M, Holsboer F. Treatment of depression with the CRH-1-receptor antagonist R121919: endocrine changes and side effects. J Psychiatr Res. 2003. Nov-Dec;37(6):525–33. doi: 10.1016/s0022-3956(03)00070-0. [DOI] [PubMed] [Google Scholar]
- Lee SH, Jung EM. Adverse effects of early-life stress: focus on the rodent neuroendocrine system. Neural Regen Res. 2024. Feb;19(2):336–341. doi: 10.4103/1673-5374.377587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y, Wen J, Fang Y, Zhang H, Zhang J. Corticotropin-releasing factor receptor 1 (CRF-R1) antagonists: Promising agents to prevent visceral hypersensitivity in irritable bowel syndrome. Peptides. 2022. Jan;147:170705. doi: 10.1016/j.peptides.2021.170705. Epub 2021 Nov 22. [DOI] [PubMed] [Google Scholar]
- Martz J, Shelton MA, Geist L, Seney ML, Kentner AC. Sex differences in offspring risk and resilience following 11β-hydroxylase antagonism in a rodent model of maternal immune activation. Neuropsychopharmacology. 2024. Jun;49(7):1078–1090. doi: 10.1038/s41386-023-01771-5. Epub 2023 Nov 25. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavroconstanti T, Johansson S, Winge I, Knappskog PM, Haavik J. Functional properties of rare missense variants of human CDH13 found in adult attention deficit/hyperactivity disorder (ADHD) patients. PLoS One. 2013. Aug 1;8(8):e71445. doi: 10.1371/journal.pone.0071445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron. 2015. Aug 5;87(3):605–20. doi: 10.1016/j.neuron.2015.07.002. Epub 2015 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod F, Salinas PC. Wnt proteins as modulators of synaptic plasticity. Curr Opin Neurobiol. 2018. Dec;53:90–95. doi: 10.1016/j.conb.2018.06.003. Epub 2018 Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown S, Xia S, Baumgärtel K, Barido R, Anderson G, Dyck B, Scott R, Peters M. Phosphodiesterase 1b (PDE1B) Regulates Spatial and Contextual Memory in Hippocampus. Front Mol Neurosci. 2019. Feb 7;12:21. doi: 10.3389/fnmol.2019.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles J, Shevlin M. (2001). Applying regression and correlation: a guide for students and researchers. London: Sage. [Google Scholar]
- Monday HR, Bourdenx M, Jordan BA, Castillo PE. CB1-receptor-mediated inhibitory LTD triggers presynaptic remodeling via protein synthesis and ubiquitination. Elife. 2020. Sep 9;9:e54812. doi: 10.7554/eLife.54812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossink B, van Rhijn JR, Wang S, Linda K, Vitale MR, Zöller JEM, van Hugte EJH, Bak J, Verboven AHA, Selten M, Negwer M, Latour BL, van der Werf I, Keller JM, Klein Gunnewiek TM, Schoenmaker C, Oudakker A, Anania A, Jansen S, Lesch KP, Frega M, van Bokhoven H, Schubert D, Nadif Kasri N. Cadherin-13 is a critical regulator of GABAergic modulation in human stem-cell-derived neuronal networks. Mol Psychiatry. 2022. Jan;27(1):1–18. doi: 10.1038/s41380-021-01117-x. Epub 2021 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NC3Rs (n.d.). The 3Rs. Accessed January 13th, 2024: https://www.nc3rs.org.uk/who-we-are/3rs#reduction.
- Núñez Estevez KJ, Rondón-Ortiz AN, Nguyen JQT, Kentner AC. Environmental influences on placental programming and offspring outcomes following maternal immune activation. Brain Behav Immun. 2020. Jan;83:44–55. doi: 10.1016/j.bbi.2019.08.192. Epub 2019 Sep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordoñes Sanchez E, Bavley CC, Deutschmann AU, Carpenter R, Peterson DR, Karbalaei R, Flowers J 2nd, Rogers CM, Langrehr MG, Ardekani CS, Famularo ST, Bongiovanni AR, Knouse MC, Floresco SB, Briand LA, Wimmer ME, Bangasser DA. Early life adversity promotes resilience to opioid addiction-related phenotypes in male rats and sex-specific transcriptional changes. Proc Natl Acad Sci U S A. 2021. Feb 23;118(8):e2020173118. doi: 10.1073/pnas.2020173118. doi: 10.1073/pnas.2204210119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Álvaro A, Navarrete F, Aracil-Fernández A, Navarro D, Berbel P, Manzanares J. Differential Pharmacological Regulation of Sensorimotor Gating Deficit in CB1 Knockout Mice and Associated Neurochemical and Histological Alterations. Neuropsychopharmacology. 2015. Oct;40(11):2639–47. doi: 10.1038/npp.2015.113. Epub 2015 Apr 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007. Feb 15;61(4):482–6. doi: 10.1016/j.biopsych.2005.09.025. Epub 2006 Feb 7. [DOI] [PubMed] [Google Scholar]
- Piccin A, Contarino A. Sex-linked roles of the CRF1 and the CRF2 receptor in social behavior. J Neurosci Res. 2020. Aug;98(8):1561–1574. doi: 10.1002/jnr.24629. Epub 2020 May 29. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004. Apr 7;24(14):3471–9. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajbhandari AK, Baldo BA, Bakshi VP. Predator Stress-Induced CRF Release Causes Enduring Sensitization of Basolateral Amygdala Norepinephrine Systems that Promote PTSD-Like Startle Abnormalities. J Neurosci. 2015. Oct 21;35(42):14270–85. doi: 10.1523/JNEUROSCI.5080-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004. Jul 21;24(29):6545–52. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruat J, Hartmann A, Heinz DE, Nemcova P, Stoffel R, Deussing JM, Chen A, Wotjak CT. CB1 receptors in corticotropin-releasing factor neurons selectively control the acoustic startle response in male mice. Genes Brain Behav. 2021. Nov;20(8):e12775. doi: 10.1111/gbb.12775. Epub 2021 Oct 21. [DOI] [PubMed] [Google Scholar]
- Salvatore M, Wiersielis KR, Luz S, Waxler DE, Bhatnagar S, Bangasser DA. Sex differences in circuits activated by corticotropin releasing factor in rats. Horm Behav. 2018. Jan;97:145–153. doi: 10.1016/j.yhbeh.2017.10.004. Epub 2017 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, Clark M, Zweifel LS. A Central Amygdala CRF Circuit Facilitates Learning about Weak Threats. Neuron. 2017. Jan 4;93(1):164–178. doi: 10.1016/j.neuron.2016.11.034. Epub 2016 Dec 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San-Martin R, Castro LA, Menezes PR, Fraga FJ, Simões PW, Salum C. Meta-Analysis of Sensorimotor Gating Deficits in Patients With Schizophrenia Evaluated by Prepulse Inhibition Test. Schizophr Bull. 2020. Dec 1;46(6):1482–1497. doi: 10.1093/schbul/sbaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough J, Mueller F, Arban R, Dorner-Ciossek C, Weber-Stadlbauer U, Rosenbrock H, Meyer U, Richetto J. Preclinical validation of the micropipette-guided drug administration (MDA) method in the maternal immune activation model of neurodevelopmental disorders. Brain Behav Immun. 2020. Aug;88:461–470. doi: 10.1016/j.bbi.2020.04.015. Epub 2020 Apr 9. [DOI] [PubMed] [Google Scholar]
- Schreiber AL, Gilpin NW. Corticotropin-Releasing Factor (CRF) Neurocircuitry and Neuropharmacology in Alcohol Drinking. Handb Exp Pharmacol. 2018;248:435–471. doi: 10.1007/164_2017_86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short AK, Maras PM, Pham AL, Ivy AS, Baram TZ. Blocking CRH receptors in adults mitigates age-related memory impairments provoked by early-life adversity. Neuropsychopharmacology. 2020. Feb;45(3):515–523. doi: 10.1038/s41386-019-0562-x. Epub 2019 Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões AP, Machado NJ, Gonçalves N, Kaster MP, Simões AT, Nunes A, Pereira de Almeida L, Goosens KA, Rial D, Cunha RA. Adenosine A2A Receptors in the Amygdala Control Synaptic Plasticity and Contextual Fear Memory. Neuropsychopharmacology. 2016. Nov;41(12):2862–2871. doi: 10.1038/npp.2016.98. Epub 2016 Jun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Pollak SD. Early life stress and development: potential mechanisms for adverse outcomes. J Neurodev Disord. 2020. Dec 16;12(1):34. doi: 10.1186/s11689-020-09337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Rubenstein JLR. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol Psychiatry. 2019. Sep;24(9):1248–1257. doi: 10.1038/s41380-019-0426-0. Epub 2019 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland JE, Conti LH. Restraint stress-induced reduction in prepulse inhibition in Brown Norway rats: role of the CRF2 receptor. Neuropharmacology. 2011. Mar;60(4):561–71. doi: 10.1016/j.neuropharm.2010.12.022. Epub 2010 Dec 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M, Gresack JE, Bangasser DA, Plona Z, Valentino RJ, Flandreau EI, Mansuy IM, Merlo-Pich E, Geyer MA, Risbrough VB. Forebrain-specific CRF overproduction during development is sufficient to induce enduring anxiety and startle abnormalities in adult mice. Neuropsychopharmacology. 2014. May;39(6):1409–19. doi: 10.1038/npp.2013.336. Epub 2013 Dec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandael D, Wierda K, Vints K, Baatsen P, De Groef L, Moons L, Rybakin V, Gounko NV. Corticotropin-releasing factor induces functional and structural synaptic remodelling in acute stress. Transl Psychiatry. 2021. Jul 7;11(1):378. doi: 10.1038/s41398-021-01497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos M, Stein DJ, Gallas-Lopes M, Landau L, de Almeida RMM. Corticotropin-releasing factor receptor signaling and modulation: implications for stress response and resilience. Trends Psychiatry Psychother. 2020. Jun;42(2):195–206. doi: 10.1590/2237-6089-2018-0027. Epub 2020 Jul 17. [DOI] [PubMed] [Google Scholar]
- Veenit V, Riccio O, Sandi C. CRHR1 links peripuberty stress with deficits in social and stress-coping behaviors. J Psychiatr Res. 2014. Jun;53:1–7. doi: 10.1016/j.jpsychires.2014.02.015. Epub 2014 Feb 28. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Swerdlow NR. The basolateral amygdala regulates sensorimotor gating of acoustic startle in the rat. Neuroscience. 1997. Feb;76(3):715–24. doi: 10.1016/s0306-4522(96)00218-7. [DOI] [PubMed] [Google Scholar]
- Wang XD, Labermaier C, Holsboer F, Wurst W, Deussing JM, Müller MB, Schmidt MV. Early-life stress-induced anxiety-related behavior in adult mice partially requires forebrain corticotropin-releasing hormone receptor 1. Eur J Neurosci. 2012. Aug;36(3):2360–7. doi: 10.1111/j.1460-9568.2012.08148.x. Epub 2012 Jun 4. [DOI] [PubMed] [Google Scholar]
- Wolfe SA, Sidhu H, Patel RR, Kreifeldt M, D’Ambrosio SR, Contet C, Roberto M. Molecular, Morphological, and Functional Characterization of Corticotropin-Releasing Factor Receptor 1-Expressing Neurons in the Central Nucleus of the Amygdala. eNeuro. 2019. Jun 18;6(3):ENEURO.0087-19.2019. doi: 10.1523/ENEURO.0087-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, McFadden KV, Grigoriadis D, Bhatnagar S, Valentino RJ. Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology (Berl). 2012. Jul;222(2):325–36. doi: 10.1007/s00213-012-2648-6. Epub 2012 Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano Y, Yoshioka M, Toda Y, Oshida Y, Chaki S, Hamamoto K, Morishima I. Regulation of CRF, POMC and MC4R gene expression after electrical foot shock stress in the rat amygdala and hypothalamus. J Vet Med Sci. 2004. Nov;66(11):1323–7. doi: 10.1292/jvms.66.1323. [DOI] [PubMed] [Google Scholar]
- Yan S, Kentner AC. Mechanical allodynia corresponds to Oprm1 downregulation within the descending pain network of male and female rats exposed to neonatal immune challenge. Brain Behav Immun. 2017. Jul;63:148–159. doi: 10.1016/j.bbi.2016.10.007. Epub 2016 Oct 11. [DOI] [PubMed] [Google Scholar]
- Zhao X, Mohammed R, Tran H, Erickson M, Kentner AC. Poly (I:C)-induced maternal immune activation modifies ventral hippocampal regulation of stress reactivity: prevention by environmental enrichment. Brain Behav Immun. 2021. Jul;95:203–215. doi: 10.1016/j.bbi.2021.03.018. Epub 2021 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010. May;15(9–10):371–83. doi: 10.1016/j.drudis.2010.02.011. Epub 2010 Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Graphs display male (left) and female (right) data for adult A) adolescent and B) adult body weights (g). Data are expressed as mean ±SEM. *p < 0.05, **p < 0.01, Saline versus adolescent CRFR1 antagonism, n = 8.
Graphs display male (left) and female (right) data for adolescent (top panel) and adult (bottom panel) A,C) social discrimination and B,D) mechanical allodynia threshold (g) on the von Frey test. Data are expressed as mean ±SEM. *p < 0.05, **p < 0.01, Saline versus adolescent CRFR1 antagonism, n = 8.
A) Top pathways represented by transcripts differentially expressed in females (A) and males (B) as a function of CRFR1a.
Heatmaps of A) glucocorticoid binding-, B) oxytocin-, C) cannabinoid-, D) glutamate (Glu)/GABA -, E) parvalbumin-, and F) microglia-related genes, with effects represented as fold change (FC) between CRFR1a and Saline exposed males, and between CRFR1a and Saline exposed females. *Genes that reached the Benjamini-Hochberg corrected effect of 20%. +Genes with p<0.05 and FC>1.3.
Supplemental Figure 5. Threshold-free RRHO analysis showed a high degree of overlap between the effect of sex in controls and the effect of sex in CRFR1a exposed rats.
Supplemental Results Table 1. Gene Expression in Saline vs. CRFR1a Amygdala
Supplemental Results Table 2. Females CRFR1a vs Sal
Supplemental Results Table 3. Males CRFR1a vs Sal