Abstract
Hippocampal long-term potentiation (LTP) and long-term depression (LTD) are Hebbian forms of synaptic plasticity that are widely believed to comprise the physiological correlates of associative learning. They comprise a persistent, input-specific increase or decrease, respectively, in synaptic efficacy that, in rodents, can be followed for days and weeks in vivo. Persistent (>24 h) LTP and LTD exhibit distinct frequency-dependencies and molecular profiles in the hippocampal subfields. Moreover, causal and genetic studies in behaving rodents indicate that both LTP and LTD fulfil specific and complementary roles in the acquisition and retention of spatial memory. LTP is likely to be responsible for the generation of a record of spatial experience, which may serve as an associative schema that can be re-used to expedite or facilitate subsequent learning. In contrast, LTD may enable modification and dynamic updating of this representation, such that detailed spatial content information is included and the schema is rendered unique and distinguishable from other similar representations. Together, LTP and LTD engage in a dynamic interplay that supports the generation of complex associative memories that are resistant to generalization.
This article is part of a discussion meeting issue ‘Long-term potentiation: 50 years on’.
Keywords: synaptic plasticity, hippocampus, learning and memory, rodent cognition, LTP, LTD
1. Introduction
There is broad consensus that persistent, use-driven and experience-dependent modifications of synaptic strength form the cellular basis of long-term information storage and memory updating by the hippocampus. This derives from an abundance of evidence acquired in recent decades that indicates that associative learning drives long-lasting changes in synaptic efficacy [1–3]. Particularly relevant in this regard are forms of synaptic plasticity that persist over periods of hours or days, thereby putatively allowing the retention of information for prolonged periods and opening up time-windows for the building of associations between past and prospective experiences. This, in turn, may be supported by processes such as metaplasticity [4], synaptic tagging [5] and homeostatic plasticity [6]. Persistent forms of synaptic plasticity are likely to enable the generation of linked neuronal ensembles that support the creation and updating of discriminable associative experience. The primary candidates for this process are two forms of Hebbian, homosynaptic synaptic plasticity that can persist for periods of 24 h and longer, namely hippocampal long-term potentiation (LTP) and long-term depression (LTD). These comprise physiological phenomena that are observed in a laboratory setting that reflect activity-dependent increases or decreases in synaptic efficacy. In this review, the current understanding of the molecular basis and subfield-specific differences of persistent hippocampal LTP and LTD is described. In addition, insights gained from studies of persistent (>24 h) LTP and LTD in the dorsal hippocampus of freely behaving rodents in the presence or absence of learning paradigms shall be considered from the perspective of the delineation of their putative roles and their dynamic interplay in the creation of associative (spatial) memories that last for several hours or days in an experimental setting.
2. Temporal phases of hippocampal synaptic plasticity
(a). When is LTP, LTP?
LTP describes a persistent strengthening of synaptic efficacy that occurs in response to specific patterns of stimulation of afferent inputs [7,8]. The vast majority of studies on hippocampal LTP have been conducted in vitro, which, on the one hand, has provided extensive knowledge about the molecular mechanisms that enable this process [9], but, on the other hand, restricts observations of changes in synaptic strength (and their related mechanisms) to periods of maximally hours [10] and typically involves scrutiny for circa (ca) 60 min (electronic supplementary material, table S1). Customarily, LTP is therefore described as an increase in synaptic strength that lasts for at least 1 h. However, in behaving animals, hippocampal LTP can be monitored for days [11] and months [12], and induction protocols can discriminate between synaptic potentiation of different durations (electronic supplementary material, table S2). These differences in the timelines of LTP studied in vivo and in vitro have created difficulties in the definitions of what is truly long-term potentiation, as opposed to shorter forms that may comprise separate phenomena (short-term potentiation (STP)) [13], or an interim phase distinguishing LTP that lasts for hours [10] from one that lasts for days and weeks [11,12]. Studies of the molecular basis of persistent (>24 h) LTP, for example, in the cornu ammonis-1 (CA1) region in vivo have revealed that it can be disambiguated into temporal components comprising STP that requires activation of N-methyl-d-aspartate receptors (NMDAR) and lasts for 30–90 min, depending on the subunit composition of the NMDAR [14]. This early phase of LTP then segues into LTP that lasts for 90–180 min in behaving rodents and requires activation of the metabotropic glutamate receptor, mGlu5 [15]. LTP that lasts for more than 3 h requires brain-derived neurotrophic factor (BDNF) [16], whereas CA1 LTP that lasts for longer than 8 h (referred to as late-LTP) requires both protein translation and transcription [17]. Scrutiny of the NMDAR-dependent component of STP (<90 min) in vitro has revealed that it can also be disambiguated into different components: a transient (30 min) form that depends on GluN2D-containing NMDAR [13] and a longer-lasting STP that depends on GluN2A-containing NMDAR and can last for at least 90 min [13,14]. This latter STP can be distinguished from LTP that endures for a similar duration in the CA1 region in vitro [18]. Taken together, this suggests that the terminology used to define hippocampal LTP needs to be revisited and made more precise. In the context of this review, LTP will be defined as synaptic potentiation that persists for at least 4 h, whereas persistent LTP refers to synaptic potentiation that lasts for at least 24 h.
(b) Hippocampal LTD also occurs in temporal phases
Scrutiny of hippocampal LTD has revealed that it too can be segregated into temporal components. In the CA1 region, for example, antagonism of NMDAR prevents LTD in anaesthetized rats [19] and radically reduces the magnitude of induced synaptic depression in freely behaving rats, as well as curtailing it to a duration of roughly 30 min [20]. Short-term depression (STD) requires BDNF in vivo [21] and LTD in freely behaving rats, which is induced after low-frequency afferent stimulation is limited to a period of ca 2 h by antagonism of group 1 mGlu receptors [20]. Furthermore, LTD that lasts for longer than 4 h requires immediate early gene (IEG) expression [22] and protein translation [23].
3. The cellular and molecular basis of LTP and LTD: there is no single form of LTP or LTD in the hippocampus, and species differences are also evident
Although interpretations of the cellular or molecular basis of LTP or LTD in the hippocampus (and their relationship to associative learning) are often merged to reflect a holistic view of hippocampal synaptic plasticity, an examination of the frequency-dependency of LTP in different synaptic subcompartments of the hippocampus reveals very distinct outcomes (figure 1). In this section, focus will be placed on hippocampal synaptic plasticity (>4 h) induced in freely behaving rats and mice, owing to the fact that differences in bath temperature, the composition of perfusion media, the inclusion of excitability suppressants such as picrotoxin in bath media, electrode placement, electrode resistance and slice thickness (to name but a few aspects) make it very difficult to compare outcomes derived in vitro [1] (electronic supplementary material, table S1). In general, in vitro papers also do not specify whether slices were taken from dorsal, intermediate or ventral hippocampus, which is problematic because not only are the NMDAR complements different in these longitudinal areas of the hippocampus, but LTP in the slice preparation also differs according to the longitudinal axis of the hippocampus [30].
Figure 1.
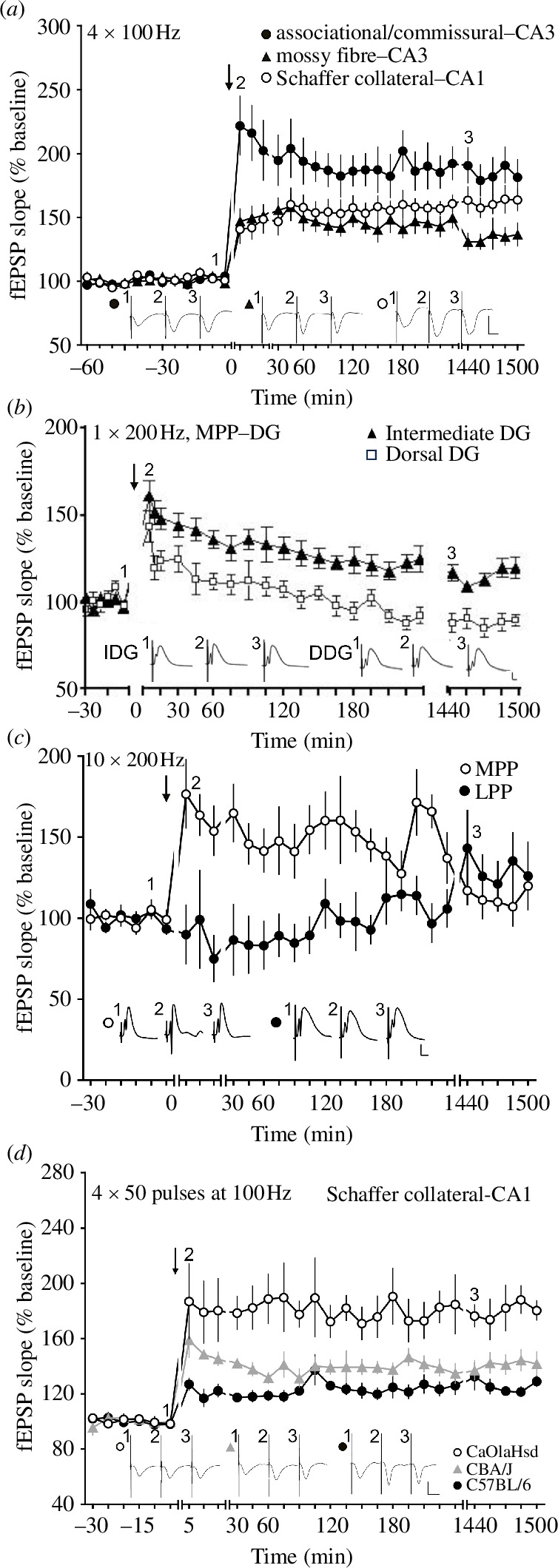
LTP profiles in the cornu ammonis (CA) and dentate gyrus (DG) subfields of the hippocampus of freely behaving young adult rats and mice. (a) LTP evoked with high-frequency stimulation (HFS, 100 Hz, four trains of 100 pulses each, 5 min intertrain interval) results in different LTP profiles in associational/commissural (AC)–CA3, mossy fibre (MF)–CA3 and Schaffer collateral (SC)–CA1 synapses of the cornu ammonis of dorsal rat hippocampus. Vertical scale bar: 2 mV, horizontal scale bar: 10 ms. (b) Persistent (>24h) LTP can be evoked with a single train of stimuli delivered at 100 Hz in the intermediate DG (closed triangles), but not the dorsal rat DG (open squares). Vertical scale bar: 5 mV, horizontal scale bar: 5 ms. (c) Persistent LTP in medial perforant path (MPP) inputs to the dorsal rat DG can be induced with 10 trains of stimuli at 100 Hz and 10 s intertrain intervals. The same stimulation pattern fails to induce LTP at lateral perforant path (LPP)-DG inputs. Vertical scale bar: 4 mV, horizontal scale bar: 3 ms. (d) HFS (four trains of 50 pulses each at 100 Hz, 5 min intertrain interval) results in LTP of different magnitudes in CaOlaHsd (open circles), CBA/J (grey triangles) and C57BL/6 (closed circles) mice in SC–CA1 synapses in vivo. To note: C57BL/6 mice develop progressive deafness beginning in the 4th postnatal week; CBA/J mice become blind within 4 weeks after birth; CaOlaHsd mice have no appreciable sensory deficits in their first 12 months of life (see citations below). Vertical scale bar: 2 mV, horizontal scale bar: 10 ms. Black arrows depict the time-points of stimulation. Insets show the analogue examples of field excitatory postsynaptic potentials (fEPSPs) recorded at the time points indicated by the numbers in the graphs. Line breaks indicate change in time scale. Figures modified from [15,24–29].
(a) Frequency-dependency of homosynaptic LTP in hippocampal inputs in freely behaving rats
In freely behaving rats, synaptic potentiation of differing durations can be induced in the dorsal hippocampus by using afferent stimulation frequencies in the range of 100 through 400 Hz (electronic supplementary material, table S2) [31]. Use of frequencies higher than 100 Hz in cornu ammonis synapses will tend to induce epileptiform seizures [31]. Weaker afferent stimulation protocols that induce STP in SC–CA1 synapses induce LTP that lasts for over 4 h at temporoammonic (TA)–CA1 synapses in vivo [32] that is NMDAR-dependent [33]. Strikingly, the same 100 Hz protocol that induces LTP at SC–CA1 synapses in vivo induces LTP of differing profiles depending on whether mossy fibre (MF)–CA3, commissural–associational (AC)–CA3 or Schaffer collateral (SC)–CA1 synapses are stimulated (figure 1a ) [11,24]. While MF–CA3 LTP is NMDAR-independent in freely behaving rats [34], it requires activation of NMDAR at AC–CA3 [15] or SC–CA1 synapses [20]. Furthermore, although reciprocal heterosynaptic LTP can be induced in lateral perforant path (LPP) and medial perforant path (MPP) inputs to the CA3 in vivo [35], differences in the conditions leading to homosynaptic LTP have been reported in these synapses [36].
To induce persistent LTP in dentate gyrus (DG) synapses, higher afferent stimulation frequencies (typically 200 Hz) are required. At MPP–DG synapses LTP (>24 h) that is NMDAR-dependent can be induced by repetitive stimulation at 200 Hz, whereas LTP (>24 h) that is induced by 400 Hz stimulation is dependent on activation of L-type voltage-gated calcium channels [37]. The frequency-dependency of LTP in DG synapses is not uniform, however: one burst of 200 Hz stimulation induces STP (<2 h) at dorsal MPP–DG synapses, whereas the same afferent patterns induce persistent LTP (>24 h) at intermediate MPP–DG synapses (figure 1b ) [25]. Furthermore, 10 bursts of 200 Hz stimulation induce persistent LTP at suprapyramidal dorsal MPP–DG synapses, whereas the same afferent pattern delivered to infrapyramidal dorsal MPP–DG synapses fails to induce LTP [38]. The frequency-dependency of synaptic plasticity also differs substantially at dorsal MPP–DG and LPP–DG synapses in freely behaving rats; for example, repeated bursts at 200 Hz result in potent LTP at MPP–DG synapses that lasts for at least 24 h, whereas the same protocol when applied to LPP–DG synapses results in synaptic depression that lasts for about 2 h (figure 1c ) [39]. Moreover, Abraham & Goddard [40] have reported that MPP–DG and LPP synapses express heterosynaptic forms of synaptic plasticity, such that potentiation in one input is accompanied by depression in the other. In addition, examination of morphological changes of DG synapses after induction of LTP in vivo revealed input-specific increases in perforated axospinous synapses in MPP–DG, but not LPP–DG inputs [41] that were accompanied by an input-specific increase in axodendritic synapse density in synapses that expressed heterosynaptic LTD. These findings indicate that all three processes (MPP–LTP, LPP–LTP and heterosynaptic LTD) may be functionally and morphologically distinct. Taken together, these findings highlight that, depending on the synapses/hippocampal subfields involved, LTP in the hippocampus is highly frequency-dependent, which argues against using only one afferent frequency when scrutinizing LTP experimentally and argues for the likelihood that LTP in the different hippocampal synapses is distinct.
(b) Frequency-dependency of homosynaptic LTP in hippocampal inputs in freely behaving mice
The dorsal CA1 region of freely behaving mice exhibits a very different response profile with regard to LTP induction in compared to rats. In the CA1 region, afferent frequencies that are lower or higher than 100 Hz are ineffective (electronic supplementary material, table S2) [42], whereas it is the impulse number and pattern delivered at 100 Hz (rather than the stimulus frequency) that determine whether STP or LTP is induced [43]. Moreover, theta-burst stimulation is ineffective in vivo [43]. At dorsal MPP–DG synapses, 400 Hz stimulation is needed to induce persistent LTP [44]. Here, however, a confound emerges in terms of the interpretation of LTP data obtained from mouse hippocampus: the majority of studies both in vitro and in vivo have been conducted in C57BL/6 mice that were established as a mouse strain by the Jackson Laboratory in 1948 and have since given rise to well over 220 generations [45]. Drift in the resultant genetic pools has led to the Jackson substrain being referred to as C57BL/6J and the NIH substrain as C57BL/6N, which differ, in turn, from European or Asian variants of these breeding lines [42]. The C57BL/6J and C57BL/6N substrains differ in terms of their responses to sensorimotor and fear conditioning [46,47], as well as spatial memory [48]. Most of the inbred mouse strains used in brain research show differences in their stress sensitivity and anxiety [48–51], brain amine levels [52] and differences in spatial and non-spatial memory [48,53,54]. They also exhibit differences in the distribution of hippocampal afferents and synapses [53]. This makes a general interpretation of the conditions leading to synaptic plasticity in mouse hippocampus and its relevance for associative learning very difficult.
But there is a further confound to using C57BL/6J mice for studies of synaptic plasticity and associated learning behaviour: this mouse strain exhibits presbycusis, whereby it begins losing its auditory acuity at four weeks of age starting with behaviourally relevant high ultrasound frequencies [55]. By five months of age, the mice can only hear frequencies in the range of stress vocalizations, as well as sonic frequencies, and over the course of their adult lifetime become completely deaf [56]. This has consequences for both synaptic plasticity and spatial learning: C57BL/6J mice exhibit significantly reduced LTP both in vitro and in vivo, as well as impaired spatial memory compared to a mouse strain that exhibits no sensory deficits and a mouse strain that becomes blind shortly after birth [26] (figure 1d ). C57BL/6J mice also exhibit different social behaviour compared to outbred mice [57] and impaired attention compared to 129SvEv mice [58] that do not display age-related hearing loss [59]. These confounds emphasize the necessity to examine plasticity-related processes and synaptic plasticity in multiple strains and species of rodents, as well as using a variety of afferent stimulation frequencies, in order to better understand their relevance for learning and memory.
(c) Molecular characteristics of persistent forms of LTP in different hippocampal subfields
The differences in persistent (>24 h) forms of LTP across the hippocampal subfields also become apparent when one compares their molecular dependencies (electronic supplementary material, table S3a). Induction of persistent LTP at dorsal SC–CA1, TA–CA1, AC–CA3, MPP–CA3 and MPP–DG synapses typically depends on activation of NMDAR [20,36,37], whereas persistent dorsal MF–CA3 LTP is NMDAR-independent [34] in line with in vitro studies that have shown that this form of LTP depends on activation of pre-synaptic kainate receptors in vitro [60]. LPP–CA3 LTP is opioid receptor-dependent and not NMDAR-dependent [36]. Although persistent LTP in all of the hippocampal subfields depends on protein synthesis (electronic supplementary material, table S3a), one striking difference is evident with regard to MF–CA3 synapses in vivo, which also show a requirement of protein synthesis for STP [61]. Furthermore, although LTP at SC–CA1 synapses is arguably predominantly mediated by post-synaptic mechanisms [62–64,7], strong evidence exists that MF–CA3 LTP is induced by pre-synaptic mechanisms [65] and perforant path (PP)–LTP may have both pre- and post-synaptic components depending on whether MPP [37] or LPP inputs [66] are activated. A putative pre-synaptic component to PP–DG LTP has not yet been verified in vivo, however.
(d) Frequency-dependency of homosynaptic LTD in hippocampal inputs in freely behaving rats and mice
In contrast to hippocampal LTP in freely behaving rats, the frequencies with which persistent LTD can be induced in hippocampal subfields in rats are quite limited both in vitro and in vivo (electronic supplementary material, table S4a,c). In freely behaving rats, regardless of the subfield, 1 Hz afferent stimulation induces persistent (>24 h) input-specific LTD in all dorsal subfields [24,67,68] (figure 2a–c ), whereby STD can be induced by reducing the pulse number from typically 900 to 600 [24,71] or 300 [72]. Faster frequencies of 3 or 5 Hz induce STD in freely behaving rats [73]. In the murine hippocampal slice preparation, LTD that lasts for up to 2 h can be induced using different variations of 1 Hz stimulation (electronic supplementary material, table S4b). In contrast, it is really difficult to induce LTD by afferent stimulation alone in the dorsal hippocampus of freely behaving mice (electronic supplementary material, table S4d) [42–44]: different afferent stimulation frequencies (1–10 Hz) and impulse numbers (100–1800) result in STD of differing magnitudes and durations [74], although input-specific NMDAR-dependent LTD can be easily induced in mice by coupling afferent stimulation with spatial learning [75].
Figure 2.
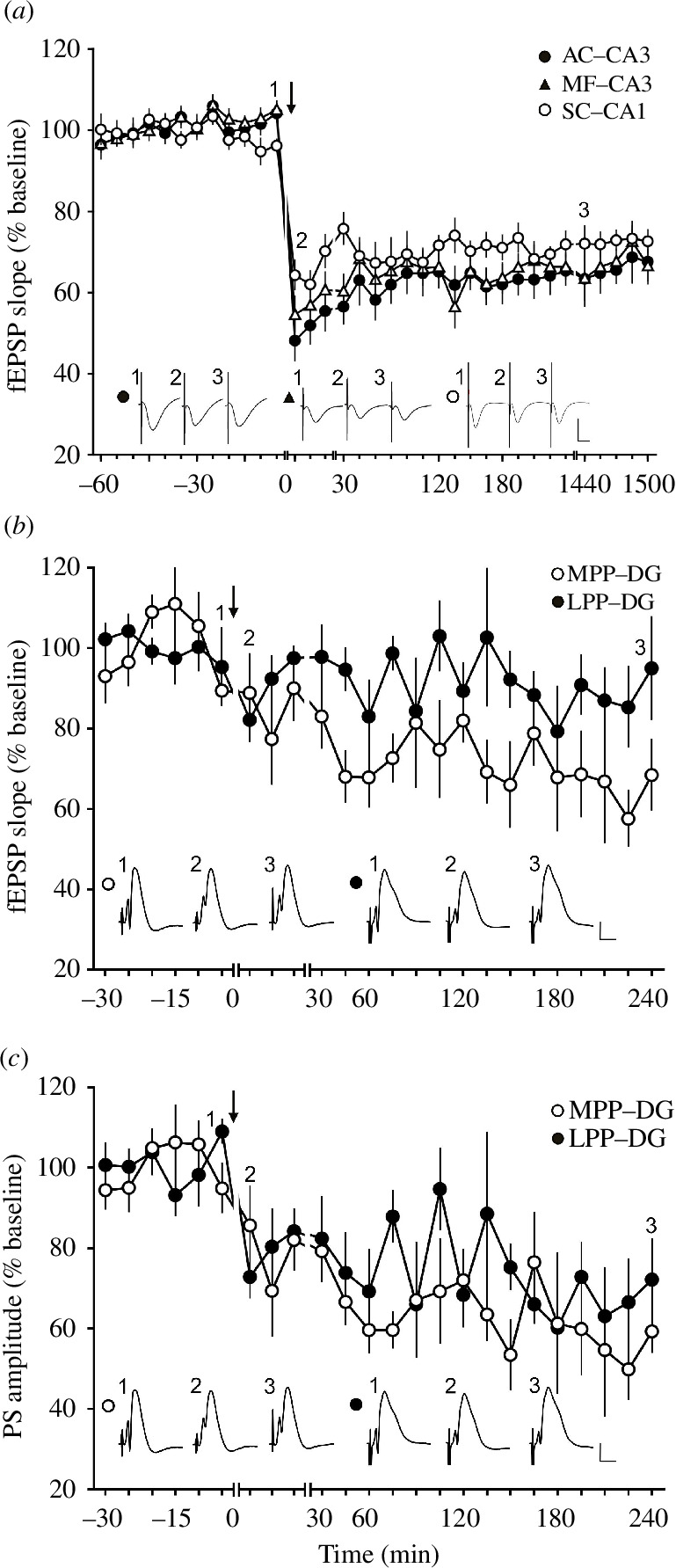
LTD profiles in the cornu ammonis (CA) and dentate gyrus (DG) subfields of the rat hippocampus. (a) LTD evoked with low-frequency stimulation (LFS, 900 pulses at 1 Hz) results in different LTD profiles at AC–CA3, MF–CA3 and SC–CA1 synapses in the hippocampus in freely behaving rats. Vertical scale bar: 2 mV, horizontal scale bar: 10 ms. (b,c) Result of the same stimulation protocol (900 pulses at 1 Hz) at lateral perforant path (LPP, closed black circles) and medial perforant path (MPP, open circles)–DG synapses. Vertical scale bar: 5 mV, horizontal scale bar: 5 ms. Black arrow depicts the time-point of stimulation. Insets show analogue examples of fEPSPs recorded at the time points indicated by the numbers in the graphs. Line breaks indicate changes in time scale. Figures modified from: (a) [69,70]. (b,c) [39,71].
(e) Molecular characteristics of persistent forms of LTD in different hippocampal subfields
Despite the homogeneity of its electrophysiological induction conditions, the molecular dependency of persistent LTD is very different depending on the hippocampal subfield (electronic supplementary material, table S3b). Whereas persistent SC–CA1 and AC–CA3 LTD depend on the activation of NMDAR [15,20], persistent LTD at MF–CA3 synapses [61] or MPP–DG synapses [76] are NMDAR-independent. Furthermore, persistent MPP–DG LTD does not require protein synthesis [76], whereas SC–CA1 and AC–CA1 LTD do [23,61]. Additionally, protein synthesis inhibition curtails STD at MF–CA3 synapses in vivo [61]. Other differences emerge when the involvement of catecholaminergic receptors in persistent LTD is considered: pharmacological antagonism of β-adrenergic receptors has no effect on LTD that is induced by 1 Hz (900 pulses) stimulation of SC–CA1 synapses in rats [1], but it prevents the maintenance of LTD beyond 4 h at MPP–DG synapses [77]. Pharmacological antagonism of dopamine D1/D5 receptors prevents LTD (>24 h) at SC–CA1 and MPP–DG synapses [1], but has no effect on persistent LTD at MF–CA3 [78] synapses in freely behaving rats.
Taken together, it is clear that there are multiple forms of persistent LTP and persistent LTD in the distinct hippocampal subfields that are characterized by their different frequency-dependencies, expression profiles and molecular dependencies and that are likely to subserve different kinds of information encoding. From an intuitive perspective, this makes sense: the DG is likely to have evolved ahead of the cornu ammonis and may thus fulfil functions that are distinct from those of this structure, such as the acquisition of information about spatial bearing relative to allocentric information [79] and pattern separation [80,81]. All subfields of the hippocampus receive inputs from the entorhinal cortex (EC) [82], theoretically receiving similar information at any given moment in time. However, depending on the synaptic targets ofthe EC within the hippocampal subfields [83], EC afferent terminations within compartments of the respective dendritic trees of the recipient neurons [84], and coupling of EC input with information transferred within the trisynaptic circuit [85,86], this information will be transformed in different ways, resulting in different outcomes in terms of the magnitude, persistency and direction of change of synaptic strength depending on the hippocampal subfield concerned. This interpretation is also consistent with the widespread belief that the different hippocampal subfields fulfil different roles in the storage and recall of associative memories [87].
4. Synaptic plasticity is not the only change in synaptic efficacy that occurs in the hippocampus
It would be remiss to fail to mention that synaptic plasticity is not the only physiological process that modulates synaptic strength in the hippocampus. In addition to LTP and LTD, two other cellular phenomena have been described in the dorsal hippocampus that either indirectly or directly instigate changes in synaptic efficacy, namely metaplasticity [88] and slow-onset potentiation (SOP) [89]. Both phenomena exhibit features that are mechanistically distinct from LTP; for example, inhibitory autophosphorylation of calcium calmodulin-dependent kinase II (CAMKII) is required for hippocampal metaplasticity that is induced by patterned afferent stimulation of LPP, but not MPP–DG synapses [90], although LTP typically requires CAMKII. This may relate to neuroregulatory control of metaplasticity by neurogranin [91]. SOP cannot be induced by homosynaptic afferent stimulation parameters [92] but rather occurs in response to activation of G-protein-coupled receptors [89,93,94].
Metaplasticity reflects the property that the prior experience of a synapse has an impact on the characteristics of the synapse’s plasticity response to a subsequent synaptic experience [95]. This can mean, for example, that the same afferent stimulation pattern can result in hippocampal LTP or LTD, depending on the recent past experience of the synapse [1]. Also, the frequency-dependency of synaptic plasticity can shift (to a higher or lower frequency) depending on the prior experience of the synapse [90,96,97]. One striking feature of this phenomenon is that effects occur within a limited time-window after a metaplastic event [98,99]. Moreover, metaplasticity may require synaptic tagging [100] and, thus, could form a substrate for the binding of temporally proximal events into an associative representation. Hippocampal information processing is highly state-dependent, and effects are reflected by changes in the coupling of neuronal oscillations at theta–gamma frequencies [101]. In freely moving rats, identical afferent stimulation frequencies can result in robust LTP, STP, or no change in synaptic efficacy in the absence of any overt change in behaviour, although changes in the coupling of theta- and gamma-frequency neuronal oscillations during the stimulation predict the plasticity outcome [15]. Furthermore, stress changes the thresholds for the induction and maintenance of synaptic plasticity [102–104], as can alterations of the action of neuromodulators in the hippocampus [105]. Thus, metaplasticity is very likely to occur in response to changes in hippocampal homeostasis that can either be experience-dependent or state-dependent, which in turn determines if, and how, recent experience is stored in the form of synaptic plasticity.
SOP describes a gradual potentiation of field potentials that emerges with a latency of several minutes and continues to develop and reach a plateau hours after the initiating event [89,93]. A caveat is that SOP always appears with a delay of several minutes in vivo after its initiation [93,106], raising the question as to what the functional relevance of the delayed and incremental increase in excitability could be. LTP, but not SOP, in the CA1 region involves an input-specific increase in synaptic efficacy [92], although the latter may occur in the DG in conjunction with SOP [107]. A striking characteristic of SOP is that it can be induced in anaesthetized rodents [67,106] by afferent stimulation parameters and/or neuromodulatory conditions that induce hippocampal LTD in freely behaving rodents [20,76,77,108], suggesting that it may play a state-dependent role in homeostatic plasticity processes [6]. Thus, the elevation of synaptic excitability enabled by SOP may facilitate the integration and association of information along a prospective timescale by lowering the threshold for the induction of synaptic plasticity for a period of minutes or hours after SOP has been initiated.
5. Causal evidence that LTP and LTD support the acquisition and retention of long-term associative experience
Early reports that pointed to a role for LTP in the encoding of associative memory were inferred through experiments conducted in parallel: interventions that prevented hippocampal LTP also impaired spatial learning in separately conducted investigations in rodents (electronic supplementary material, table S5) [109–111], or it was reported, in transgenic animal models, that deficient LTP was associated with deficient spatial memory (electronic supplementary material, table S5) [112–114]. Subsequent studies described the direct relationship of learning with LTP (electronic supplementary material, table S6a). For example, recordings from multiple field electrodes placed in the CA1 region of freely behaving rats revealed that a proportion of the recording sites exhibited synaptic potentiation and/or LTP after one-trial foot shock learning [3]. Coupling afferent stimulation of the perforant path afferents with spatial learning events has revealed that cumulative spatial learning [115] and appetitive task-specific learning [116] promote the expression of LTP in the DG. In contrast, learning about novel space facilitates the expression of input-specific hippocampal LTP at MPP–DG, AC–CA3, MF–CA3 and SC–CA1 synapses in freely behaving rats [11,24,71] (electronic supplementary material, table S6a). Despite the widespread belief that LTP comprises the cellular basis of learning, ‘proof of principle’ studies of this kind are surprisingly rare, however.
Nonetheless, considering the abovementioned findings that hippocampal LTP may encode one-trial fear memory [3] and that neurons that encode fear memory can generalize easily to integrate other experiences [117], one possibility is that hippocampal LTP may enable the rapid recording of the general schema of an associative experience. This possibility is corroborated by reports that LTP expands the informational content of hippocampal synapses [118] and prompts structural plasticity [119], as well as by findings that hippocampal LTP is facilitated by cumulative spatial learning experience [2] and that temporally spaced inductions of LTP are cumulative and serve to improve spatial learning [120]. Moreover, LTP can be induced by burst depolarizations of one second or less [121,122], suggesting that LTP induction could plausibly occur as soon as an associative experience is perceived as being novel. Reinforcement of the initially acquired LTP may then occur cumulatively: afferent volleys (arguably triggered by increases in attention and arousal driven by the initial LTP-inducing experience) occurring on the backdrop of post-synaptic depolarization (which is mediated by the initial LTP) could then serve to reinforce and prolong LTP [123].
In contrast to LTP, where a substantial change in the appearance of the spatial environment is needed to promote LTP that lasts for more than 24 h [1], LTD is facilitated by changes in spatial content, even within a familiar environment (electronic supplementary material, table S6b) [2]. The most robust behavioural instigators of hippocampal LTD to be identified thus far are de novo item–place experience and the updating (spatial re-configuration) of item–place information. In SC–CA1 synapses of freely behaving rats, persistent LTD is enabled when brief afferent stimulation (which is sufficient to induce transient synaptic depression when applied in the absence of learning) is coupled with novel (or updated) learning of information about spatial constellations of visual [11,68], odour [1] or auditory information [1] (figure 3a,b, f ). For this, physical motion in space is not needed: the presentation of spatial configurations of items on a computer screen is also sufficient to prime for induction of LTD (>24 h) at SC–CA1 synapses [1]. Furthermore, effects are not species-specific: mice also respond with LTD to novel or updated item–place information, whereby in this case only test–pulse stimulation of SC–CA1 synapses in conjunction with novel spatial content learning is needed for LTD to become manifest by this event [75].
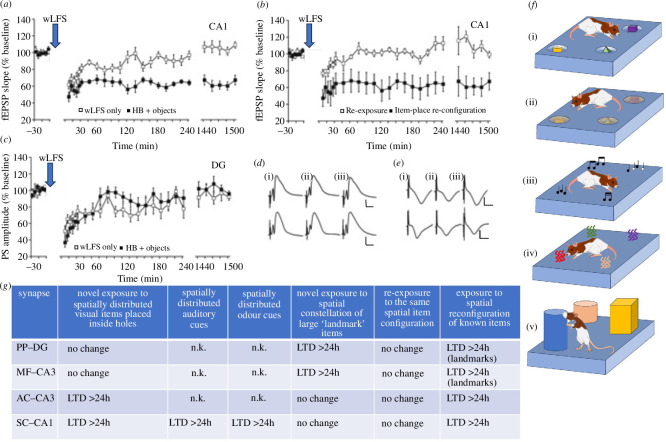
Figure 3.
Functional differentiation of the facilitation of LTD by spatial content learning. (a) Novel exploration by rats of objects placed in holeboard (HB) holes, during weak low-frequency stimulation (wLFS, 1 Hz, 900 pulses) results in LTD (>24 h) in SC–CA1 synapses. Afferent stimulation with wLFS only results in STD that lasts for ca 30 min. Arrows in (a–c) indicate when wLFS was applied (in the presence or absence of item–place exploration). (b) Re-exposure to the same objects in the same holeboard positions in conjunction with wLFS fails to result in LTD. However, exposure of the animals to a spatial re-configuration of the same objects results in LTD (>24 h). (c) Stimulation of PP inputs to the DG with wLFS results in STD that persists for <60 min. Novel exposure of rats to objects placed in holeboard holes in conjunction with wLFS fails to induce LTD. (d,e) Analogue examples of evoked potentials recorded from the DG (d) or the CA1 region (e) prior to wLFS (i), 5 min post-wLFS (ii) and 24 h after wLFS (iii) in an animal that received wLFS only (top row) and in an animal that received wLFS during the exploration of objects in the holeboard holes (bottom row). (f) In SC–CA1 synapses, LTD is expressed when wLFS is applied during novel exploration of novel objects placed in holeboard holes (i) [68], or novel objects concealed under sand inside holeboard holes [11] (ii). LTD is also expressed when wLFS is applied in conjunction with the novel exploration of spatially discriminable auditory frequencies that emanate from loudspeakers placed under holes in the floor (iii) [124] , or spatially distributed odours that diffuse through holes in the floor (iv) [125]. The DG does not respond with a change in synaptic strength to any of these conditions: rather, it expresses LTD when exploration of novel constellations of large landmark features of the environment occurs in conjunction with wLFS (v) [1]. (g) Summary of responses of different hippocampal synapses to the abovementioned conditions. PP–DG synapses and MF–CA3 synapses express LTD following exploration of novel configurations of landmark items in space. The AC–CA3 and SC–CA1 synapses do not respond to this kind of information. In contrast, LTD is expressed in these synapses following exploration of novel constellations of items concealed in holeboard holes. PP–DG and MF–CA3 synapses do not respond to subtle item–place information. n.k.: not known. Panels (a–f) are modified from [11].
Effects are input-specific [1], but differ depending on the hippocampal subfield concerned: while SC–CA1 synapses express LTD in response to learning about spatial constellations of discretely placed cues that can only be discovered if the animal is right beside them, the DG expresses LTD when rats learn about the locations of large, overt items in space [71] (figure 3a–e ). The CA3 region displays a hybrid function in this regard: MF–CA3 synapses express LTD in response to large overt item–place features, but AC–CA3 synapses behave like SC–CA1 synapses and only express LTD in response to subtly placed items that have to be proximately viewed in order to be localized in space [24] (figure 3). These findings not only indicate that a functional differentiation is evident in hippocampal subfields with regard to the kinds of spatial content that facilitate LTD, but they also show that the hippocampus can use odour and auditory items as a substitute for, or in addition to, visual items to create a record of spatial experience that is supported by persistent (>24 h) LTD.
6. Interplay of LTP and LTD enables the acquisition and retention of detailed spatial representations
Fluorescence in situ hybridization, used to exploit the property of immediate early genes (IEGs) to exhibit time-dependent peaks in somatic expression that are specifically linked to a behavioural or physiological event [126], has opened up opportunities to examine to what extent the abovementioned forms of learning-related LTP and LTD result in somatic information encoding and/or structural plasticity associated with synapse remodelling [119,127]. While the IEG, Arc, exhibits peak somatic expression 5–6 min after a specific experience [128], Homer1a exhibits peak somatic expression 30–40 min after a specific event [129]. Immediately after peak somatic expression, the IEG diffuses into the cytoplasm [126], meaning that the detection of somatic IEG expression can serve as an accurate indicator of which neurons engaged in a specific experience-dependent event.
Homer1a is involved in experience-dependent remodelling of the post-synaptic density [130], including increasing both the clustering of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR) [131], as well as the proportion of GluA2-containing AMPAR [132]. In contrast, Arc reduces surface AMPAR expression on synapses [133]. Moreover, Arc accumulates in non-potentiated synapses [134] and contributes to the weakening of synapses by promoting AMPAR endocytosis [135]. To what extent Arc specifically accumulates in synapses that undergo LTD is unclear. The facilitation of LTP by spatial learning results in widespread somatic Homer1a expression across hippocampal subfields [136]. In contrast, learning-facilitation of LTD results in somatic Homer1a expression that is limited to the subfields that express LTD; for example, LTD facilitation by learning about landmark configurations is limited to neurons of the DG and CA3 regions [136]. Examination of somatic IEG expression triggered by exploration of novel items in the holes of a novel holeboard revealed that fusing both novel (LTP-related and LTD-related) events triggers subfield-specific increases in Arc and Homer1a expression [137] that correspond to the subfields in which LTD is expressed [71]. This raises the possibility that LTP and LTD occur in a dynamic partnership: the one (LTP) serving to identify the neural network in which information will be stored and the other (LTD) serving to modify this representation.
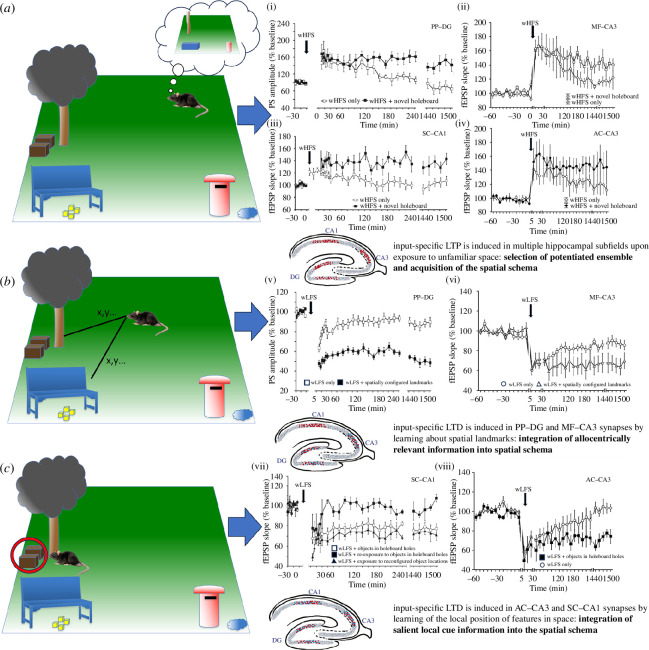
This interpretation is supported by data from an electrophysiological study where a novel holeboard containing novel items was presented to rats during test–pulse stimulation of SC–CA1 synapses [68]. What emerged was a potentiation of synaptic responses that segued into synaptic depression. Mechanistically, one could envisage that LTP selects the pan-hippocampal neuronal network that serves as the primary scaffold for information storage and that LTD acts to dynamically enhance the resolution and uniqueness of these potentiated synapses (see, for example, [138]), thereby permitting the storage and disambiguation of similar experiences. When an animal is exposed to a novel environment, LTP is rapidly induced at selected synapses throughout the hippocampus [11,71] (figure 4). If the environment is salient enough or enough time is spent exploring the environment, LTD in PP–DG and MF–CA3 synapses serves to modify the ensemble such that allocentric orientational details are included, whereas LTD at AC–CA3 and SC–CA1 synapses enables the retention of more localized information about the content of the environment [24,71] (figure 4). We propose that this interplay between LTP and LTD is functionally very meaningful: after childhood and arguably young adulthood, most of what we learn is likely to use schemata of past associative experiences [139,140] as the basis for new associative learning. After our first exposure to, e.g. a city park, our next exposure to a park in a different city will use the previous schema to create a new representation that can be disambiguated from the last. We do this over and over in life. It is feasible that LTP serves as the basis for the schema that is re-used for the encoding of similar or updated associative events and that LTD ensures that each generated representation is nonetheless unique.
Figure 4.
In this concept, a rat is introduced to a completely novel spatial environment where high-contrast items (tree, park bench and letterbox) can be perceived from their initial positions without the need for visual acuity. Movement in the environment allows the animal to acquire metric information about its position relative to these landmark features, as well as to locate salient local features that can only be discovered when the animal is close to them (such as crates that could be used for shelter, food remnants that are under the park bench or water that is beside the letterbox). (a) Initial exposure to a novel spatial environment results in immediate induction of LTP in an input-specific manner through hippocampal subfields (red neurons within the top hippocampus schema). By this means a neuronal ensemble is selected in a distributed hippocampal network that serves to retain a schematic representation of the spatial environment. (i–iv) Input-specific LTP (>24 h) is induced in PP–DG (i), MF–CA3 (ii), SC–CA1 (iii) and AC–CA3 (iv) synapses when exposure to entirely novel space is coupled with weak afferent stimulation (weak high-frequency stimulation, wHFS). (b) If time is spent moving within the environment, allocentric representations are cumulatively created that permit the acquisition of dimensional, orientational and directional (e.g. landmark) information that allows the integration of allocentrically relevant details into the initially acquired spatial schema. (v-vi) This information is encoded by means of LTD (>24h) in PP–DG (v) and MF–CA3 (vi) synapses (blue neurons within the middle hippocampus schema). (c) Salient local details of the environment (i.e. information that can only be found by means of proximal exploration and which is integrated into the allocentric reference frame) are acquired by means of LTD (> 24h) that is expressed in SC–CA1 (vii) and AC–CA3 synapses (viii) (blue neurons within bottom hippocampus schema). By this means the spatial representation is modified and refined, such that it can be discriminated from other similar representations. Graphs are modified from [1,11,24].
An interesting possibility is that the sensory features of, e.g. a novel city park result in the recruitment of hippocampal engram cells [141], via LTP, into an ensemble that will repeatedly respond to these cues when the individual is exposed to any generic city park in the future . Depending on the circumstance, this will result in retrieval of the original park memory (pattern completion) or the creation of a new city park memory (pattern separation, driving modifications of the representation). The site of access of the original schema is likely to be the hippocampus (CA1, CA3, DG subfields) [142] even if remote memory of the schema is stored in the neocortex [143], whereas the updating of the schema to integrate new information recruits information processing in both the hippocampus and cortical regions such as the retrosplenial cortex [142,144].
With the caveat in mind that the experiments were done in experience-naive animals, it was shown that LTP can be maintained for months in the hippocampus of rats [145], thus raising the possibility that the scaffold of the schema may be retained in the hippocampus for prolonged periods and reactivated by appropriate sensory input from the EC. Here, the possibility exists that LTD not only serves to modify the schema to enable the creation of a new representation that is distinguishable from other similar memories, but also that it plays a role in driving changes in neocortical representations: induction of LTD in the CA1 region in association with spatial content learning drives somatic IEG expression in the retrosplenial cortex [146].
7. How is the hippocampus instructed to express either persistent LTP or persistent LTD?
Although the frequency-dependency of electrophysiologically induced persistent LTP and LTD largely determines the direction of change in synaptic strength (electronic supplementary material, tables S1–S2), this is not the case for endogenously induced synaptic plasticity. Either STD or LTD can be induced by test–pulse stimulation of hippocampal afferents in conjunction with spatial content learning [68,75]. This process can even curtail LTP [75]. Afferent stimulation that emulates theta frequency oscillations in the hippocampus (which typically occur during spatial exploration: [147,148]) can either promote or interfere with LTP [149,150]. While stimulation on the peak of theta induces LTP, stimulation on its trough induces LTD [151,152]. This raises the question as to the means by which the hippocampus interprets incoming signals such that information encoding in the form of LTP or LTD occurs.
As mentioned earlier, hippocampal information processing is state-dependent. Elevations in attention trigger increased medial septal release of acetylcholine and glutamate in the hippocampus [153], which change network excitability, drive theta oscillations [154,155] and lower the threshold of LTP induction [96]. Action of septal acetylcholine in the hippocampus also permits that stimuli, that are otherwise subthreshold for induction of synaptic plasticity to successfully induce LTP [154] and supports the generation of spatial representations [156]. The ventral tegmental area (VTA) is a midbrain structure that plays an important role in the perception and binding of reward and punishment-related stimuli [157]. It has been proposed to engage in a feedback loop with the hippocampus that enhances novelty-related firing of cells in both structures [158]. VTA activity may modulate both hippocampal LTP [159] and hippocampus-dependent associative memories [160]. Furthermore, activation of midbrain inputs to the hippocampus fosters the persistence of spatial memory and leads to reactivation of neuronal ensembles that were recruited during spatial learning [161]. These observations suggest that septal and VTA inputs to the hippocampus may facilitate LTP induction during increased arousal related to associative experience.
The locus coeruleus (LC) is a strong candidate for endogenous promotion of hippocampal LTD. Test–pulse stimulation of hippocampal afferents in conjunction with electrophysiological activation of the LC results in input-specific hippocampal LTD that is NMDAR-dependent [105,108,162]. Stimulation of the LC also improves episodic-like memory in rats [108], facilitates spatial memory retention [106] and supports spatial contextual memory updating [163]. The timing of LC activity relative to the induction of LTP in vivo can either have no effect on, or depotentiate, recently induced LTP [164]. LC-mediated hippocampal LTD can be induced by a variety of LC frequencies [162] and is evident in both PP–DG and SC–CA1 synapses of freely behaving rats [108], suggesting that this is a very robust phenomenon. Taken together, these findings could suggest that changes in LC firing that are driven by saliency or novelty could enable hippocampal LTD and related encoding of spatial content. The coincidence of informational inputs from other sources is essential; however, LC stimulation fails to induce LTD in freely behaving rats in the absence of test–pulse stimulation of hippocampal afferents [108] and in anaesthetized rodents, in which excitatory responses are consequently depressed [165,166] and the thresholds for induction of synaptic plasticity are increased [167], LC stimulation can induce either SOP [106] or inhibit LTP [163].
8. Conclusion
Causal evidence is accumulating that persistent forms of LTP and LTD enable the acquisition and retention of associative memories. While LTP enables the acquisition of the associative schema and initial spatial representations, LTD appears to support the refinement and optimization of the representation such that allocentric and subtle spatial content details are included in the representation [71]. LTD may also enable dynamic updating and adaptive flexibility of engram ensembles [138], thereby ensuring that similar representations can be disambiguated and remain unique. Through this dynamic interplay of hippocampal LTP and LTD, associative representations can be linked and updated, thereby allowing the creation and retention of reliable records of complex experience.
Acknowledgements
We are really grateful to current and past members of the Department of Neurophysiology for their contributions. Many thanks to Jens Collitti-Klausnitzer for rat drawings.
Contributor Information
Hardy Hagena, Email: hardy.hagena@rub.de.
Denise Manahan-Vaughan, Email: denise.manahan-vaughan@rub.de.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
Supplementary material is available online [168].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
H.H.: investigation, validation, writing—review and editing; D.M.-V.: conceptualization, funding acquisition, investigation, validation, writing—original draft, writing—review and editing.
Both authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
German Research Foundation (Deutsche Forschungsgemeinschaft), Grant no.: SFB1280/A04, project no.: 31 68 03 389.
References
- 1. Manahan-Vaughan D. 2017. Learning-related hippocampal long-term potentiation and long-term depression. In Learning and memory: a comprehensive reference (second edition). reference Module in Neuroscience and Biobehavioral psychology (ed. Byrne JH), pp. 585–609. Elsevier. ( 10.1016/b978-0-12-809324-5.21104-8) [DOI] [Google Scholar]
- 2. Stacho M, Manahan-Vaughan D. 2022. The intriguing contribution of hippocampal long-term depression to spatial learning and long-term memory. Front. Behav. Neurosci. 16 , 806356. ( 10.3389/fnbeh.2022.806356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whitlock JR, Heynen AJ, Shuler MG, Bear MF. 2006. Learning induces long-term potentiation in the hippocampus. Science 313 , 1093–1097. ( 10.1126/science.1128134) [DOI] [PubMed] [Google Scholar]
- 4. Hulme SR, Jones OD, Raymond CR, Sah P, Abraham WC. 2014. Mechanisms of heterosynaptic metaplasticity. Phil. Trans. R. Soc. B 369 , 20130148, ( 10.1098/rstb.2013.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rogerson T, Cai DJ, Frank A, Sano Y, Shobe J, Lopez-Aranda MF, Silva AJ. 2014. Synaptic tagging during memory allocation. Nat. Rev. Neurosci. 15 , 157–169. ( 10.1038/nrn3667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yee AX, Hsu YT, Chen L. 2017. A metaplasticity view of the interaction between homeostatic and Hebbian plasticity. Phil. Trans. R. Soc. B 372 , 20160155. ( 10.1098/rstb.2016.0155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bliss TVP, Collingridge GL. 2013. Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Mol. Brain 6 , 5. ( 10.1186/1756-6606-6-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nicoll RA. 2017. A brief history of long-term potentiation. Neuron 93 , 281–290. ( 10.1016/j.neuron.2016.12.015) [DOI] [PubMed] [Google Scholar]
- 9. Ibrahim MZB, Benoy A, Sajikumar S. 2022. Long‐term plasticity in the hippocampus: maintaining within and ‘tagging’ between synapses. FEBS J. 289 , 2176–2201. ( 10.1111/febs.16065) [DOI] [PubMed] [Google Scholar]
- 10. Reymann KG, Frey JU. 2007. The late maintenance of hippocampal LTP: requirements, phases, ‘synaptic tagging’, ‘late-associativity’ and implications. Neuropharmacology 52 , 24–40. ( 10.1016/j.neuropharm.2006.07.026) [DOI] [PubMed] [Google Scholar]
- 11. Kemp A, Manahan-Vaughan D. 2004. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc. Natl Acad. Sci. USA 101 , 8192–8197. ( 10.1073/pnas.0402650101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abraham WC. 2003. How long will long-term potentiation last? Phil. Trans. R. Soc. B 358 , 735–744. ( 10.1098/rstb.2002.1222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park P, Volianskis A, Sanderson TM, Bortolotto ZA, Jane DE, Zhuo M, Kaang BK, Collingridge GL. 2014. NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation. Phil. Trans. R. Soc. B 369 , 20130131. ( 10.1098/rstb.2013.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ballesteros JJ, Buschler A, Köhr G, Manahan-Vaughan D. 2016. Afferent input selects NMDA receptor subtype to determine the persistency of hippocampal LTP in freely behaving mice. Front. Synaptic Neurosci. 8 , 33. ( 10.3389/fnsyn.2016.00033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagena H, Manahan-Vaughan D. 2022. Role of mGlu5 in persistent forms of hippocampal synaptic plasticity and the encoding of spatial experience. Cells 11 , 3352. ( 10.3390/cells11213352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barco A, Patterson SL, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER. 2005. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron 48 , 123–137. ( 10.1016/j.neuron.2005.09.005) [DOI] [PubMed] [Google Scholar]
- 17. Frey U, Frey S, Schollmeier F, Krug M. 1996. Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro. J. Physiol. 490 , 703–711. ( 10.1113/jphysiol.1996.sp021179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. France G, et al. 2022. Differential regulation of STP, LTP and LTD by structurally diverse NMDA receptor subunit-specific positive allosteric modulators. Neuropharmacology 202 , 108840. ( 10.1016/j.neuropharm.2021.108840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thiels E, Xie X, Yeckel MF, Barrionuevo G, Berger TW. 1996. NMDA receptor-dependent LTD in different subfields of hippocampus in vivo and in vitro. Hippocampus 6 , 43–51. () [DOI] [PubMed] [Google Scholar]
- 20. Manahan-Vaughan D. 1997. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J. Neurosci. 17 , 3303–3311. ( 10.1523/JNEUROSCI.17-09-03303.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aarse J, Herlitze S, Manahan‐Vaughan D. 2016. The requirement of BDNF for hippocampal synaptic plasticity is experience‐dependent. Hippocampus 26 , 739–751. ( 10.1002/hipo.22555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kemp A, Tischmeyer W, Manahan-Vaughan D. 2013. Learning-facilitated long-term depression requires activation of the immediate early gene, c-fos, and is transcription dependent. Behav. Brain Res. 254 , 83–91. ( 10.1016/j.bbr.2013.04.036) [DOI] [PubMed] [Google Scholar]
- 23. Manahan-Vaughan D, Kulla A, Frey JU. 2000. Requirement of translation but not transcription for the maintenance of long-term depression in the CA1 region of freely moving rats. J. Neurosci. 20 , 8572–8576. ( 10.1523/JNEUROSCI.20-22-08572.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hagena H, Manahan-Vaughan D. 2011. Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural–associational synapses reveals different roles in information processing. Cereb. Cortex 21 , 2442–2449. ( 10.1093/cercor/bhq271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kenney J, Manahan-Vaughan D. 2013. NMDA receptor-dependent synaptic plasticity in dorsal and intermediate hippocampus exhibits distinct frequency-dependent profiles. Neuropharmacology 74 , 108–118. ( 10.1016/j.neuropharm.2013.02.017) [DOI] [PubMed] [Google Scholar]
- 26. Beckmann D, Feldmann M, Shchyglo O, Manahan-Vaughan D. 2020. Hippocampal synaptic plasticity, spatial memory, and neurotransmitter receptor expression are profoundly altered by gradual loss of hearing ability. Cereb. Cortex 30 , 4581–4596. ( 10.1093/cercor/bhaa061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hagena H, Stacho M, Laja A, Manahan-Vaughan D. 2022. Strain-dependent regulation of hippocampal long-term potentiation by dopamine D1/D5 receptors in mice. Front. Behav. Neurosci. 16 , 1023361. ( 10.3389/fnbeh.2022.1023361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hagena H, Feldmann M, Manahan-Vaughan D. 2022. Lifelong changes of neurotransmitter receptor expression and debilitation of hippocampal synaptic plasticity following early postnatal blindness. Sci. Rep. 12 , 9142. ( 10.1038/s41598-022-13127-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kulla A, Manahan-Vaughan D. 2002. Modulation by serotonin 5-HT(4) receptors of long-term potentiation and depotentiation in the dentate gyrus of freely moving rats. Cereb. Cortex 12 , 150–162. ( 10.1093/cercor/12.2.150) [DOI] [PubMed] [Google Scholar]
- 30. Maggio N, Segal M. 2007. Unique regulation of long term potentiation in the rat ventral hippocampus. Hippocampus 17 , 10–25. ( 10.1002/hipo.20237) [DOI] [PubMed] [Google Scholar]
- 31. Manahan-Vaughan D. 2019. Chapter 1 - Recording field potentials and synaptic plasticity from freely behaving rodents. Handb. Behav. Neurosci. 28 , 1–42. ( 10.1016/b978-0-12-812028-6.00001-x) [DOI] [Google Scholar]
- 32. Aksoy-Aksel A, Manahan-Vaughan D. 2013. The temporoammonic input to the hippocampal CA1 region displays distinctly different synaptic plasticity compared to the Schaffer collateral input in vivo: significance for synaptic information processing. Front. Synaptic Neurosci. 5 , 5. ( 10.3389/fnsyn.2013.00005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gonzalez J, Villarreal DM, Morales IS, Derrick BE. 2016. Long-term potentiation at temporoammonic path-CA1 synapses in freely moving rats. Front. Neural Circuits 10 , 2. ( 10.3389/fncir.2016.00002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hagena H, Manahan-Vaughan D. 2010. Frequency facilitation at mossy fiber–CA3 synapses of freely behaving rats contributes to the induction of persistent LTD via an adenosine-A1 receptor-regulated mechanism. Cereb. Cortex 20 , 1121–1130. ( 10.1093/cercor/bhp184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinez CO, Do VH, Martinez JL, Derrick BE. 2002. Associative long-term potentiation (LTP) among extrinsic afferents of the hippocampal CA3 region in vivo. Brain Res. 940 , 86–94. ( 10.1016/S0006-8993(02)02598-2) [DOI] [PubMed] [Google Scholar]
- 36. Do VH, Martinez CO, Martinez JL, Derrick BE. 2002. Long-term potentiation in direct perforant path projections to the hippocampal CA3 region in vivo. J. Neurophysiol. 87 , 669–678. ( 10.1152/jn.00938.2000) [DOI] [PubMed] [Google Scholar]
- 37. Manahan-Vaughan D, Braunewell KH, Reymann KG. 1998. Subtype-specific involvement of metabotropic glutamate receptors in two forms of long-term potentiation in the dentate gyrus of freely moving rats. Neuroscience 86 , 709–721. ( 10.1016/S0306-4522(98)00111-0) [DOI] [PubMed] [Google Scholar]
- 38. Strauch C, Böge J, Shchyglo O, Dubovyk V, Manahan-Vaughan D. 2024. The suprapyramidal and infrapyramidal blades of the dentate gyrus exhibit different GluN subunit content and dissimilar frequency-dependent synaptic plasticity in vivo. Biorxiv. ( 10.1101/2024.01.08.574587) [DOI]
- 39. Collitti-Klausnitzer J, Hagena H, Dubovyk V, Manahan-Vaughan D. 2021. Preferential frequency-dependent induction of synaptic depression by the lateral perforant path and of synaptic potentiation by the medial perforant path inputs to the dentate gyrus. Hippocampus 31 , 957–981. ( 10.1002/hipo.23338) [DOI] [PubMed] [Google Scholar]
- 40. Abraham WC, Goddard GV. 1983. Asymmetric relationships between homosynaptic long-term potentiation and heterosynaptic long-term depression. Nature 305 , 717–719. ( 10.1038/305717a0) [DOI] [PubMed] [Google Scholar]
- 41. Mezey S, Doyère V, De Souza I, Harrison E, Cambon K, Kendal CE, Davies H, Laroche S, Stewart MG. 2004. Long-term synaptic morphometry changes after induction of long-term potentiation and long-term depression in the dentate gyrus of awake rats are not simply mirror phenomena. Eur. J. Neurosci. 19 , 2310–2318. ( 10.1111/j.0953-816X.2004.03334.x) [DOI] [PubMed] [Google Scholar]
- 42. Manahan-Vaughan D. 2019. Chapter 3 - Special considerations when using mice for in vivo electrophysiology and long-term studies of hippocampal synaptic plasticity during behavior. Handb. Behav. Neurosci 28 , 63–84. ( 10.1016/b978-0-12-812028-6.00003-3) [DOI] [Google Scholar]
- 43. Buschler A, Goh JJ, Manahan-Vaughan D. 2012. Frequency dependency of NMDA receptor-dependent synaptic plasticity in the hippocampal CA1 region of freely behaving mice. Hippocampus 22 , 2238–2248. ( 10.1002/hipo.22041) [DOI] [PubMed] [Google Scholar]
- 44. Jansen S, Gottschling C, Faissner A, Manahan-Vaughan D. 2017. Intrinsic cellular and molecular properties of in vivo hippocampal synaptic plasticity are altered in the absence of key synaptic matrix molecules. Hippocampus 27 , 920–933. ( 10.1002/hipo.22742) [DOI] [PubMed] [Google Scholar]
- 45. Simon MM, et al. 2013. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 14 , R82. ( 10.1186/gb-2013-14-7-r82) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Radulovic J, Kammermeier J, Spiess J. 1998. Generalization of fear responses in C57BL/6N mice subjected to one-trial foreground contextual fear conditioning. Behav. Brain Res. 95 , 179–189. ( 10.1016/S0166-4328(98)00039-4) [DOI] [PubMed] [Google Scholar]
- 47. Stiedl O, Palve M, Radulovic J, Birkenfeld K, Spiess J. 1999. Differential impairment of auditory and contextual fear conditioning by protein synthesis inhibition in C57BL/6N mice. Behav. Neurosci. 113 , 496–506. ( 10.1037//0735-7044.113.3.496) [DOI] [PubMed] [Google Scholar]
- 48. Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T. 2010. Behavioral profiles of three C57BL/6 substrains. Front. Behav. Neurosci. 4 , 29. ( 10.3389/fnbeh.2010.00029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Andolina D, Maran D, Viscomi MT, Puglisi-Allegra S. 2014. Strain-dependent variations in stress coping behavior are mediated by a 5-HT/GABA interaction within the prefrontal corticolimbic system. Int. J. Neuropsychopharmacol. 18 , pyu074. ( 10.1093/ijnp/pyu074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. 2007. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One 2 , e351. ( 10.1371/journal.pone.0000351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Panksepp JB, Lahvis GP. 2007. Social reward among juvenile mice. Genes Brain Behav. 6 , 661–671. ( 10.1111/j.1601-183X.2006.00295.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yochum CL, Medvecky CM, Cheh MA, Bhattacharya P, Wagner GC. 2010. Differential development of central dopaminergic and serotonergic systems in BALB/c and C57BL/6J mice. Brain Res. 1349 , 97–104. ( 10.1016/j.brainres.2010.06.031) [DOI] [PubMed] [Google Scholar]
- 53. Bertholet JY, Crusio WE. 1991. Spatial and non-spatial spontaneous alternation and hippocampal mossy fibre distribution in nine inbred mouse strains. Behav. Brain Res. 43 , 197–202. ( 10.1016/s0166-4328(05)80071-3) [DOI] [PubMed] [Google Scholar]
- 54. Wahlsten D, Cooper SF, Crabbe JC. 2005. Different rankings of inbred mouse strains on the Morris maze and a refined 4-arm water escape task. Behav. Brain Res. 165 , 36–51. ( 10.1016/j.bbr.2005.06.047) [DOI] [PubMed] [Google Scholar]
- 55. Park SN, Back SA, Park KH, Kim DK, Park SY, Oh JH, Park YS, Yeo SW. 2010. Comparison of cochlear morphology and apoptosis in mouse models of presbycusis. Clin. Exp. Otorhinolaryngol. 3 , 126. ( 10.3342/ceo.2010.3.3.126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mikaelian DO. 1979. Development and degeneration of hearing in the C57/b16 mouse: relation of electrophysiologic responses from the round window and cochlear nucleus to cochlear anatomy and behavioral responses. Laryngoscope 89 , 1–15. ( 10.1288/00005537-197901000-00001) [DOI] [PubMed] [Google Scholar]
- 57. Kopachev N, Netser S, Wagner S. 2022. Sex-dependent features of social behavior differ between distinct laboratory mouse strains and their mixed offspring. iScience 25 , 103735. ( 10.1016/j.isci.2022.103735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Colacicco G, Welzl H, Lipp HP, Würbel H. 2002. Attentional set-shifting in mice: modification of a rat paradigm, and evidence for strain-dependent variation. Behav. Brain Res. 132 , 95–102. ( 10.1016/s0166-4328(01)00391-6) [DOI] [PubMed] [Google Scholar]
- 59. Yoshida N, Hequembourg SJ, Atencio CA, Rosowski JJ, Liberman MC. 2000. Acoustic injury in mice: 129/SvEv is exceptionally resistant to noise-induced hearing loss. Hear. Res. 141 , 97–106. ( 10.1016/S0378-5955(99)00210-5) [DOI] [PubMed] [Google Scholar]
- 60. Bortolotto ZA, et al. 1999. Kainate receptors are involved in synaptic plasticity. Nature 402 , 297–301. ( 10.1038/46290) [DOI] [PubMed] [Google Scholar]
- 61. Hagena H, Manahan-Vaughan D. 2013. Differentiation in the protein synthesis-dependency of persistent synaptic plasticity in mossy fiber and associational/commissural CA3 synapses in vivo. Front. Integr. Neurosci. 7 , 10. ( 10.3389/fnint.2013.00010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nicoll RA, Schulman H. 2023. Synaptic memory and CaMKII. Physiol. Rev. 103 , 2897–2945. ( 10.1152/physrev.00034.2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Park M. 2018. AMPA receptor trafficking for postsynaptic potentiation. Front. Cell. Neurosci. 12 , 361. ( 10.3389/fncel.2018.00361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Soderling TR, Derkach VA. 2000. Postsynaptic protein phosphorylation and LTP. Trends Neurosci. 23 , 75–80. ( 10.1016/S0166-2236(99)01490-3) [DOI] [PubMed] [Google Scholar]
- 65. Bortolotto ZA, Lauri S, Isaac JTR, Collingridge GL. 2003. Kainate receptors and the induction of mossy fibre long-term potentiation. Phil. Trans. R. Soc. B 358 , 657–666. ( 10.1098/rstb.2002.1216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Amani M, Lauterborn JC, Le AA, Cox BM, Wang W, Quintanilla J, Cox CD, Gall CM, Lynch G. 2021. Rapid aging in the perforant path projections to the rodent dentate gyrus. J. Neurosci. 41 , 2301–2312. ( 10.1523/JNEUROSCI.2376-20.2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gonzalez J, Morales IS, Villarreal DM, Derrick BE. 2014. Low-frequency stimulation induces long-term depression and slow onset long-term potentiation at perforant path-dentate gyrus synapses in vivo. J. Neurophysiol. 111 , 1259–1273. ( 10.1152/jn.00941.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Manahan-Vaughan D, Braunewell KH. 1999. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc. Natl Acad. Sci. USA 96 , 8739–8744. ( 10.1073/pnas.96.15.8739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hagena H, Manahan-Vaughan D. 2015. mGlu5 acts as a switch for opposing forms of synaptic plasticity at mossy fiber-CA3 and commissural associational-CA3 synapses. J. Neurosci. 35 , 4999–5006. ( 10.1523/JNEUROSCI.3417-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Twarkowski H, Hagena H, Manahan-Vaughan D. 2016. The 5-hydroxytryptamine4 receptor enables differentiation of informational content and encoding in the hippocampus. Hippocampus 26 , 875–891. ( 10.1002/hipo.22569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kemp A, Manahan-Vaughan D. 2008. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb. Cortex 18 , 968–977. ( 10.1093/cercor/bhm136) [DOI] [PubMed] [Google Scholar]
- 72. Popkirov SG, Manahan-Vaughan D. 2011. Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb. Cortex 21 , 501–509. ( 10.1093/cercor/bhq093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kulla A, Manahan-Vaughan D. 2000. Depotentiation in the dentate gyrus of freely moving rats is modulated by D1/D5 dopamine receptors. Cereb. Cortex 10 , 614–620. ( 10.1093/cercor/10.6.614) [DOI] [PubMed] [Google Scholar]
- 74. Goh JJ, Manahan-Vaughan D. 2013. Synaptic depression in the CA1 region of freely behaving mice is highly dependent on afferent stimulation parameters. Front. Integr. Neurosci. 7 , 1. ( 10.3389/fnint.2013.00001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Goh JJ, Manahan-Vaughan D. 2013. Spatial object recognition enables endogenous LTD that curtails LTP in the mouse hippocampus. Cereb. Cortex 23 , 1118–1125. ( 10.1093/cercor/bhs089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pöschel B, Manahan-Vaughan D. 2007. Persistent (>24h) long-term depression in the dentate gyrus of freely moving rats is not dependent on activation of NMDA receptors, L-type voltage-gated calcium channels or protein synthesis. Neuropharmacology 52 , 46–54. ( 10.1016/j.neuropharm.2006.07.019) [DOI] [PubMed] [Google Scholar]
- 77. Hansen N, Manahan-Vaughan D. 2015. Locus coeruleus stimulation facilitates long-term depression in the dentate gyrus that requires activation of β-adrenergic receptors. Cereb. Cortex 25 , 1889–1896. ( 10.1093/cercor/bht429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hagena H, Manahan-Vaughan D. 2012. Learning-facilitated long-term depression and long-term potentiation at mossy fiber—CA3 synapses requires activation of β-adrenergic receptors. Front. Integr. Neurosci. 6 , 23. ( 10.3389/fnint.2012.00023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jacobs LF, Schenk F. 2003. Unpacking the cognitive map: the parallel map theory of hippocampal function. Psychol. Rev. 110 , 285–315. ( 10.1037/0033-295x.110.2.285) [DOI] [PubMed] [Google Scholar]
- 80. Kesner RP. 2013. An analysis of the dentate gyrus function. Behav. Brain Res. 254 , 1–7. ( 10.1016/j.bbr.2013.01.012) [DOI] [PubMed] [Google Scholar]
- 81. Wang HS, Rosenbaum RS, Baker S, Lauzon C, Batterink LJ, Köhler S. 2023. Dentate gyrus integrity is necessary for behavioral pattern separation but not statistical learning. J. Cogn. Neurosci 35 , 900–917. ( 10.1162/jocn_a_01981) [DOI] [PubMed] [Google Scholar]
- 82. Amaral DG, Witter MP. 1989. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31 , 571–591. ( 10.1016/0306-4522(89)90424-7) [DOI] [PubMed] [Google Scholar]
- 83. Yamamoto J, Tonegawa S. 2017. Direct medial entorhinal cortex input to hippocampal CA1 is crucial for extended quiet awake replay. Neuron 96 , 217–227.( 10.1016/j.neuron.2017.09.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. van Groen T, Kadish I, Wyss JM. 2002. Species differences in the projections from the entorhinal cortex to the hippocampus. Brain Res. Bull. 57 , 553–556. ( 10.1016/S0361-9230(01)00683-9) [DOI] [PubMed] [Google Scholar]
- 85. Fernández-Ruiz A, Oliva A, Nagy GA, Maurer AP, Berényi A, Buzsáki G. 2017. Entorhinal-CA3 dual-input control of spike timing in the hippocampus by theta-gamma coupling. Neuron 93 , 1213–1226.( 10.1016/j.neuron.2017.02.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Remondes M, Schuman EM. 2002. Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature 416 , 736–740. ( 10.1038/416736a) [DOI] [PubMed] [Google Scholar]
- 87. Rolls ET. 2018. The storage and recall of memories in the hippocampo-cortical system. Cell Tissue Res. 373 , 577–604. ( 10.1007/s00441-017-2744-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Abraham WC. 2008. Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 9 , 387–387. ( 10.1038/nrn2356) [DOI] [PubMed] [Google Scholar]
- 89. Bashir ZI, et al. 1993. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature 363 , 347–350. ( 10.1038/363347a0) [DOI] [PubMed] [Google Scholar]
- 90. Zhang L, Kirschstein T, Sommersberg B, Merkens M, Manahan-Vaughan D, Elgersma Y, Beck H. 2005. Hippocampal synaptic metaplasticity requires inhibitory autophosphorylation of Ca2+/calmodulin-dependent kinase II. J. Neurosci. 25 , 7697–7707. ( 10.1523/JNEUROSCI.2086-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhong L, Gerges NZ. 2019. Neurogranin Regulates Metaplasticity. Front. Mol. Neurosci. 12 , 322. ( 10.3389/fnmol.2019.00322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chinestra P, Diabira D, Urban NN, Barrionuevo G, Ben-Ari Y. 1994. Major differences between long-term potentiation and ACPD-induced slow onset potentiation in hippocampus. Neurosci. Lett. 182 , 177–180. ( 10.1016/0304-3940(94)90791-9) [DOI] [PubMed] [Google Scholar]
- 93. Manahan-Vaughan D, Reymann KG. 1995. 1S,3R-ACPD dose-dependently induces a slow-onset potentiation in the rat hippocampal CA1 region in vivo. Neuropharmacology 34 , 1103–1105. ( 10.1016/0028-3908(95)00108-I) [DOI] [PubMed] [Google Scholar]
- 94. Shetty MS, Sajikumar S. 2017. Differential involvement of Ca2+/calmodulin-dependent protein kinases and mitogen-activated protein kinases in the dopamine D1/D5 receptor-mediated potentiation in hippocampal CA1 pyramidal neurons. Neurobiol. Learn. Mem. 138 , 111–120. ( 10.1016/j.nlm.2016.07.020) [DOI] [PubMed] [Google Scholar]
- 95. Mockett BG, Hulme SR. 2008. Metaplasticity: new insights through electrophysiological investigations. J. Integr. Neurosci. 7 , 315–336. ( 10.1142/s0219635208001782) [DOI] [PubMed] [Google Scholar]
- 96. Benoy A, Ibrahim MZB, Behnisch T, Sajikumar S. 2021. Metaplastic reinforcement of long-term potentiation in hippocampal area CA2 by cholinergic receptor activation. J. Neurosci. 41 , 9082–9098. ( 10.1523/JNEUROSCI.2885-20.2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yang Q, Liao ZH, Xiao YX, Lin QS, Zhu YS, Li ST. 2011. Hippocampal synaptic metaplasticity requires the activation of NR2B-containing NMDA receptors. Brain Res. Bull. 84 , 137–143. ( 10.1016/j.brainresbull.2010.12.009) [DOI] [PubMed] [Google Scholar]
- 98. Kulla A, Reymann KG, Manahan-Vaughan D. 1999. Time-dependent induction of depotentiation in the dentate gyrus of freely moving rats: involvement of group 2 metabotropic glutamate receptors. Eur. J. Neurosci. 11 , 3864–3872. ( 10.1046/j.1460-9568.1999.00807.x) [DOI] [PubMed] [Google Scholar]
- 99. Wang H, Wagner JJ. 1999. Priming-induced shift in synaptic plasticity in the rat hippocampus. J. Neurophysiol. 82 , 2024–2028. ( 10.1152/jn.1999.82.4.2024) [DOI] [PubMed] [Google Scholar]
- 100. Sajikumar S, Navakkode S, Frey JU. 2007. Identification of compartment- and process-specific molecules required for “synaptic tagging” during long-term potentiation and long-term depression in hippocampal CA1. J. Neurosci. 27 , 5068–5080. ( 10.1523/JNEUROSCI.4940-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Amemiya S, Redish AD. 2018. Hippocampal theta-gamma coupling reflects state-dependent information processing in decision making. Cell Rep. 22 , 3328–3338. ( 10.1016/j.celrep.2018.02.091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ahmed T, Frey JU, Korz V. 2006. Long-term effects of brief acute stress on cellular signaling and hippocampal LTP. J. Neurosci. 26 , 3951–3958. ( 10.1523/JNEUROSCI.4901-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Diamond DM, Park CR, Woodson JC. 2004. Stress generates emotional memories and retrograde amnesia by inducing an endogenous form of hippocampal LTP. Hippocampus 14 , 281–291. ( 10.1002/hipo.10186) [DOI] [PubMed] [Google Scholar]
- 104. Xu L, Holscher C, Anwyl R, Rowan MJ. 1998. Glucocorticoid receptor and protein/RNA synthesis-dependent mechanisms underlie the control of synaptic plasticity by stress. Proc. Natl Acad. Sci. USA 95 , 3204–3208. ( 10.1073/pnas.95.6.3204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hagena H, Hansen N, Manahan-Vaughan D. 2016. β-Adrenergic control of hippocampal function: subserving the choreography of synaptic information storage and memory. Cereb. Cortex 26 , 1349–1364. ( 10.1093/cercor/bhv330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tse D, Privitera L, Norton AC, Gobbo F, Spooner P, Takeuchi T, Martin SJ, Morris RGM. 2023. Cell-type-specific optogenetic stimulation of the locus coeruleus induces slow-onset potentiation and enhances everyday memory in rats. Proc. Natl Acad. Sci. USA 120 , e2307275120. ( 10.1073/pnas.2307275120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Williams JH, Errington ML, Lynch MA, Bliss TVP. 1989. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature 341 , 739–742. ( 10.1038/341739a0) [DOI] [PubMed] [Google Scholar]
- 108. Lemon N, Aydin-Abidin S, Funke K, Manahan-Vaughan D. 2009. Locus coeruleus activation facilitates memory encoding and induces hippocampal LTD that depends on β-adrenergic receptor activation. Cereb. Cortex 19 , 2827–2837. ( 10.1093/cercor/bhp065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Balschun D, Wetzel W. 2002. Inhibition of mGluR5 blocks hippocampal LTP in vivo and spatial learning in rats. Pharmacol. Biochem. Behav. 73 , 375–380. ( 10.1016/s0091-3057(02)00847-x) [DOI] [PubMed] [Google Scholar]
- 110. Morris RG. 1989. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J. Neurosci. 9 , 3040–3057. ( 10.1523/JNEUROSCI.09-09-03040.1989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Naie K, Manahan‐Vaughan D. 2005. Pharmacological antagonism of metabotropic glutamate receptor 1 regulates long‐term potentiation and spatial reference memory in the dentate gyrus of freely moving rats via N‐methyl‐D‐aspartate and metabotropic glutamate receptor‐dependent mechanisms. Eur. J. Neurosci. 21 , 411–421. ( 10.1111/j.1460-9568.2005.03864.x) [DOI] [PubMed] [Google Scholar]
- 112. Tallent MK, et al. 2005. Cortistatin overexpression in transgenic mice produces deficits in synaptic plasticity and learning. Mol. Cell. Neurosci. 30 , 465–475. ( 10.1016/j.mcn.2005.08.010) [DOI] [PubMed] [Google Scholar]
- 113. Thiels E, Urban NN, Gonzalez-Burgos GR, Kanterewicz BI, Barrionuevo G, Chu CT, Oury TD, Klann E. 2000. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J. Neurosci. 20 , 7631–7639. ( 10.1523/JNEUROSCI.20-20-07631.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AMM, Lombardi TL, Abel T. 2005. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn. Mem. 12 , 111–119. ( 10.1101/lm.86605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Straube T, Korz V, Frey JU. 2003. Bidirectional modulation of long-term potentiation by novelty-exploration in rat dentate gyrus. Neurosci. Lett. 344 , 5–8. ( 10.1016/S0304-3940(03)00349-5) [DOI] [PubMed] [Google Scholar]
- 116. Uzakov S, Frey JU, Korz V. 2005. Reinforcement of rat hippocampal LTP by holeboard training. Learn. Mem. 12 , 165–171. ( 10.1101/lm.89305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C, Mayford M. 2012. Generation of a synthetic memory trace. Science 335 , 1513–1516. ( 10.1126/science.1214985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bromer C, et al. 2018. Long-term potentiation expands information content of hippocampal dentate gyrus synapses. Proc. Natl Acad. Sci. USA 115 , E2410–E2418. ( 10.1073/pnas.1716189115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Harris KM. 2020. Structural LTP: from synaptogenesis to regulated synapse enlargement and clustering. Curr. Opin. Neurobiol. 63 , 189–197. ( 10.1016/j.conb.2020.04.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Jeffery KJ, Morris RGM. 1993. Cumulative long-term potentiation in the rat dentate gyrus correlates with, but does not modify, performance in the water maze. Hippocampus 3 , 133–140. ( 10.1002/hipo.450030205) [DOI] [PubMed] [Google Scholar]
- 121. Remy S, Spruston N. 2007. Dendritic spikes induce single-burst long-term potentiation. Proc. Natl Acad. Sci. USA 104 , 17192–17197. ( 10.1073/pnas.0707919104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wigström H, Gustafsson B. 1986. Postsynaptic control of hippocampal long-term potentiation. J. Physiol. (Paris) 81 , 228–236. [PubMed] [Google Scholar]
- 123. Abraham WC, Gustafsson B, Wigström H. 1986. Single high strength afferent volleys can produce long-term potentiation in the hippocampus in vitro. Neurosci. Lett. 70 , 217–222. ( 10.1016/0304-3940(86)90466-0) [DOI] [PubMed] [Google Scholar]
- 124. Dietz B, Manahan-Vaughan D. 2017. Hippocampal long-term depression is facilitated by the acquisition and updating of memory of spatial auditory content and requires mGlu5 activation. Neuropharmacology 115 , 30–41. ( 10.1016/j.neuropharm.2016.02.026) [DOI] [PubMed] [Google Scholar]
- 125. André MAE, Manahan-Vaughan D. 2013. Spatial olfactory learning facilitates long-term depression in the hippocampus. Hippocampus 23 , 963–968. ( 10.1002/hipo.22158) [DOI] [PubMed] [Google Scholar]
- 126. Guzowski JF, Worley PF. 2001. Cellular compartment analysis of temporal activity by fluorescence in situ hybridization (catFISH). Curr. Protoc. Neurosci. 15 , 1.8.1-1.8.16. ( 10.1002/0471142301.ns0108s15) [DOI] [PubMed] [Google Scholar]
- 127. Bailey CH, Kandel ER, Harris KM. 2015. Structural components of synaptic plasticity and memory consolidation. Cold Spring Harb. Perspect. Biol. 7 , a021758. ( 10.1101/cshperspect.a021758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Guzowski JF, McNaughton BL, Barnes CA, Worley PF. 1999. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 2 , 1120–1124. ( 10.1038/16046) [DOI] [PubMed] [Google Scholar]
- 129. Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. 2002. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J. Neurosci. 22 , 10067–10071. ( 10.1523/JNEUROSCI.22-23-10067.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Clifton NE, Trent S, Thomas KL, Hall J. 2019. Regulation and function of activity-dependent homer in synaptic plasticity. Mol. Neuropsychiatry 5 , 147–161. ( 10.1159/000500267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hennou S, Kato A, Schneider EM, Lundstrom K, Gähwiler BH, Inokuchi K, Gerber U, Ehrengruber MU. 2003. Homer-1a/Vesl-1S enhances hippocampal synaptic transmission. Eur. J. Neurosci. 18 , 811–819. ( 10.1046/j.1460-9568.2003.02812.x) [DOI] [PubMed] [Google Scholar]
- 132. Rozov A, Zivkovic AR, Schwarz MK. 2012. Homer1 gene products orchestrate Ca2+-permeable AMPA receptor distribution and LTP expression. Front. Syn. Neurosci 4 , 4. ( 10.3389/fnsyn.2012.00004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. El-Boustani S, Ip JPK, Breton-Provencher V, Knott GW, Okuno H, Bito H, Sur M. 2018. Locally coordinated synaptic plasticity of visual cortex neurons in vivo. Science 360 , 1349–1354. ( 10.1126/science.aao0862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Okuno H, et al. 2012. Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIβ. Cell 149 , 886–898. ( 10.1016/j.cell.2012.02.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. 2006. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron 52 , 445–459. ( 10.1016/j.neuron.2006.08.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hoang TH, Böge J, Manahan-Vaughan D. 2021. Hippocampal subfield-specific Homer1a expression is triggered by learning-facilitated long-term potentiation and long-term depression at medial perforant path synapses. Hippocampus 31 , 897–915. ( 10.1002/hipo.23333) [DOI] [PubMed] [Google Scholar]
- 137. Hoang TH, Aliane V, Manahan-Vaughan D. 2018. Novel encoding and updating of positional, or directional, spatial cues are processed by distinct hippocampal subfields: evidence for parallel information processing and the “what” stream. Hippocampus 28 , 315–326. ( 10.1002/hipo.22833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Malleret G, Alarcon JM, Martel G, Takizawa S, Vronskaya S, Yin D, Chen IZ, Kandel ER, Shumyatsky GP. 2010. Bidirectional regulation of hippocampal long-term synaptic plasticity and its influence on opposing forms of memory. J. Neurosci. 30 , 3813–3825. ( 10.1523/JNEUROSCI.1330-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. van Kesteren MTR, Rijpkema M, Ruiter DJ, Morris RGM, Fernández G. 2014. Building on prior knowledge: Schema-dependent encoding processes relate to academic performance. J. Cogn. Neurosci. 26 , 2250–2261. ( 10.1162/jocn_a_00630) [DOI] [PubMed] [Google Scholar]
- 140. Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM. 2007. Schemas and memory consolidation. Science 316 , 76–82. ( 10.1126/science.1135935) [DOI] [PubMed] [Google Scholar]
- 141. Ghandour K, et al. 2019. Orchestrated ensemble activities constitute a hippocampal memory engram. Nat. Commun. 10 , 2637. ( 10.1038/s41467-019-10683-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Takeuchi T, Tamura M, Tse D, Kajii Y, Fernández G, Morris RGM. 2022. Brain region networks for the assimilation of new associative memory into a schema. Mol. Brain 15 , 24. ( 10.1186/s13041-022-00908-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Bayley PJ, Gold JJ, Hopkins RO, Squire LR. 2005. The neuroanatomy of remote memory. Neuron 46 , 799–810. ( 10.1016/j.neuron.2005.04.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Stacho M, Manahan-Vaughan D. 2022. Mechanistic flexibility of the retrosplenial cortex enables its contribution to spatial cognition. Trends Neurosci. 45 , 284–296. ( 10.1016/j.tins.2022.01.007) [DOI] [PubMed] [Google Scholar]
- 145. Abraham WC, Logan B, Greenwood JM, Dragunow M. 2002. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J. Neurosci. 22 , 9626–9634. ( 10.1523/JNEUROSCI.22-21-09626.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hoang TH, Manahan-Vaughan D. 2024. Differentiated somatic gene expression is triggered in the dorsal hippocampus and the anterior retrosplenial cortex by hippocampal synaptic plasticity prompted by spatial content learning. Brain Struct. Funct. 229 , 639–655. ( 10.1007/s00429-023-02694-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Buzsáki G. 2005. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15 , 827–840. ( 10.1002/hipo.20113) [DOI] [PubMed] [Google Scholar]
- 148. O’Keefe J, Recce ML. 1993. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3 , 317–330. ( 10.1002/hipo.450030307) [DOI] [PubMed] [Google Scholar]
- 149. Barr DS, Lambert NA, Hoyt KL, Moore SD, Wilson WA. 1995. Induction and reversal of long-term potentiation by low- and high-intensity theta pattern stimulation. J. Neurosci. 15 , 5402–5410. ( 10.1523/JNEUROSCI.15-07-05402.1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Larson J, Lynch G. 1989. Theta pattern stimulation and the induction of LTP: the sequence in which synapses are stimulated determines the degree to which they potentiate. Brain Res. 489 , 49–58. ( 10.1016/0006-8993(89)90007-3) [DOI] [PubMed] [Google Scholar]
- 151. Huerta PT, Lisman JE. 1996. Low-frequency stimulation at the troughs of theta-oscillation induces long-term depression of previously potentiated CA1 synapses. J. Neurophysiol. 75 , 877–884. ( 10.1152/jn.1996.75.2.877) [DOI] [PubMed] [Google Scholar]
- 152. Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo ME. 2003. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J. Neurosci. 23 , 11725–11731. ( 10.1523/JNEUROSCI.23-37-11725.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tsanov M. 2017. Speed and oscillations: medial septum integration of attention and navigation. Front. Syst. Neurosci. 11 , 67. ( 10.3389/fnsys.2017.00067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Huerta PT, Lisman JE. 1993. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature 364 , 723–725. ( 10.1038/364723a0) [DOI] [PubMed] [Google Scholar]
- 155. Tsanov M. 2015. Septo-hippocampal signal processing: breaking the code. Prog. Brain Res. 219 , 103–120. ( 10.1016/bs.pbr.2015.04.002) [DOI] [PubMed] [Google Scholar]
- 156. Ikonen S, McMahan R, Gallagher M, Eichenbaum H, Tanila H. 2002. Cholinergic system regulation of spatial representation by the hippocampus. Hippocampus 12 , 386–397. ( 10.1002/hipo.1109) [DOI] [PubMed] [Google Scholar]
- 157. Geisler CE, Hayes MR. 2023. Metabolic hormone action in the VTA: Reward-directed behavior and mechanistic insights. Physiol. Behav. 268 , 114236. ( 10.1016/j.physbeh.2023.114236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lisman JE, Grace AA. 2005. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46 , 703–713. ( 10.1016/j.neuron.2005.05.002) [DOI] [PubMed] [Google Scholar]
- 159. Ghanbarian E, Motamedi F. 2013. Ventral tegmental area inactivation suppresses the expression of CA1 long term potentiation in anesthetized rat. PLoS One 8 , e58844. ( 10.1371/journal.pone.0058844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Tsetsenis T, Badyna JK, Li R, Dani JA. 2022. Activation of a locus coeruleus to dorsal hippocampus noradrenergic circuit facilitates associative learning. Front. Cell. Neurosci 16 , 887679. ( 10.3389/fncel.2022.887679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. McNamara CG, Tejero-Cantero Á, Trouche S, Campo-Urriza N, Dupret D. 2014. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat. Neurosci. 17 , 1658–1660. ( 10.1038/nn.3843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Babushkina N, Manahan-Vaughan D. 2022. Frequency-dependency of the involvement of dopamine D1/D5 and beta-adrenergic receptors in hippocampal LTD triggered by locus coeruleus stimulation. Hippocampus 32 , 449–465. ( 10.1002/hipo.23419) [DOI] [PubMed] [Google Scholar]
- 163. Gálvez-Márquez DK, Salgado-Ménez M, Moreno-Castilla P, Rodríguez-Durán L, Escobar ML, Tecuapetla F, Bermudez-Rattoni F. 2022. Spatial contextual recognition memory updating is modulated by dopamine release in the dorsal hippocampus from the locus coeruleus. Proc. Natl Acad. Sci. USA 119 , e2208254119. ( 10.1073/pnas.2208254119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Hansen N, Manahan-Vaughan D. 2015. Hippocampal long-term potentiation that is elicited by perforant path stimulation or that occurs in conjunction with spatial learning is tightly controlled by beta-adrenoreceptors and the locus coeruleus. Hippocampus 25 , 1285–1298. ( 10.1002/hipo.22436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bieda MC, Su H, Maciver MB. 2009. Anesthetics discriminate between tonic and phasic gamma-aminobutyric acid receptors on hippocampal CA1 neurons. Anesth. Analg. 108 , 484–490. ( 10.1213/ane.0b013e3181904571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Zhao W, et al. 2021. Isoflurane suppresses hippocampal high-frequency ripples by differentially modulating pyramidal neurons and interneurons in mice. Anesthesiology 135 , 122–135. ( 10.1097/ALN.0000000000003803) [DOI] [PubMed] [Google Scholar]
- 167. Riedel G, Seidenbecher T, Reymann KG. 1994. LTP in hippocampal CA1 of urethane-narcotized rats requires stronger tetanization parameters. Physiol. Behav. 55 , 1141–1146. ( 10.1016/0031-9384(94)90401-4) [DOI] [PubMed] [Google Scholar]
- 168. Hagena H, Manahan-Vaughan D. 2024. Supplementary material from: Interplay of hippocampal long-term potentiation and long-term depression in enabling memory representations. Figshare ( 10.6084/m9.figshare.c.7249508) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supplementary material is available online [168].