Abstract
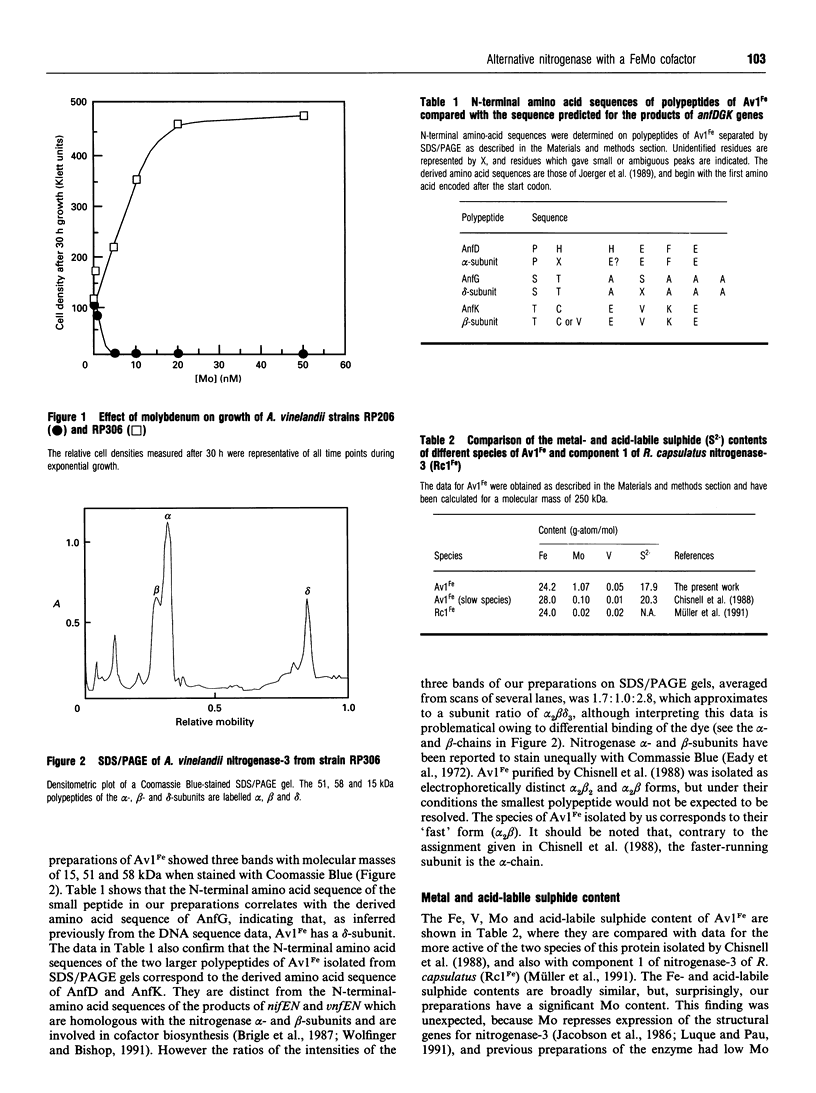
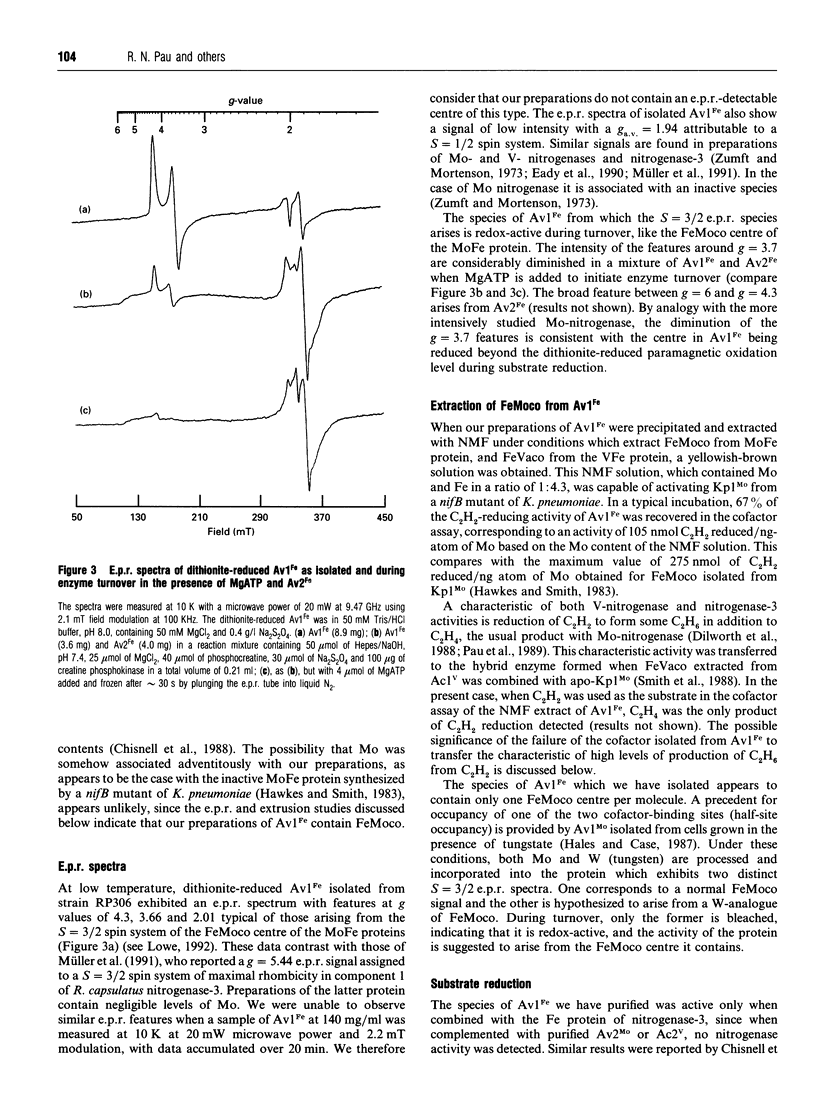
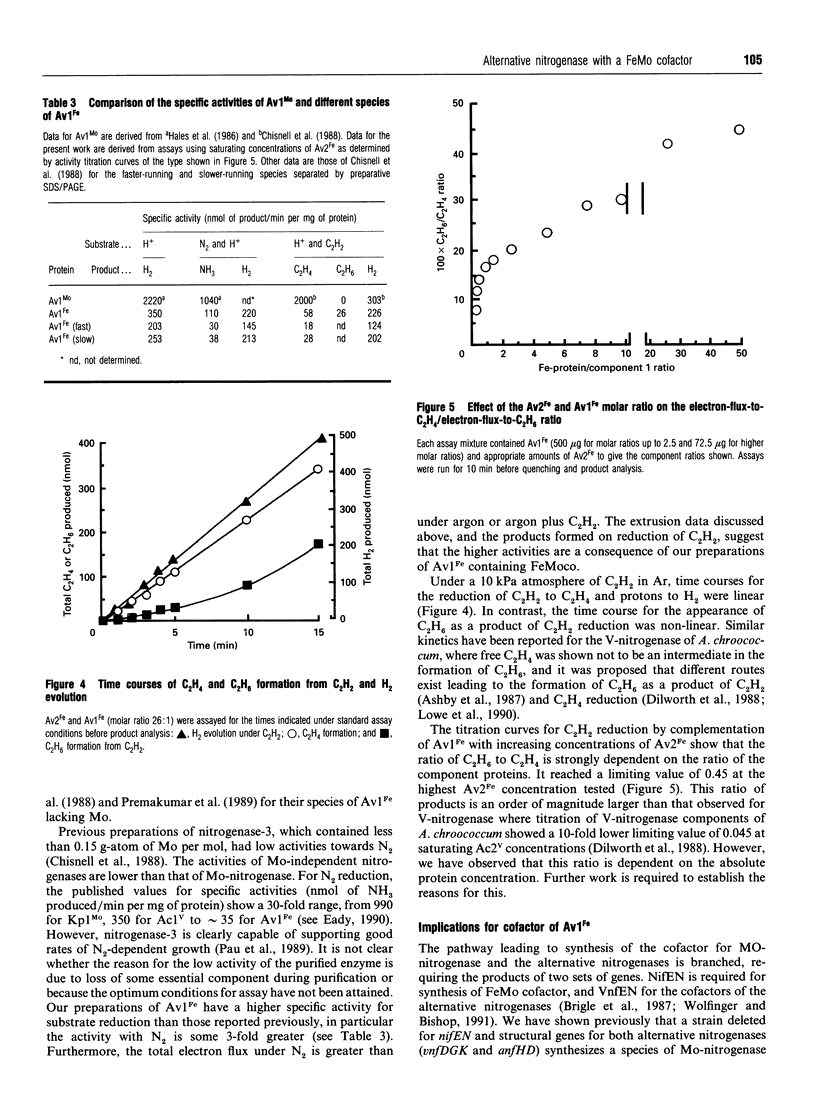
Nitrogenase-3 of Azotobacter vinelandii is synthesized under conditions of molybdenum and vanadium deficiency. The minimal metal requirement for its synthesis, and its metal content, indicated that the only transition metal in nitrogenase-3 was iron [Chisnell, Premakumar and Bishop (1988) J. Bacteriol. 170, 27-33; Pau, Mitchenall and Robson (1989) J. Bacteriol. 171, 124-129]. A new species of nitrogenase-3 has been purified from a strain of A. vinelandii (RP306) lacking structural genes for the Mo- and V-nitrogenases and containing a mutation which enables nitrogenase-3 to be synthesized in the presence of molybdenum. SDS/PAGE showed that component 1 contained a 15 kDa polypeptide which N-terminal amino acid sequence determination showed to be encoded by anfG. This confirms that nitrogenase-3, like V-nitrogenase, comprises three subunits. Preparations of the nitrogenase-3 from strain RP306 contained 24 Fe atoms and 1 Mo atom per molecule. Characterization of the cofactor centre of the enzyme by e.p.r. spectroscopy and an enzymic cofactor assay, together with stimulation of the growth of strain RP306 by Mo, showed that nitrogenase-3 can incorporate the Mo-nitrogenase cofactor (FeMoco) to form a functional enzyme. The specific activities (nmol of product produced/min per mg of protein) determined from activity titration curves were: under N2, NH3 formation 110, with concomitant H2 evolution of 220; under argon, H2 evolution 350; under 10% acetylene (C2H2) in argon, ethylene (C2H4) 58, ethane (C2H6) 26, and concomitant H2 evolution 226. The rate of formation of C2H6 was non-linear, and the C2H6/C2H4 ratio strongly dependent on the ratio of nitrogenase components.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby G. A., Dilworth M. J., Thorneley R. N. Klebsiella pneumoniae nitrogenase. Inhibition of hydrogen evolution by ethylene and the reduction of ethylene to ethane. Biochem J. 1987 Nov 1;247(3):547–554. doi: 10.1042/bj2470547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigle K. E., Weiss M. C., Newton W. E., Dean D. R. Products of the iron-molybdenum cofactor-specific biosynthetic genes, nifE and nifN, are structurally homologous to the products of the nitrogenase molybdenum-iron protein genes, nifD and nifK. J Bacteriol. 1987 Apr;169(4):1547–1553. doi: 10.1128/jb.169.4.1547-1553.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisnell J. R., Premakumar R., Bishop P. E. Purification of a second alternative nitrogenase from a nifHDK deletion strain of Azotobacter vinelandii. J Bacteriol. 1988 Jan;170(1):27–33. doi: 10.1128/jb.170.1.27-33.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. R., Setterquist R. A., Brigle K. E., Scott D. J., Laird N. F., Newton W. E. Evidence that conserved residues Cys-62 and Cys-154 within the Azotobacter vinelandii nitrogenase MoFe protein alpha-subunit are essential for nitrogenase activity but conserved residues His-83 and Cys-88 are not. Mol Microbiol. 1990 Sep;4(9):1505–1512. [PubMed] [Google Scholar]
- Dilworth M. J., Eady R. R., Eldridge M. E. The vanadium nitrogenase of Azotobacter chroococcum. Reduction of acetylene and ethylene to ethane. Biochem J. 1988 Feb 1;249(3):745–751. doi: 10.1042/bj2490745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth M. J., Eldridge M. E., Eady R. R. The molybdenum and vanadium nitrogenases of Azotobacter chroococcum: effect of elevated temperature on N2 reduction. Biochem J. 1993 Jan 15;289(Pt 2):395–400. doi: 10.1042/bj2890395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Richardson T. H., Miller R. W., Hawkins M., Lowe D. J. The vanadium nitrogenase of Azotobacter chroococcum. Purification and properties of the Fe protein. Biochem J. 1988 Nov 15;256(1):189–196. doi: 10.1042/bj2560189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Robson R. L., Richardson T. H., Miller R. W., Hawkins M. The vanadium nitrogenase of Azotobacter chroococcum. Purification and properties of the VFe protein. Biochem J. 1987 May 15;244(1):197–207. doi: 10.1042/bj2440197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales B. J., Case E. E., Morningstar J. E., Dzeda M. F., Mauterer L. A. Isolation of a new vanadium-containing nitrogenase from Azotobacter vinelandii. Biochemistry. 1986 Nov 18;25(23):7251–7255. doi: 10.1021/bi00371a001. [DOI] [PubMed] [Google Scholar]
- Hales B. J., Case E. E. Nitrogen fixation by Azotobacter vinelandii in tungsten-containing medium. J Biol Chem. 1987 Nov 25;262(33):16205–16211. [PubMed] [Google Scholar]
- Hawkes T. R., McLean P. A., Smith B. E. Nitrogenase from nifV mutants of Klebsiella pneumoniae contains an altered form of the iron-molybdenum cofactor. Biochem J. 1984 Jan 1;217(1):317–321. doi: 10.1042/bj2170317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes T. R., Smith B. E. Purification and characterization of the inactive MoFe protein (NifB-Kp1) of the nitrogenase from nifB mutants of Klebsiella pneumoniae. Biochem J. 1983 Jan 1;209(1):43–50. doi: 10.1042/bj2090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. R., Premakumar R., Bishop P. E. Transcriptional regulation of nitrogen fixation by molybdenum in Azotobacter vinelandii. J Bacteriol. 1986 Aug;167(2):480–486. doi: 10.1128/jb.167.2.480-486.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger R. D., Bishop P. E. Nucleotide sequence and genetic analysis of the nifB-nifQ region from Azotobacter vinelandii. J Bacteriol. 1988 Apr;170(4):1475–1487. doi: 10.1128/jb.170.4.1475-1487.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger R. D., Jacobson M. R., Premakumar R., Wolfinger E. D., Bishop P. E. Nucleotide sequence and mutational analysis of the structural genes (anfHDGK) for the second alternative nitrogenase from Azotobacter vinelandii. J Bacteriol. 1989 Feb;171(2):1075–1086. doi: 10.1128/jb.171.2.1075-1086.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger R. D., Loveless T. M., Pau R. N., Mitchenall L. A., Simon B. H., Bishop P. E. Nucleotide sequences and mutational analysis of the structural genes for nitrogenase 2 of Azotobacter vinelandii. J Bacteriol. 1990 Jun;172(6):3400–3408. doi: 10.1128/jb.172.6.3400-3408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C., Dean D. The nifU, nifS and nifV gene products are required for activity of all three nitrogenases of Azotobacter vinelandii. Mol Gen Genet. 1992 Feb;231(3):494–498. doi: 10.1007/BF00292722. [DOI] [PubMed] [Google Scholar]
- Kent H. M., Baines M., Gormal C., Smith B. E., Buck M. Analysis of site-directed mutations in the alpha- and beta-subunits of Klebsiella pneumoniae nitrogenase. Mol Microbiol. 1990 Sep;4(9):1497–1504. [PubMed] [Google Scholar]
- Kent H. M., Ioannidis I., Gormal C., Smith B. E., Buck M. Site-directed mutagenesis of the Klebsiella pneumoniae nitrogenase. Effects of modifying conserved cysteine residues in the alpha- and beta-subunits. Biochem J. 1989 Nov 15;264(1):257–264. doi: 10.1042/bj2640257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Rees D. C. Structural models for the metal centers in the nitrogenase molybdenum-iron protein. Science. 1992 Sep 18;257(5077):1677–1682. doi: 10.1126/science.1529354. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Lowe D. J. ENDOR and EPR of metalloproteins. Prog Biophys Mol Biol. 1992;57(1):1–22. doi: 10.1016/0079-6107(92)90002-n. [DOI] [PubMed] [Google Scholar]
- Lowe D. J., Fisher K., Thorneley R. N. Klebsiella pneumoniae nitrogenase. Mechanism of acetylene reduction and its inhibition by carbon monoxide. Biochem J. 1990 Dec 15;272(3):621–625. doi: 10.1042/bj2720621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque F., Pau R. N. Transcriptional regulation by metals of structural genes for Azotobacter vinelandii nitrogenases. Mol Gen Genet. 1991 Jul;227(3):481–487. doi: 10.1007/BF00273941. [DOI] [PubMed] [Google Scholar]
- May H. D., Dean D. R., Newton W. E. Altered nitrogenase MoFe proteins from Azotobacter vinelandii. Analysis of MoFe proteins having amino acid substitutions for the conserved cysteine residues within the beta-subunit. Biochem J. 1991 Jul 15;277(Pt 2):457–464. doi: 10.1042/bj2770457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A., Schneider K., Knüttel K., Hagen W. R. EPR spectroscopic characterization of an 'iron only' nitrogenase. S = 3/2 spectrum of component 1 isolated from Rhodobacter capsulatus. FEBS Lett. 1992 May 25;303(1):36–40. doi: 10.1016/0014-5793(92)80472-s. [DOI] [PubMed] [Google Scholar]
- Pau R. N., Mitchenall L. A., Robson R. L. Genetic evidence for an Azotobacter vinelandii nitrogenase lacking molybdenum and vanadium. J Bacteriol. 1989 Jan;171(1):124–129. doi: 10.1128/jb.171.1.124-129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienkos P. T., Brill W. J. Molybdenum accumulation and storage in Klebsiella pneumoniae and Azotobacter vinelandii. J Bacteriol. 1981 Feb;145(2):743–751. doi: 10.1128/jb.145.2.743-751.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Woodley P. R., Pau R. N., Eady R. R. Structural genes for the vanadium nitrogenase from Azotobacter chroococcum. EMBO J. 1989 Apr;8(4):1217–1224. doi: 10.1002/j.1460-2075.1989.tb03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Müller A., Johannes K. U., Diemann E., Kottmann J. Selective removal of molybdenum traces from growth media of N2-fixing bacteria. Anal Biochem. 1991 Mar 2;193(2):292–298. doi: 10.1016/0003-2697(91)90024-n. [DOI] [PubMed] [Google Scholar]
- Schneider K., Müller A., Schramm U., Klipp W. Demonstration of a molybdenum- and vanadium-independent nitrogenase in a nifHDK-deletion mutant of Rhodobacter capsulatus. Eur J Biochem. 1991 Feb 14;195(3):653–661. doi: 10.1111/j.1432-1033.1991.tb15750.x. [DOI] [PubMed] [Google Scholar]
- Scott D. J., May H. D., Newton W. E., Brigle K. E., Dean D. R. Role for the nitrogenase MoFe protein alpha-subunit in FeMo-cofactor binding and catalysis. Nature. 1990 Jan 11;343(6254):188–190. doi: 10.1038/343188a0. [DOI] [PubMed] [Google Scholar]
- Smith B. E., Eady R. R., Lowe D. J., Gormal C. The vanadium-iron protein of vanadium nitrogenase from Azotobacter chroococcum contains an iron-vanadium cofactor. Biochem J. 1988 Feb 15;250(1):299–302. doi: 10.1042/bj2500299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfinger E. D., Bishop P. E. Nucleotide sequence and mutational analysis of the vnfENX region of Azotobacter vinelandii. J Bacteriol. 1991 Dec;173(23):7565–7572. doi: 10.1128/jb.173.23.7565-7572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft W. G., Mortensson L. E. Evidence for a catalytic-centre heterogeneity of molybdoferredoxin from Clostridium pasteurianum. Eur J Biochem. 1973 Jun 15;35(3):401–409. doi: 10.1111/j.1432-1033.1973.tb02852.x. [DOI] [PubMed] [Google Scholar]


