Abstract
Group I metabotropic glutamate receptors (Gp1-mGluRs) exert a host of effects on cellular functions, including enhancement of protein synthesis and the associated facilitation of long-term potentiation (LTP) and induction of long-term depression (LTD). However, the complete cascades of events mediating these events are not fully understood. Gp1-mGluRs trigger α-secretase cleavage of amyloid precursor protein, producing soluble amyloid precursor protein-α (sAPPα), a known regulator of LTP. However, the α-cleavage of APP has not previously been linked to Gp1-mGluR’s actions. Using rat hippocampal slices, we found that the α-secretase inhibitor tumour necrosis factor-alpha protease inhibitor-1, which inhibits both disintegrin and metalloprotease 10 (ADAM10) and 17 (ADAM17) activity, blocked or reduced the ability of the Gp1-mGluR agonist (R,S)-3,5-dihydroxyphenylglycine (DHPG) to stimulate protein synthesis, metaplastically prime future LTP and elicit sub-maximal LTD. In contrast, the specific ADAM10 antagonist GI254023X did not affect the regulation of plasticity, suggesting that ADAM17 but not ADAM10 is involved in mediating these effects of DHPG. However, neither drug affected LTD that was strongly induced by either high-concentration DHPG or paired-pulse synaptic stimulation. Our data suggest that moderate Gp1-mGluR activation triggers α-secretase sheddase activity targeting APP or other membrane-bound proteins as part of a more complex signalling cascade than previously envisioned.
This article is part of a discussion meeting issue ‘Long-term potentiation: 50 years on’.
Keywords: metabotropic glutamate receptors, synaptic plasticity, hippocampus, alpha-secretase, protein synthesis
1. Introduction
Metabotropic glutamate receptors, especially group I metabotropic glutamate receptors (Gp1-mGluRs), are well-known regulators of hippocampal long-term potentiation (LTP) and long-term depression (LTD). In particular, Gp1-mGluRs play a key role in facilitating LTP induction and persistence, whether activated during or before LTP-inducing stimulation [1–3]; moreover, Gp1-mGluR activation can also directly induce LTD [4–6]. Notably, both phenomena are protein synthesis-dependent.
Gp1-mGluRs are principally located in the perisynaptic and extrasynaptic membranes and contain seven transmembrane domains canonically linked to the intracellular Gαq protein subunit. Receptor activation by glutamate leads to increased phospholipase C (PLC) activity, which then initiates a series of molecular signalling transduction cascades that exert many molecular effects, including activation of de novo protein synthesis [1,7]. Numerous studies have demonstrated that blocking protein synthesis during Gp1-mGluR activation blocks the priming of subsequently induced LTP [1] and blocks, or at least impairs, the induction of Gp1-mGluR-LTD depending on the protocol and age of the animals used [4,8,9]. Thus, newly synthesized proteins play an essential role in the full expression of Gp1-mGluR-mediated LTP and LTD.
Soluble amyloid precursor protein-alpha (sAPPα) is another signalling molecule that exerts widespread regulation of cellular functions. sAPPα is a 612 amino acid protein derived from the predominantly neuronal 695 amino acid isoform of APP through cleavage by membrane-bound α-secretases. These secretases are members of the ADAM (a disintegrin and metalloprotease) family of transmembrane Zn-proteases that are located at the cell surface. Within this secretase family, ADAM9, ADAM10 and ADAM17 can cleave APP at the α-secretase site. In neurons, ADAM10 serves as the major constitutive α-secretase as demonstrated by the reduction in sAPPα levels caused by pharmacological inhibition and knockdown in vitro, as well as by brain-specific knockout in vivo [10–13], while ADAM17, which also contributes to constitutive cleavage [14], mediates much of the ligand-stimulated alpha cleavage of APP [15].
Once liberated, sAPPα diffuses through the extracellular space and interacts with a range of extracellular and plasma membrane-bound proteins. Through these interactions and downstream signalling cascades, sAPPα plays a key role in regulating a wide range of fundamental cellular processes, including cell proliferation and adult neurogenesis, neurite outgrowth, synaptogenesis and dendrite spine development, facilitation of synaptic plasticity and protein synthesis as well as regulation of gene expression, although a specific receptor has not yet been identified that mediates these effects [16,17]. For these reasons, it is also under consideration as a therapeutic target for neurological disorders [16].
Interestingly, sAPPα, like Gp1-mGluRs [1], upregulates de novo protein synthesis in the hippocampus [18] and primes hippocampal CA1 LTP in a protein synthesis-dependent manner that also involves the synthesis and trafficking of glutamatergic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors [19,20]. This commonality of effects raises the question of whether Gp1-mGluR and sAPPα signal transduction pathways are independent or whether they are intrinsically linked through serial or intersecting signalling cascades. Indeed, many of the signalling molecules downstream of Gp1-mGluR activation are known to stimulate α-secretase activity, and Gp1-mGluR activation itself has been shown to increase sAPPα levels [21,22]. Activators of α-secretase also include many other G-protein-coupled receptors such as P2Y2 receptors [23], muscarinic acetylcholine M1 and M3 receptors [14,24], angiotensin AT1 receptors [25] and receptor tyrosine kinases [26]. Importantly, however, l-glutamate significantly increases detectable levels of sAPPα in hippocampal slices in a manner that is blocked by MCPG, a broad-spectrum Gp1-mGluR antagonist [22]. This effect may be driven by downstream activation of protein kinase C (PKC) [22,27], although other cascades may also be involved [28]. We hypothesize therefore that the activation of α-secretases (ADAM10/17) is an intermediate step in the signalling cascade activated by glutamate binding to Gp1-mGluRs that leads to elevated protein synthesis and protein synthesis-dependent synaptic plasticity. We report here that a global α-secretase inhibitor does inhibit the ability of the Gp1-mGluR agonist (R,S)-3,5-dihydroxyphenylglycine (DHPG) to enhance de novo protein synthesis, to facilitate LTP and elicit at least one form of Gp1-mGluR-LTD in the rat hippocampus.
2. Methods
(a). Animals
All experiments used hippocampal slices prepared from 6 to 12 week old male Sprague-Dawley rats (Rattus norvegicus), as previously described [9,19]. Rats were sourced from the University of Otago Biomedical Research Facility and group-housed using a 12 : 12 light/dark cycle. All experiments were approved by the University of Otago Animal Ethics Committee. Animal use was recorded using Integrated Animal Research Management System software https://www.a-tune.com/ (a-tune Software AG, Darmstadt, Germany).
(b). Slice preparation
Rats were anaesthetized with ketamine (100 mg kg−1), injected intraperitoneally, decapitated and the brain removed. The brain was immediately chilled by submerging it in an ice-cold cutting solution (in mM: 210 sucrose, 26 NaHCO3, 20 d-glucose, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2 and 3 MgCl2) bubbled with carbogen (95% O2/5% CO2). The hippocampus was isolated, and CA3 was removed by a manual knife cut [3]. Transverse hippocampal slices (400 µm) were cut from the dorsal two-thirds of the structure, excluding the very dorso-anterior portions, using a vibratome (VT1000, Leica) and incubated at the interface in carbogenated artificial cerebrospinal fluid (aCSF, in mM: 10 d-glucose, 124 NaCl, 3.2 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2.5 CaCl2 and 1.3 MgCl2) at 32°C for 30 min. The incubation chamber was then allowed to cool to room temperature, and the slices were recovered for a further 90 min.
(c). Electrophysiology
Following at least 2 h of incubation, slices were submerged in a recording chamber through which aCSF equilibrated with carbogen was superfused continuously at a rate of 2 ml min–1 and maintained at a temperature of 32.5°C. Baseline field excitatory postsynaptic potentials (EPSP) (fEPSPs) were elicited in area CA1 by stimulation of the Schaffer collateral–commissural pathway at 0.017 Hz (diphasic pulses, 0.1 ms half-wave duration) using a Teflon-coated 50 μm tungsten wire monopolar electrode (A-M Systems). Pulses were delivered either individually or in pairs separated by 50 ms to enable the measurement of paired-pulse facilitation (PPF). Evoked responses were recorded with a glass microelectrode filled with 2 M NaCl (1–2 MΩ) and placed in stratum radiatum of area CA1. During periods of baseline recording, the stimulation intensity was adjusted to elicit an fEPSP with a slope of 40% of the maximum response where single pulses were delivered, or 1.0 mV amplitude for the initial pulse where paired pulses were delivered. Baseline stability was visually assessed immediately before bath perfusion of the drugs and by linear regression after the conclusion of each experiment [1]. Group averages included only those slices whose baseline slope, as determined by linear regression, changed by <10% over the 30 min baseline. Chemically mediated Gp1-mGluR-LTD and Gp1-mGluR priming of electrically evoked LTP were induced by bathing with DHPG (20 or 100 μM) for 10 min [1,9]. Non-saturated LTP was induced by applying a train of theta-burst stimulation (TBS, five bursts of five pulses at 100 Hz delivered at 200 ms intervals) at baseline stimulus intensity [1]. This stimulation protocol has previously shown the biggest magnitude of DHPG-induced LTP facilitation compared to controls [3]. Synaptically mediated Gp1-mGluR-LTD was induced by paired-pulse stimulation (1200 pairs) delivered at 1 Hz with a 50 ms interpulse interval. Stimulus intensity was increased during this time from that used to evoke a 1 mV fEPSP during baseline to evoke a 2 mV fEPSP, which we have shown to be important for generating LTD in adult rat CA1 slices [29]. For these experiments, d-2-amino-5-phosphonovaleric acid (D-APV, 50 μM) was bath-perfused throughout.
(d). SUnSET assay of de novo protein synthesis
De novo protein synthesis was assessed using the SUnSET (surface sensing of translation) technique [30,31]. This technique uses the incorporation of puromycin, a structural analogue of tRNAs, into nascent polypeptide chains as an indicator of global protein synthesis during the period when puromycin is present. The overall rate of protein synthesis is not affected at the concentrations used (10 µg ml–1) and thus enables the quantification of rates of global protein synthesis in vitro in hippocampal slices. Puromycin incorporation prevents further polypeptide elongation, thus labelling only polypeptides already undergoing synthesis during puromycin exposure. The puromycin ‘tag’ is detected immunologically using western blotting.
Mini-slices of area CA1 were prepared from rat hippocampal slices as previously described [30]. In brief, transverse hippocampal slices (400 µm) were prepared and further dissected using manual knife cuts under stereomicroscope guidance to yield mini-slices that encompassed only area CA1. Mini-slices were then transferred to 35 mm culture dishes (four to five per dish) containing 1 ml of aCSF equilibrated with carbogen and held at 32.5°C in a custom-designed incubation chamber [30,32]. Following a 2 h recovery period, the aCSF was replaced with identical aCSF differing only by the additional inclusion of either DHPG (20 µM), sAPPα (1 nM), tumour necrosis factor-alpha protease inhibitor-1 (TAPI-1, 20 µM), LY341495 (100 µM) or a combination of DHPG and TAPI-1 or sAPPα and LY341495. The new aCSF medium always contained puromycin [10 μg/ml (18 μM)]. Control dishes were switched to aCSF with puromycin only. At the end of the incubation period, slices were snap-frozen on dry ice and stored at −80°C.
To extract soluble and insoluble proteins, slices were homogenized by pestle 30× in a detergent-containing buffer [100 mM phosphate buffered saline pH 7.4, 1 mM EDTA, 1 mM erthylene-bis(oxyethylenenitrilo)tetraacetic acid (EGTA), 0.1 mM phenylmethylsulfonyl fluoride, 1% (v/v) Triton-X, 0.1% (w/v) sodium dodecyl sulfate and a protease inhibitor (cOmplete Ultra Mini Tablet, Roche) used as per the manufacturer’s instructions] and solubilized by probe sonication (10 pulses at 1 s each; Qsonica, CT, USA) to produce a lysate mixture. A DC protein assay (Bio-Rad) was then used to quantify protein concentrations using bovine serum albumin (BSA, Sigma Aldrich) as a standard.
Relative levels of de novo protein synthesis were assessed by western blot. Protein samples were separated on 12% (w/v) bis-acrylamide (Bio-Rad) gels before being transferred to a nitrocellulose membrane (GE Healthcare). Blots were incubated in Odyssey blocking buffer (LI-COR) at room temperature for 1 h. The primary antibodies, mouse anti-puromycin (1:1000, Kerafast EQ0001) and rabbit anti-tubulin (1:6666, Abcam ab4074) were prepared in PBS-Tween, 0.1% (w/v) BSA and 0.1% (v/v) normal goat serum. The blots were incubated with the primary antibodies overnight at 4°C with shaking. The secondary antibody solution was composed of IRDye goat anti-rabbit 680 (1:10 000, LI-COR) and IRDye goat anti-mouse 800 (1:15 000, LI-COR) in PBS/Tween. The blots were incubated in this solution for 1 h at room temperature. Blots were imaged on a LI-COR Odyssey imaging system and quantified using Image Studio Lite 5.2 (LI-COR) after normalizing to the loading control protein (tubulin). To quantify each lane, an identical length box was used to determine the relative intensity of the puromycin labelling. As labelled nascent peptides ranged in size depending on when their synthesis was halted by puromycin incorporation, the analysis box covered almost the entirety of each lane within a blot. A small box (3 mm) above and below each band was used to define the background and the overall signal was determined as area-normalized background subtracted from the average density of the box.
(e). Drugs and reagents
D-APV (HelloBio, UK) and DHPG (Hellobio, UK) were dissolved in water and stored at −18°C. GI254023X ((2R)-N-[(1S)-2,2-dimethyl-1-[(methylamino)carbonyl]propyl]-2-[(1S)-1-(N-hydroxyformamido)ethyl]-5-phenylpentanamide; MedChemExpress, NJ, USA) was dissolved in dimethylsulfoxide (DMSO) and stored at −80°C. TAPI-1 was purchased from Tocris (Bristol, UK), dissolved in water and aliquots stored at −80°C. All stock drug solutions were diluted 1:1000 with aCSF for the final working concentration. All salts were supplied by Sigma-Aldrich (St. Louis, USA).
(f). Data and statistical analysis
The initial slopes of the fEPSPs were measured using in-house software constructed using Labview graphical programming software https://www.ni.com/en/shop/labview.html (NI, TX, USA) and expressed as a percentage change from the baseline level, calculated as the average of the last 15 min of the baseline recording period [19]. The initial magnitude of DHPG-induced LTD was calculated using the lowest average of five consecutive slope values within the first 15 min of DHPG delivery. The initial magnitude of LTP was calculated using the average of the first five slope values immediately following theta-burst stimulation. The degree of LTD or LTP for each experiment was measured as the average of the last 5 min of the post-DHPG or TBS recording period, respectively. Group means were expressed as the percentage change ± s.e.m. PPF was calculated as the ratio of the initial slope of the second versus the first evoked paired-pulse responses and expressed as a percentage change from the average of the last 15 min of the baseline recording period. For each western blot, the relative degree of de novo protein synthesis was analysed by expressing the degree of puromycin immunoreactivity for each experimental lane relative to the untreated control lane. For all experiments, data were imported into a statistical software package (Prism, GraphPad, v.9 https://www.graphpad.com/ ) and group means were compared statistically by two-tailed independent Student’s t-tests when only two groups were compared and one-way ANOVAs followed by Dunnett’s post hoc tests when comparing against a single control group or Tukey’s post hoc tests when comparisons were made across all groups, at the p < 0.05 significance level. The n’s represent numbers of slices, with one or two slices used per animal. The data that support the findings of this study are available in the supplementary materials [33].
3. Results
(a). α-Secretase inhibition blocks Gp1-mGluR-stimulated protein synthesis
As an initial test that α-secretase activity may be mediating protein synthesis-dependent actions of Gp1-mGluRs, we employed the SUnSET technique for assessing de novo protein synthesis, as applied to CA1 mini-slices. One-way ANOVA revealed an overall treatment effect (F 3,20 = 31.4, p < 0.0001) such that the Gp1-mGluR agonist DHPG (20 µM, 30 min) increased puromycin immunoreactivity relative to controls by 1.95 ± 0.10 fold (n = 6, Tukey’s post hoc p < 0.0001; figure 1a,b ). This effect was completely blocked by co-administration of the ADAM10/17 α-secretase inhibitor TAPI-1 (20 µM) (−0.05 ± 0.10 fold, n = 6, p = 0.969 relative to controls). TAPI-1 by itself did not affect basal rates of puromycin incorporation (1.11 ± 0.08 fold, n = 6, p = 0.774). These data indicate that α-secretase activity is necessary for Gp1-mGluR activation to stimulate protein synthesis.
Figure 1.
Regulation of protein synthesis by DHPG and sAPPα, as assessed using the puromycin-based SUnSET technique. (a) Representative western blot illustrating the enhancement of puromycin incorporation in CA1 mini-slices across the various treatment groups, as well as an α-tubulin band separately shown for the same blot and used as a loading control. (b) Quantification showing that 30 min DHPG (20 µM) increased the puromycin immunoreactivity almost twofold. The effect was completely abolished by co-administration of TAPI-1 (20 µM), which by itself did not affect basal protein synthesis. (c) Representative western blot as in (a). (d) Quantification showing that sAPPα (30 min, 1 nM) enhanced protein synthesis by almost threefold. The global mGluR antagonist LY341495 (100 µM) did not significantly inhibit the effect of sAPPα, nor did it affect basal protein synthesis. *p < 0.05, **p < 0.01, ****p < 0.0001 (Tukey’s post hoc tests) compared to untreated control slices. Cont, control CA1 mini-slices; D+T, combined DHPG and TAPI-1 treatment; LY, LY341495; M, marker lane; S+LY, combined sAPPα and LY341495 treatment. Data are mean ± s.e.m.
The above data could be explained by either α-secretase and Gp1-mGluR signalling cascades converging to upregulate protein synthesis or by α-secretase activity sitting downstream of Gp1-mGluR activity. Accordingly, we undertook the reverse experiment that addressed the question of whether Gp1-mGluR inhibition would block the ability of sAPPα to elevate protein synthesis, which it would do if both were working together. One-way ANOVA revealed an overall treatment effect (F 3,20 = 8.746, p = 0.0007), such that treatment of the CA1 mini-slices with recombinant sAPPα (1 nM, 30 min [30]) increased puromycin immunoreactivity by 2.83 ± 0.34 fold (n = 6, Tukey’s p = 0.0019 relative to controls; figure 1c,d ). This effect was mildly but not significantly reduced by co-administration of the broad-spectrum mGluR inhibitor LY341495 (100 µM; 2.30 ± 0.43 fold, n = 6, p = 0.6084 relative to sAPPα alone; p = 0.0308 relative to controls). LY341495 alone did not affect basal protein synthesis (1.12 ± 0.25 fold, n = 6, p = 0.9912 relative to controls; figure 1c,d ). Thus, Gp1-mGluR activity is not required for sAPPα-mediated facilitation of protein synthesis. Taken together, these data support the hypothesis that α-secretase activity sits downstream of Gp1-mGluR activation and is a necessary step for its stimulation of protein synthesis.
(b). α-Secretase inhibition blocks Gp1-mGluR priming of LTP
We have previously reported that priming activation of Gp1-mGluRs by DHPG (20 µM, 10 min), 20 min before delivery of a mild TBS, metaplastically facilitates the persistence of the induced LTP in a protein synthesis-dependent manner, i.e. from LTP1 to LTP2 [1]. To determine whether DHPG-mediated priming of LTP requires α-secretase activity, we investigated the effects of co-administration of DHPG with one of two α-secretase inhibitors: the specific ADAM10 inhibitor GI254023X (20 µM) [13] and the global inhibitor TAPI-1 (20 µM) [14]. One-way ANOVA revealed that there was an overall treatment effect (F 3,29 = 4.139, p = 0.015), such that DHPG treatment alone caused a near-significant enhancement of the LTP measured 55–60 min after TBS (control: 6.8 ± 3.6%, n = 9; DHPG: 24.4 ± 4.7%, n = 8, Dunnett’s p = 0.059; figure 2a,b ), obscured in part by the LTD induced by DHPG alone (cf. figure 3). GI254023X administration 30 min before and during DHPG perfusion did not significantly affect the degree of LTP induced by DHPG (17.7 ± 8.3%, n = 7, p = 0.722). In contrast, TAPI-1 delivered in the same way completely prevented any LTP facilitation by DHPG (0.7 ± 4.6%, n = 9, p = 0.009; figure 2a,b ), an effect that began even in the first 5 min post-TBS (figure 2b ). These results suggest that an α-secretase other than ADAM10, likely ADAM17, mediates the priming facilitation of LTP by DHPG.
Figure 2.
Block of DHPG priming of LTP in area CA1 of hippocampal slices by TAPI-1. (a) Plot showing the time course of the effects of DHPG (20 µM, 10 min) on LTP induced by theta-burst stimulation in CA1 stratum radiatum, along with the effects of the α-secretase inhibitors. Representative waveforms shown at the top are averages of 10 sweeps taken just before the TBS (dotted lines) and at the end of the experiment (solid lines). Calibration bars: 1 mV, 10 ms. (b) Quantification of the early potentiation response revealed that TAPI-1 (20 µM) completely blocked the enhancement of LTP by DHPG, while GI254023X (20 µM) had no effect. *p < 0.05; (Tukey’s post hoc tests). Data are mean ± s.e.m.
Figure 3.
Impairment of DHPG-induced moderate LTD by TAPI-1. (a) Plot showing the time course of the effects of DHPG on hippocampal field potentials evoked in CA1 stratum radiatum, along with the effects of the α-secretase inhibitors. Representative waveforms at the top are averages of 10 sweeps taken just before the time of DHPG administration (dotted lines) and at the end of the experiment (solid lines). Calibration bars: 1 mV, 10 ms. (b) Bar chart showing the quantification of the drug effects on the LTD, delivered for the times and concentrations as in figure 2. TAPI-1 but not GI254023X significantly impaired the DHPG-induced LTD. *p < 0.05; **p < 0.01 (Dunnett’s post hoc tests). Data are mean ± s.e.m.
(c). Effects of α-secretase inhibition on DHPG-induced mGluR-LTD
The 20 µM dose of DHPG used in the above experiments for stimulating protein synthesis and priming LTP is also known to produce a sub-maximal amplitude of mGluR-LTD, albeit recorded for only 20 min [1,3]. Accordingly, we tested the ability of α-secretase inhibitors to block the LTD induced by a 10 min perfusion of DHPG. Treatment with DHPG alone resulted in a rapid-onset depression of fEPSPs (−36.3 ± 6.5%, n = 14; figure 3a,b ) that decayed briefly after drug washout before stabilizing for the duration of the 80 min post-drug recording period (final depression −23.1 ± 3.3%). To determine whether ADAM10 mediates this LTD, we again bath-perfused the highly specific ADAM10 inhibitor GI254023X (20 μM) for 30 min before and during DHPG application. One-way ANOVA revealed a significant overall treatment effect (F 2,27 = 4.419, p = 0.022), but that GI254023X had no effect on the magnitude of the depression measured 75–80 min after DHPG administration (+GI254023X, −21.1 ± 4.2%, n = 7; Dunnett’s p = 0.896). In contrast, TAPI-1 significantly reduced the degree of DHPG-induced mGluR-LTD (−9.8 ± 2.3%, n = 9, p = 0.014, figure 3a,b ). Note that the partial block of LTD by TAPI-1 is consistent with the partial block of LTD that we have observed previously using protein synthesis inhibitors [9]. These data show that for sub-maximal mGluR-LTD induced by 20 µM DHPG, ADAM10 does not contribute to the LTD effect, and thus, similar to the priming of LTP, ADAM17 is the likely α-secretase mediating this effect of DHPG.
(d). ADAM10/17 activity does not mediate strongly induced mGluR-LTD
We also tested whether ADAM10 and/or ADAM17 activity contributes to strongly induced mGluR-LTD. We investigated this by bath perfusion of 100 μM DHPG and continued recording fEPSPs for a further 60 min. Baseline stimulation consisted of paired pulses (50 ms interstimulus interval) to allow analysis of presynaptic effects. We first tested whether GI254023X administered at least 30 min before and during DHPG administration affected LTD. There was no statistically significant difference in either the depression during the initial induction period (DHPG only: −71.8 ± 7.3%, n = 7; +GI254023X: −85.3 ± 4.7%, n = 8; Student’s t (13) = 1.588, p = 0.136) or LTD measured at the end of the recording (DHPG only: −49.6 ± 3.5%; +GI254023X: −44.5 ± 7.2%; t (13) = 0.603, p = 0.557; figure 4a,b ). There was also no difference in the final degree of enhancement of PPF caused by the drugs (p = 0.738; figure 4c ). We then tested whether administration of GI254023X throughout the experiment would inhibit LTD, given evidence that there can be an active signalling cascade driving LTD even after DHPG washout [5]. However, once again, we observed no effect of the ADAM10 inhibitor on the final degree of LTD (DHPG only: −51.8 ± 3.3%, n = 9; +GI254023X: −46.0 ± 5.6%, n = 7; Student’s t (14) = 0.943, p = 0.362; figure 4d,e ), nor on the PPF change (p = 0.710; figure 4f ). We next tested whether TAPI-1 would be more effective than GI254023X in blocking such LTD, as in the above experiments. However, in this case, administration of TAPI-1 for 30 min before and co-terminating with DHPG did not significantly affect the final level of LTD (DHPG only: −38.8 ± 6.3%, n = 7; +TAPI-1: −40.1 ± 3.1%, n = 4; t (9) = 0.156, p = 0.880; figure 4g,h ) nor the degree of PPF change (p = 0.326; figure 4i ).
Figure 4.
Lack of effect of α-secretase inhibitors on strongly induced LTD. (a, b) Lack of effect of GI254023X on LTD and associated PPF change (c) induced by a high concentration of DHPG (100 µM). Representative waveforms are averages of 10 sweeps taken just before the time of DHPG administration (dotted lines) and at the end of the experiment (solid lines). Calibration bars: 1 mV, 10 ms. (d, e) Lack of effect of GI254023X administration throughout the experiment on strongly induced LTD and the associated PPF change (f). Waveforms as in (a). (g, h) Lack of effect of TAPI-1 on strongly induced LTD and associated PPF change (i). Data are mean ± s.e.m.
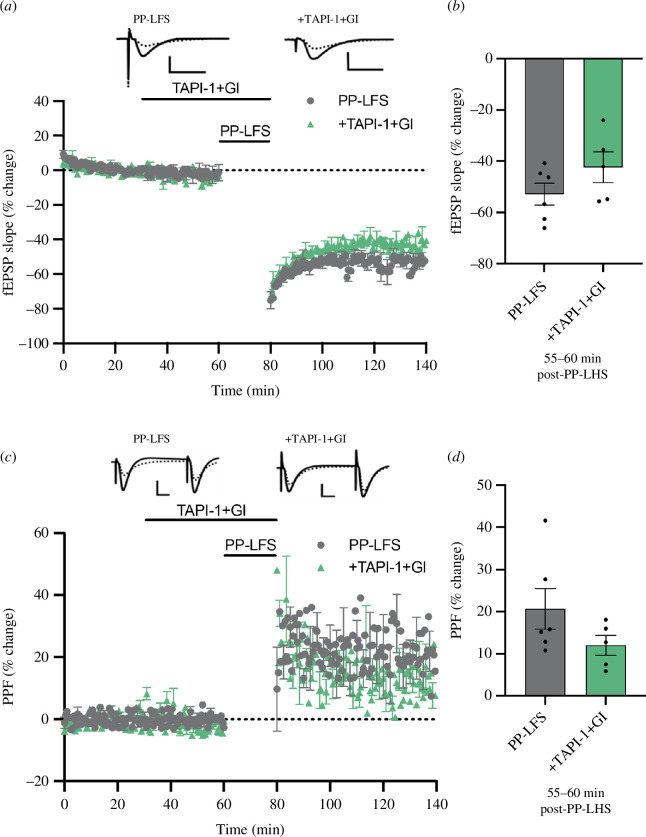
In a final experiment, we induced a strong mGluR-LTD using a synaptic stimulation protocol rather than pharmacologically with DHPG. Evidence suggests that these alternative approaches to inducing mGluR-LTD may engage different if overlapping molecular pathways [34,35] and there may thus be a differential reliance on α-secretase activity. We observed, however, that the application of combined TAPI-1 (20 µM) and GI254023X (20 µM) for 20 min before paired-pulse low-frequency stimulation (PP-LFS) until the end of the PP-LFS, all in the presence of D-APV, did not significantly affect the expression of mGluR-LTD measured 55–60 min post-PP-LFS (PP-LFS alone: −52.9 ± 4.2%, n = 6; +TAPI-1+GI254023X: −42.4 ± 6.0%, n = 5; t (9) = 1.466, p = 0.177; figure 5a,b ). As a final check, we assessed whether the percentage increase in presynaptic PPF generated by synaptic mGluR-LTD was affected by the α-secretase inhibitors, even though this increase is not a protein synthesis-dependent process [9]. However, this correlate of mGluR-LTD was also not affected by the combined drug treatment (PP-LFS alone: 20.7 ± 4.8%; +TAPI-1+GI254023X: 12.0 ± 2.4 %; t (9) = 1.501, p = 0.168; figure 5c,d ). Thus, the α-secretase inhibitors did not affect either the presynaptic or the postsynaptic components of strongly induced mGluR-LTD. These data indicate that α-secretase activity is not necessary for the expression of this strong form of mGluR-LTD, supporting the result obtained above using the high dose of DHPG.
Figure 5.
Lack of effect of TAPI-1 on synaptically evoked mGluR-LTD. (a) mGluR-LTD was evoked in stratum radiatum by 1200 pairs of pulses (50 ms ISI) delivered at 1 Hz. (b) A combination of both TAPI-1 and GI254023X failed to impair the LTP induction or persistence. (c,d) There was also no effect on the degree of PPF change caused by the PP-LFS. Waveforms are as in previous figures. Data are mean ± s.e.m.
4. Discussion
Activation of Gp1-mGluRs generates a host of molecular and cellular effects both presynaptically and postsynaptically, with varying durations of effects. The canonical signal transduction pathway entails activation of PLC-β, hydrolysis of phosphatidylinositol-4,5-bisphosphate leading to the liberation of the second messengers—inositol trisphosphate and diacylglycerol—and then the release of calcium from intracellular stores and activation of PKC, respectively. One non-canonical pathway involves activation of β-arrestin2, activation of extracellular signal-related kinases (ERKs) and stimulation of protein synthesis [36]. Another entails activation of PI3 (phosphoinositide 3-kinase) kinase [37] leading to activation of the mTOR pathway [38] (although see [36]). Thus, a variety of signalling pathways can contribute either individually or together to mediate Gp1-mGluR’s cellular effects.
(a). Gp1-mGluR stimulation of protein synthesis
One well-known example of Gp1-mGluR’s cellular effects is its ability to stimulate de novo protein synthesis. An early study using the group I/II agonist ACPD (aminocyclopentane-1,3-dicarboxylic acid) to stimulate RNA accumulation in polyribosomes in cortical synaptoneurosomes found the effect was blocked by an inhibitor of PLC, indicative of canonical pathway signalling [7]. In contrast, we observed in hippocampal synaptoneurosomes that DHPG-stimulated 35S-methionine incorporation into protein was not significantly affected by inhibitors of either PKC or PLC but was blocked by an inhibitor of ERK and by the mTOR inhibitor rapamycin, suggesting non-canonical pathway signalling [9]. Consistent with this latter effect, stimulation specifically of mGlu5 receptors in mouse hippocampal slices also increased 35S-methionine incorporation into protein in a β-arrestin2-dependent manner [36]. Thus, both canonical and non-canonical pathways can be triggered by Gp1-mGluR activation, depending on the specific experimental conditions. However, the conventional thinking has been that these signalling pathways lead more or less directly to the activation of protein synthesis machinery.
In the present study, we addressed the hypothesis that Gp1-mGluR signalling entails a more complicated sequence of events, particularly with respect to protein synthesis-dependent mechanisms. Specifically, we tested whether activation of α-secretases plays a role. As a first test of this hypothesis, we confirmed that pharmacological inhibition of α-secretases by TAPI-1 blocks the stimulation of protein synthesis by a sub-maximal concentration of DHPG. This finding is consistent with α-secretases being activated by signals downstream of Gp1-mGluR activation such as PKC and ERK [22,28]. Importantly, the reverse effect did not hold, as a global mGluR inhibitor did not block the stimulation of protein synthesis by sAPPα. Taken together, this suggests that the α-secretases work downstream of mGluR activity, rather than in convergence with it, a conclusion consistent with the failure of an mGluR inhibitor to prevent the stimulation of Arc protein synthesis by sAPPα [39]. The upregulation of protein synthesis by sAPPα is dependent on ERK, protein kinase G (PKG) and calcium/calmodulin-dependent protein kinase II (CaMKII) activity [18,39], but the receptors triggering these signalling cascades remain unclear, although α7-nicotinic receptors are candidates [39].
(b). Gp1-mGluR priming of LTP
Previously, we reported that prior administration of a low concentration of DHPG could metaplastically prime subsequently induced LTP, converting it from translation-independent LTP1 to the longer-lasting and translation-dependent LTP2 [1]. Intriguingly, sAPPα exerts a similar protein synthesis-dependent priming effect on LTP [19]. In the case of sAPPα, this effect was associated with the protein synthesis-dependent trafficking of AMPA receptors to the plasma membrane. Here, we have shown that these effects may be linked since, as for the stimulation of protein synthesis, the priming of LTP by DHPG was blocked by TAPI-1, demonstrating the critical role of α-secretase in mediating this effect.
(c). Gp1-mGluR induction of LTD
To date, there has been no evidence for recombinant sAPPα directly inducing LTD, although the fact that it can drive Arc protein synthesis [39] suggests that it could be a contributor to LTD in combination with other signals. However, we found in the present experiments that TAPI-1 but not GI254023X blocks the LTD induced by 20 µM DHPG, the same concentration used in the above experiments on protein synthesis and LTP priming. Together these findings suggest a critical role for ADAM17 in mediating sub-maximal upregulation of protein synthesis and the associated priming of LTP and induction of moderate LTD. Interestingly, LTD that was more strongly induced by a much higher concentration of DHPG (100 µM) or by low-frequency paired-pulse stimulation was not affected by TAPI-1 administration. This suggests that an additional signalling pathway, perhaps the canonical pathway, is sufficiently activated by this higher concentration to engage the LTD mechanisms.
5. Conclusion
Taken together, these data give strong support for α-secretase being critically involved in Gp1-mGluR signalling that leads to upregulated protein synthesis, LTP and LTD. Given the differential effects of TAPI-1 and GI254023X, we tentatively conclude that this α-secretase is ADAM17. ADAM17 is also known as TACE (tumour necrosis factor-alpha (TNF-α)-converting enzyme), owing to its ectodomain cleavage of TNF. However, ADAM17 is a sheddase that has multiple targets in the plasma membrane, including not only APP and TNF but also TNFR1, TNFR2, interleukin-6 (IL-6) receptor, triggering receptor expressed on myeloid cells 2 (TREM2) and Notch [17,40]. Indeed, ADAM17 ectodomain cleavage of pentraxin has been shown to mediate LTD induced by the intermediate concentration of DHPG (50 µM) in juvenile animals, in part through altered AMPA receptor trafficking [41]. It is perhaps for this reason that α-secretase cleavage of APP and release of sAPPα have not been linked to LTD. However, the similarity between sAPPα and Gp1-mGluR activation in stimulating protein synthesis and priming LTP, and their joint sensitivity to TAPI-1 administration, still support our hypothesis that APP cleavage is a key step in the Gp1-mGluR signal transduction pathway leading to these particular outcomes. This interpretation is consistent with our previous research showing that the impairments caused by TAPI-1 in dentate gyrus LTP, tectonically evoked NMDA receptor currents and spatial memory can be rescued by the administration of exogenous sAPPα [42].
The major result of these experiments is that the Gp1-mGluR signalling pathways that couple to protein synthesis, LTP and LTD mechanisms do so via a sheddase likely to be ADAM17. These data suggest that there is a cascade of events triggered by intracellular signalling that releases a further extracellular ligand release that then signals back to the cell’s interior through mechanisms still to be resolved. In the case of sAPPα, there are many binding partners [17] but the receptor mediating its regulation of plasticity is unknown. Antagonists of the α7-nicotinic receptor block some of its effects such as the increase in Arc synthesis [39] and facilitation of LTP in APP-KO mice [43], but it is not clear if this represents a permissive or direct role of these receptors. sAPPα is also known to bind to epidermal growth factor (EGF) and contribute to some of its proliferation and phosphorylation effects [44,45]. Given that EGF and its associated receptor can contribute to protein synthesis, LTP and mGluR-LTD, a sAPPα-EGF signalling system may be involved in one or more of the effects shown here [46–48]. Further experiments are required, however, to confirm whether sAPPα mediates these effects of Gp1-mGluR activation or whether other shed proteins are involved.
Acknowledgements
We thank Dr K. Peppercorn and Prof. W. Tate for the supply of recombinant sAPPα.
Contributor Information
Bruce G. Mockett, Email: bruce.mockett@otago.ac.nz.
James W. T. Davies, Email: davja986@student.otago.ac.nz.
Zoë B. Mills, Email: zoe.mills@auckland.ac.nz.
Do Y. Kweon, Email: davidkweon@gmail.com.
Wickliffe C. Abraham, Email: cliff.abraham@otago.ac.nz.
Ethics
All experiments and procedures were approved by the University of Otago Animal Ethics Committee, approval number AUP19-177.
Data accessibility
The data that support the findings of this study are available in the electronic supplementary materials [33].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
B.G.M.: conceptualization, data curation, investigation, methodology, supervision, writing—original draft, writing—review and editing; J.W.T.D.: data curation, formal analysis, investigation, writing—review and editing; Z.B.M.: data curation, investigation, writing—review and editing; D.Y.K.: data curation, investigation, writing—review and editing; W.C.A.: conceptualization, funding acquisition, project administration, resources, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein
Conflict of interest declaration
We declare we have no competing interests.
Funding
The New Zealand Health Research Council (#16-597) and the New Zealand Lottery Grants Board for funding support, and the University of Otago for scholarship support for J.W.T. Davies.
References
- 1. Raymond CR, Thompson VL, Tate WP, Abraham WC. 2000. Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation. J. Neurosci. 20 , 969–976. ( 10.1523/JNEUROSCI.20-03-00969.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bortolotto ZA, Bashir ZI, Davies CH, Collingridge GL. 1994. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature 368 , 740–743. ( 10.1038/368740a0) [DOI] [PubMed] [Google Scholar]
- 3. Cohen AS, Raymond CR, Abraham WC. 1998. Priming of long-term potentiation induced by activation of metabotropic glutamate receptors coupled to phospholipase C. Hippocampus 8 , 160–170. () [DOI] [PubMed] [Google Scholar]
- 4. Huber KM, Roder JC, Bear MF. 2001. Chemical induction of mGluR5- and protein synthesis-dependent long-term depression in hippocampal area CA1. J. Neurophysiol. 86 , 321–325. ( 10.1152/jn.2001.86.1.321) [DOI] [PubMed] [Google Scholar]
- 5. Schnabel R, Kilpatrick IC, Collingridge GL. 1999. An investigation into signal transduction mechanisms involved in DHPG-induced LTD in the CA1 region of the hippocampus. Neuropharmacology 38 , 1585–1596. ( 10.1016/s0028-3908(99)00062-3) [DOI] [PubMed] [Google Scholar]
- 6. Nosyreva ED, Huber KM. 2005. Developmental switch in synaptic mechanisms of hippocampal metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 25 , 2992–3001. ( 10.1523/JNEUROSCI.3652-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiler IJ, Greenough WT. 1993. Metabotropic glutamate receptors trigger postsynaptic protein synthesis. Proc. Natl Acad. Sci. USA 90 , 7168–7171. ( 10.1073/pnas.90.15.7168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huber KM, Kayser MS, Bear MF. 2000. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288 , 1254–1257. ( 10.1126/science.288.5469.1254) [DOI] [PubMed] [Google Scholar]
- 9. Mockett BG, Guévremont D, Wutte M, Hulme SR, Williams JM, Abraham WC. 2011. Calcium/calmodulin-dependent protein kinase II mediates group I metabotropic glutamate receptor-dependent protein synthesis and long-term depression in rat hippocampus. J. Neurosci. 31 , 7380–7391. ( 10.1523/JNEUROSCI.6656-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuhn PH, Wang H, Dislich B, Colombo A, Zeitschel U, Ellwart JW, Kremmer E, Roßner S, Lichtenthaler SF. 2010. ADAM10 is the physiologically relevant, constitutive α‐secretase of the amyloid precursor protein in primary neurons. EMBO J. 29 , 3020–3032. ( 10.1038/emboj.2010.167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prox J, et al. 2013. Postnatal disruption of the disintegrin/metalloproteinase ADAM10 in brain causes epileptic seizures, learning deficits, altered spine morphology, and defective synaptic functions. J. Neurosci. 33 , 12915–12928, ( 10.1523/JNEUROSCI.5910-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colombo A, Wang H, Kuhn PH, Page R, Kremmer E, Dempsey PJ, Crawford HC, Lichtenthaler SF. 2013. Constitutive α- and β-secretase cleavages of the amyloid precursor protein are partially coupled in neurons, but not in frequently used cell lines. Neurobiol. Dis. 49 , 137–147, ( 10.1016/j.nbd.2012.08.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hundhausen C, et al. 2003. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood 102 , 1186–1195. ( 10.1182/blood-2002-12-3775) [DOI] [PubMed] [Google Scholar]
- 14. Slack BE, Ma LK, Seah CC. 2001. Constitutive shedding of the amyloid precursor protein ectodomain is up-regulated by tumour necrosis factor-α converting enzyme. Biochem. J. 357 , 787–794. ( 10.1042/0264-6021:3570787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Black RA. 2002. Tumor necrosis factor-α converting enzyme. Int. J. Biochem. Cell Biol. 34 , 1–5. ( 10.1016/S1357-2725(01)00097-8) [DOI] [PubMed] [Google Scholar]
- 16. Mockett BG, Richter M, Abraham WC, Müller UC. 2017. Therapeutic Potential of Secreted Amyloid Precursor Protein APPsα. Front. Mol. Neurosci. 10 , 30. ( 10.3389/fnmol.2017.00030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Müller UC, Deller T, Korte M. 2017. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 18 , 281–298. ( 10.1038/nrn.2017.29) [DOI] [PubMed] [Google Scholar]
- 18. Claasen AM, Guévremont D, Mason-Parker SE, Bourne K, Tate WP, Abraham WC, Williams JM. 2009. Secreted amyloid precursor protein-α upregulates synaptic protein synthesis by a protein kinase G-dependent mechanism. Neurosci. Lett. 460 , 92–96. ( 10.1016/j.neulet.2009.05.040) [DOI] [PubMed] [Google Scholar]
- 19. Mockett BG, et al. 2019. Glutamate receptor trafficking and protein synthesis mediate the facilitation of LTP by secreted amyloid precursor protein-alpha. J. Neurosci. 39 , 3188–3203. ( 10.1523/JNEUROSCI.1826-18.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livingstone RW, Elder MK, Singh A, Westlake CM, Tate WP, Abraham WC, Williams JM. 2021. Secreted amyloid precursor protein-alpha enhances LTP through the synthesis and trafficking of Ca2+-Permeable AMPA receptors. Front. Mol. Neurosci. 14 , 660208. ( 10.3389/fnmol.2021.660208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee RKK, Wurtman RJ, Cox AJ, Nitsch RM. 1995. Amyloid precursor protein processing is stimulated by metabotropic glutamate receptors. Proc. Natl Acad. Sci. USA 92 , 8083–8087. ( 10.1073/pnas.92.17.8083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ulus IH, Wurtman RJ. 1997. Metabotropic glutamate receptor agonists increase release of soluble amyloid precursor protein derivatives from rat brain cortical and hippocampal slices. J. Pharmacol. Exp. Ther. 281 , 149–154. ( 10.1073/pnas.92.17.8083) [DOI] [PubMed] [Google Scholar]
- 23. Camden JM, Schrader AM, Camden RE, González FA, Erb L, Seye CI, Weisman GA. 2005. P2Y2 nucleotide receptors enhance α-secretase-dependent amyloid precursor protein processing. J. Biol. Chem. 280 , 18696–18702. ( 10.1074/jbc.M500219200) [DOI] [PubMed] [Google Scholar]
- 24. Buxbaum JD, Ruefli AA, Parker CA, Cypess AM, Greengard P. 1994. Calcium regulates processing of the Alzheimer amyloid protein precursor in a protein kinase C-independent manner. Proc. Natl Acad. Sci. USA 91 , 4489–4493. ( 10.1073/pnas.91.10.4489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanarek AM, Wagner A, Küppers J, Gütschow M, Postina R, Kojro E. 2017. Crosstalk between angiotensin and the nonamyloidogenic pathway of Alzheimer’s amyloid precursor protein. FEBS J. 284 , 742–753. ( 10.1111/febs.14015) [DOI] [PubMed] [Google Scholar]
- 26. Holback S, Adlerz L, Iverfeldt K. 2005. Increased processing of APLP2 and APP with concomitant formation of APP intracellular domains in BDNF and retinoic acid-differentiated human neuroblastoma cells. J. Neurochem. 95 , 1059–1068. ( 10.1111/j.1471-4159.2005.03440.x) [DOI] [PubMed] [Google Scholar]
- 27. Caporaso GL, Gandy SE, Buxbaum JD, Ramabhadran TV, Greengard P. 1992. Protein phosphorylation regulates secretion of Alzheimer beta/A4 amyloid precursor protein. Proc. Natl Acad. Sci. USA 89 , 3055–3059. ( 10.1073/pnas.89.7.3055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haring R, Fisher A, Marciano D, Pittel Z, Kloog Y, Zuckerman A, Eshhar N, Heldman E. 1998. Mitogen-activated protein kinase-dependent and protein kinase C-dependent pathways link the m1 muscarinic receptor to β-amyloid precursor protein secretion. J. Neurochem. 71 , 2094–2103. ( 10.1046/j.1471-4159.1998.71052094.x) [DOI] [PubMed] [Google Scholar]
- 29. Kerr DS, Abraham WC. 1995. Cooperative interactions among afferents govern the induction of homosynaptic long-term depression in the hippocampus. Proc. Natl Acad. Sci. USA 92 , 11637–11641. ( 10.1073/pnas.92.25.11637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morrissey JA, Mockett BG, Singh A, Kweon D, Ohline SM, Tate WP, Hughes SM, Abraham WC. 2019. A C-terminal peptide from secreted amyloid precursor protein-α enhances long-term potentiation in rats and A transgenic mouse model of Alzheimer’s disease. Neuropharmacology 157 , 107670. ( 10.1016/j.neuropharm.2019.107670) [DOI] [PubMed] [Google Scholar]
- 31. Schmidt EK, Clavarino G, Ceppi M, Pierre P. 2009. SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods 6 , 275–277. ( 10.1038/nmeth.1314) [DOI] [PubMed] [Google Scholar]
- 32. Mockett BG, Guévremont D, Williams JM, Abraham WC. 2007. Dopamine D1/D5 receptor activation reverses NMDA receptor-dependent long-term depression in rat hippocampus. J. Neurosci. 27 , 2918–2926. ( 10.1523/JNEUROSCI.0838-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mockett B, Davies J, Mills Z, Kweon D, Abraham C. 2024. Supplementary Material from: Alpha-secretase inhibition impairs group I metabotropic glutamate receptor-mediated protein synthesis, long-term potentiation and long-term depression. FigShare ( 10.6084/m9.figshare.c.7202783) [DOI] [PMC free article] [PubMed]
- 34. Naie K, Tsanov M, Manahan-Vaughan D. 2007. Group I metabotropic glutamate receptors enable two distinct forms of long-term depression in the rat dentate gyrus in vivo. Eur. J. Neurosci. 25 , 3264–3275. ( 10.1111/j.1460-9568.2007.05583.x) [DOI] [PubMed] [Google Scholar]
- 35. Connelly T, Fan Y, Schulz PE. 2011. Distinct mechanisms contribute to agonist and synaptically induced metabotropic glutamate receptor long-term depression. Eur. J. Pharmacol. 667 , 160–168. ( 10.1016/j.ejphar.2011.04.063) [DOI] [PubMed] [Google Scholar]
- 36. Stoppel LJ, Auerbach BD, Senter RK, Preza AR, Lefkowitz RJ, Bear MF. 2017. β-Arrestin2 Couples metabotropic glutamate receptor 5 to neuronal protein synthesis and is a potential target to treat fragile X. Cell Rep. 18 , 2807–2814, ( 10.1016/j.celrep.2017.02.075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheng G, Yu Z, Zhou DH, Mattson MP. 2002. Phosphatidylinositol-3-kinase-Akt kinase and p42/p44 mitogen-activated protein kinases mediate neurotrophic and excitoprotective actions of a secreted form of amyloid precursor protein. Exp. Neurol. 175 , 407–414. ( 10.1006/exnr.2002.7920) [DOI] [PubMed] [Google Scholar]
- 38. Hou LF, Klann E. 2004. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 24 , 6352–6361. ( 10.1523/JNEUROSCI.0995-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Livingstone RW, Elder MK, Barrett MC, Westlake CM, Peppercorn K, Tate WP, Abraham WC, Williams JM. 2019. Secreted amyloid precursor protein-alpha promotes arc protein synthesis in hippocampal neurons. Front. Mol. Neurosci. 12 , 198. ( 10.3389/fnmol.2019.00198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murphy G. 2009. Regulation of the proteolytic disintegrin metalloproteinases, the “Sheddases.” Semin. Cell Dev. Biol. 20 , 138–145. ( 10.1016/j.semcdb.2008.09.004) [DOI] [PubMed] [Google Scholar]
- 41. Cho RW, et al. 2008. mGluR1/5-dependent long-term depression requires the regulated ectodomain cleavage of neuronal pentraxin NPR by TACE. Neuron 57 , 858–871. ( 10.1016/j.neuron.2008.01.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taylor CJ, Ireland DR, Ballagh I, Bourne K, Marechal NM, Turner PR, Bilkey DK, Tate WP, Abraham WC. 2008. Endogenous secreted amyloid precursor protein-α regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol. Dis. 31 , 250–260. ( 10.1016/j.nbd.2008.04.011) [DOI] [PubMed] [Google Scholar]
- 43. Richter MC, et al. 2018. Distinct in vivo roles of secreted APP ectodomain variants APPsα and APPsβ in regulation of spine density, synaptic plasticity, and cognition. EMBO J. 37 , e98335. ( 10.15252/embj.201798335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peavy RD, Chang MSS, Sanders-Bush E, Conn PJ. 2001. Metabotropic glutamate receptor 5-induced phosphorylation of extracellular signal-regulated kinase in astrocytes depends on transactivation of the epidermal growth factor receptor. J. Neurosci. 21 , 9619–9628. ( 10.1523/JNEUROSCI.21-24-09619.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Caillé I, Allinquant B, Dupont E, Bouillot C, Langer A, Müller U, Prochiantz A. 2004. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development 131 , 2173–2181. ( 10.1242/dev.01103) [DOI] [PubMed] [Google Scholar]
- 46. Tang Y, Ye M, Du Y, Qiu X, Lv X, Yang W, Luo J. 2015. EGFR signaling upregulates surface expression of the GluN2B-containing NMDA receptor and contributes to long-term potentiation in the hippocampus. Neuroscience 304 , 109–121, ( 10.1016/j.neuroscience.2015.07.021) [DOI] [PubMed] [Google Scholar]
- 47. Lin YT, Huang CC, Hsu KS. 2012. Oxytocin promotes long-term potentiation by enhancing epidermal growth factor receptor-mediated local translation of protein kinase Mζ. J. Neurosci. 32 , 15476–15488. ( 10.1523/JNEUROSCI.2429-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gladding CM, Fitzjohn SM, Molnár E. 2009. Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol. Rev. 61 , 395–412. ( 10.1124/pr.109.001735) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available in the electronic supplementary materials [33].