Abstract
Fragile X syndrome (FXS) is characterized by impairments in executive function including different types of learning and memory. Long-term potentiation (LTP), thought to underlie the formation of memories, has been studied in the Fmr1 mouse model of FXS. However, there have been many discrepancies in the literature with inconsistent use of littermate and non-littermate Fmr1 knockout (KO) and wild-type (WT) control mice. Here, the influence of the breeding strategy (cage effect) on short-term potentiation (STP), LTP, contextual fear conditioning (CFC), expression of N-methyl-d-aspartate receptor (NMDAR) subunits and the modulation of NMDARs, were examined. The largest deficits in STP, LTP and CFC were found in KO mice compared with non-littermate WT. However, the expression of NMDAR subunits was unchanged in this comparison. Rather, NMDAR subunit (GluN1, 2A, 2B) expression was sensitive to the cage effect, with decreased expression in both WT and KO littermates compared with non-littermates. Interestingly, an NMDAR-positive allosteric modulator, UBP714, was only effective in potentiating the induction of LTP in non-littermate KO mice and not the littermate KO mice. These results suggest that commonly studied phenotypes in Fmr1 KOs are sensitive to the cage effect and therefore the breeding strategy may contribute to discrepancies in the literature.
This article is part of a discussion meeting issue ‘Long-term potentiation: 50 years on’.
Keywords: Fmr1, LTP, STP, CFC, NMDAR PAM, littermate syndrome
1. Introduction
Fragile X syndrome (FXS) is the most common monogenic cause of inherited intellectual disability. It is caused by mutations in the fragile X messenger ribonucleoprotein 1 (FMR1) gene on the X chromosome, which lead to transcriptional silencing of the gene and no production of the fragile X messenger ribonucleoprotein (FMRP) [1–11]. FMRP has been found to be important in synaptic plasticity, including long-term potentiation (LTP), the strengthening of synaptic connections. A variety of changes in LTP have been observed in Fmr1 knockout (KO) mice, ranging from inhibition [12–52] to no effect [12,13,15,29,32,33,36,38,40,42,49,53–59] or even an increase [49,51,55,58,60,61] (see table 1). There are many possible reasons for these inconsistencies, including the induction protocol used, the age and strain of the mice and a number of environmental factors. One such potential environmental difference is whether a study uses littermate or non-littermate mice. In some experiments, wild-type (WT) and Fmr1 KO mice are born to the same parents (WT father and heterozygous (HET) mother) and these littermates are kept in the same home cage after weaning [62,63]. Alternatively, separate colonies of WTs and KOs are bred from WT–WT pairs and KO–KO pairs, respectively, and housed separately as non-littermates.
Table 1.
Summary of studies examining LTP phenotypes in littermate and non-littermate Fmr1 KO mice. (Studies examining LTP in Fmr1 KO mice were subdivided into three groups: (i) littermate, (ii) non-littermate mice, and (iii) undefined (breeding scheme could not be determined). Only the Fmr1 KO and Fmr1 KO2 (missing Fmr1 mRNA) mouse models of FXS are included. The LTP genotype and brain area studied are shown. Abbreviations: mPFC, medial pre-frontal cortex; LA, lateral amygdala; DG, dentate gyrus; ACC, anterior cingulate cortex; LPP, lateral perforant pathway; MPP, medial perforant pathway; TA, temporoammonic; aud cortex, auditory cortex; MF, mossy fibre; PP, pairing protocol; β-AR, β-adrenoceptors; HFS, high-frequency stimulation; TBS, theta-burst stimulation; mGluR, metabotropic glutamate receptor; STDP, spike timing-dependent plasticity; TBSP, TBS pairing protocol; NOR, novel object recognition; C/A, commissural/associational; LFS, low-frequency stimulation.)
| LTP phenotype | studies | ref. | brain area | induction protocol; notes | |
|---|---|---|---|---|---|
| littermate | increase | Pilpel et al. 2009a ,b | [55] | CA1 | PP (age- and protocol- dependent effects); Fmr1 KO2 |
| Connor et al. 2011a | [58] | CA1 | β-AR-dependent heterosynaptic plasticity | ||
| Martin et al. 2023 | [51] | Cerebellum | 100 stimuli, 10 Hz; presynaptic LTP mediated by β-AR (low [Ca]) | ||
| no change | Godfraind et al. 1996 | [53] | CA1 | HFS | |
| Paradee et al. 1999 | [54] | CA1 | TBS | ||
| Li et al. 2002c | [12] | CA1 | TBS (brain area-dependent effect) | ||
| Pilpel et al. 2009 | [55] | CA1 | PP (age-dependent) / TBS (protocol-dependent); Fmr1 KO2 | ||
| Zhang et al. 2009a , b | [56] | CA1 | HFS / Fmr1 KO; littermate: Fmr1+/− mother and Fxr2−/y father | ||
| Auerbach & Bear 2010 | [57] | CA1 | HFS | ||
| Connor et al. 2011a | [58] | CA1 | HFS-induced synaptic tagging and capture | ||
| Franklin et al. 2014ac | [29] | CA1 | HFS (hippocampal region-dependent effect) | ||
| Bostrom et al. 2015c | [32] | CA1 | HFS (hippocampal region-dependent effect) | ||
| Ghilan et al. 2015c | [33] | CA1 | HFS (hippocampal region-dependent effect) | ||
| Martin et al. 2016c | [36] | mPFC | TBS (area dependent); Fmr1 KO2 | ||
| reduction | Li et al. 2002c | [12] | cortex | TBS (brain area-dependent effect) | |
| Suvrathan et al. 2010 | [20] | LA | HFS; mGluR-dependent LTP | ||
| Eadie et al. 2012 | [23] | DG | HFS | ||
| Padmashri et al. 2013 | [26] | cortex | chemical LTP | ||
| Boda et al. 2014 | [27] | CA1 | TBS | ||
| Franklin et al. 2014ac | [29] | DG | HFS (hippocampal region-dependent effect) | ||
| Franklin et al. 2014b | [30] | DG | HFS | ||
| Bostrom et al. 2015c | [32] | DG | HFS (hippocampal region-dependent effect) | ||
| Ghilan et al. 2015c | [33] | DG | HFS (hippocampal region-dependent effect) | ||
| Neuhofer et al. 2015 | [35] | accumbens | STDP | ||
| Martin et al. 2016b | [36] | mPFC | TBS (age-dependent effect); Fmr1 KO2 | ||
| Yau et al. 2016 | [37] | DG | HFS; Female Fmr1 HET mice | ||
| Feuge et al.2019 | [41] | CA1 | chemical LTP; no increase in mEPSC amplitude | ||
| Hwang et al. 2022 | [46] | CA1 | PP; interneurons | ||
| Martin et al. 2023a | [51] | cerebellum | 100 stimuli, 10 Hz; presynaptic LTP mediated by β-AR (high [Ca]) | ||
| non-littermate | increase | Brager et al. 2012 | [60] | CA1 | TBSP |
| Routh et al. 2013 | [61] | CA1 | TBSP | ||
| Borreca et al. 2023a | [49] | DG | HFS; 24 hour post NOR / protocol-dependent | ||
| no change | Lauterborn et al. 2007a | [15] | CA1 | TBS (protocol-dependent effect) | |
| Seese et al. 2012 | [59] | CA1 | TBS; reported 5 min post-induction | ||
| Lundbye et al. 2018b | [38] | CA1 | TBS (protocol-dependent effect) | ||
| Wang et al. 2018a | [40] | DG | Optogenetic induction protocol; C / A pathway | ||
| Banke & Barria 2020b | [42] | CA1 | LFS; 3 Hz, 2 min (age dependent) | ||
| Borreca et al. 2023a | [49] | DG | HFS; No NOR (protocol-dependent effect) | ||
| reduction | Lauterborn et al. 2007a | [16] | CA1 | TBS (protocol-dependent effect) | |
| Meredith et al. 2007 | [17] | PFC | STDP; increased threshold | ||
| Wilson & Cox 2007 | [18] | Neocortex | HFS | ||
| Lee et al. 2011 | [21] | CA1 | TBS | ||
| Xu et al. 2012a | [24] | ACC | PP | ||
| Xu et al. 2012b | [25] | ACC | PP | ||
| Lundbye et al. 2018b | [38] | CA1 | TBS (protocol-dependent effect) | ||
| Martinez & Tejada-Simon 2018 | [39] | CA1 | TBS | ||
| Wang et al. 2018a | [40] | DG | HFS/ pairing protocol; both MPP and LPP | ||
| Banke & Barria 2020b | [42] | CA1 | LFS; 3 Hz, 2 min (age-dependent effect) | ||
| Ostrovskaya et al. 2020 | [52] | CA1 | TBS; Lower probability of late-LTP in P21 KOs (studied < 2 to 5 week) | ||
| Seese et al. 2020 | [43] | CA1 | TBS | ||
| Zhan et al. 2020 | [44] | Cerebellum | TBS | ||
| Ordemann et al. 2021 | [45] | CA1 (TA) | TBS | ||
| Jeon et al. 2022 | [47] | CA1 | TBS | ||
| Borreca et al. 2023a | [49] | DG | HFS; 1 hour post NOR (protocol-dependent effect) | ||
| Li et al. 2023 | [50] | mPFC | TBS | ||
| undefined | no change | Larson et al. 2005b | [13] | APC / CA1 | TBS (age-dependent lack of effect; APC) |
| reduction | Larson et al. 2005b | [13] | APC | TBS (age-dependent effect) | |
| Zhao et al. 2005 | [14] | ACC/LA | PP | ||
| Hu et al. 2008 | [18] | CA1 | PP; acute and cultured slices | ||
| Shang et al. 2009 | [19] | CA1 | Chemical LTP | ||
| Yun & Trommer 2011 | [22] | DG | TBS | ||
| Chen et al. 2014 | [28] | ACC | TBS | ||
| Yang et al. 2014 | [31] | Aud cortex | HFS; mGluR dependent LTP | ||
| Koga et al. 2015 | [34] | ACC | LFS; 2 Hz, 2 min; Presynaptic LTP | ||
| Monday et al. 2022 | [48] | DG | 25 Hz, 3X + d-APV; conditional KO of FMRP in Granule Cells |
Diverging results within paper due to induction protocol difference.
Diverging results within paper due to age differences.
Diverging results within paper due to brain area differences.
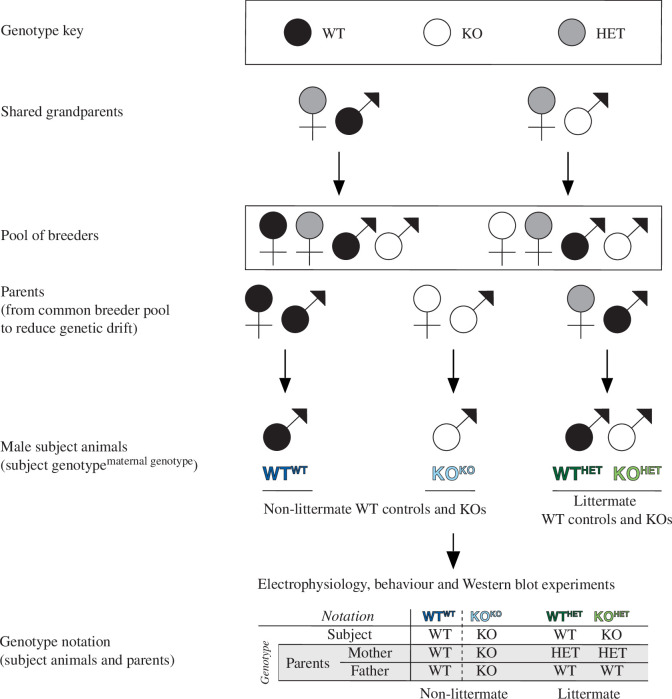
Mice may be influenced by interactions with their siblings, with their parents or a combination of the two, all of which can contribute to differences in their development. In this study, the term ‘cage effect’ encompasses any difference between the environments of non-littermate and littermate mice, including any potential gene-environment (G×E) interactions. As denoted in the format—subject genotype(maternal genotype)—non-littermate Fmr1 WTWT and KOKO, and littermate WTHET and KOHET mice, were used to examine the consequences of the cage effect on LTP, fear memory, expression of N-methyl-d-aspartate receptor (NMDAR) subunits, and the modulation of synaptic plasticity by an NMDAR-positive allosteric modulator (PAM). Previous studies have demonstrated that certain FXS phenotypes can be influenced by whether mice are bred in a littermate or non-littermate fashion. Specifically, littermate Fmr1 WTHET mice (i.e. born to Fmr1 HET mothers) show hyperactivity and social avoidance profiles that are more similar to those found in littermate and non-littermate KOs (KOHET and KOKO, respectively), than to non-littermate WT (WTWT) mice [64,65]. These results suggest that behavioural phenotypes may be influenced by the littermate environment.
Studies of LTP in Fmr1 KOs have used a relatively even split of littermate and non-littermate mice (table 1; littermate = 21, non-littermate = 19, undetermined = 9). For example, in the CA1 region of the hippocampus, there was a LTP reduction in 3 out of 15 studies using littermate mice compared with 9 out of 14 using non-littermate mice (table 1). It is important to note that there are other differences between these studies, such as the age of the mice or the strength of the induction protocol [15,38].
Besides synaptic plasticity, other measures are affected in Fmr1 KO mice in an inconsistent fashion, including the expression of NMDAR subunits. Alterations in the total expression of various NMDAR subunits have been reported to increase [38,66,67], decrease [32,50] or not change [32,68,69] in Fmr1 KO mice (table 2). Behavioural studies in Fmr1 KO mice examining long-term memory, such as through contextual fear conditioning (CFC), have also diverged, with some studies reporting impaired fear memory [39,54,70–72] and others finding intact fear memory [73–77] (table 3).
Table 2.
Summary of studies examining NMDAR expression in littermate and non-littermate Fmr1 KO mice. (Studies examining NMDAR expression in Fmr1 KO mice were subdivided into two groups: (i) littermate and (ii) non-littermate mice.)
| NMDAR phenotype | studies | ref. | |
|---|---|---|---|
| littermate | increase | — | — |
| no change | Bostrom et al. 2015 | [32] | |
| Chatterjee et al. 2018 | [68] | ||
| Yau et al. 2018 | [69] | ||
| decrease | Bostrom et al. 2015 | [32] | |
| non-littermate | increase | Schutt et al. 2009 | [66] |
| Toft et al. 2016 | [67] | ||
| Lundbye et al. 2018 | [38] | ||
| no change | — | — | |
| decrease | Li et al. 2023 | [50] |
Table 3.
Summary of studies examining CFC phenotypes in littermate and non-littermate Fmr1 KO mice. (Studies examining CFC (only context, no cued or trace conditioning) in Fmr1 KO mice were subdivided into two groups: (i) littermate and (ii) non-littermate mice.)
| CFC phenotype | studies | ref. | mouse (background); notes | |
|---|---|---|---|---|
| littermate | no change | Dobkin et al. 2000 | [77] | Fmr1 KO (FVB-129 and C57BL/6-129) |
| van Dam et al. 2000 | [73] | Fmr1 KO (C57BL/6J) | ||
| Spencer et al. 2006 | [44] | Fmr1 KO (C57BL/6J); littermate: Fmr1+/− mother and Fxr2−/y father | ||
| Uutela et al. 2012 | [75] | Fmr1 KO (C57BL/6J); littermate: Fmr1+/− mother and BDNF+/− father | ||
| Nolan et al. 2022 | [76] | Fmr1 KO (FVB-129) | ||
| decrease | Paradee et al. 1999 | [54] | Fmr1 KO (C57BL/6J) | |
| Baker et al. 2010 | [70] | Fmr1 KO (albino C57BL/6J) | ||
| Reyes et al. 2021 | [72] | Fmr1 KO2 (C57BL/6J) | ||
| non-littermate | no change | — | — | — |
| decrease | Ding et al. 2014 | [71] | Fmr1 KO (C57BL/6J); male and female | |
| Martinez & Tejada-Simon 2018 | [39] | Fmr1 KO (FVB-129) |
Accordingly, the current study aimed to systematically determine whether variations in breeding design contribute to the offspring developing differences in synaptic plasticity (short-term potentiation (STP) and LTP), NMDAR subunit expression and contextual fear memory. Cage effects were examined through comparisons of non-littermate Fmr1 WTWT and KOKO and littermate WTHET and KOHET mice. In addition, the effectiveness of a low-efficacy PAM of NMDARs, UBP714, at rescuing synaptic plasticity deficits was compared in KOKO and KOHET mice.
2. Methods
(a). Animals
Experiments were performed at the Lunenfeld–Tanenbaum Research Institute (LTRI, Sinai Health System, Toronto, Ontario, Canada) and the Neurobehaviour Core at The Centre for Phenogenomics (TCP), according to an animal use protocol approved by the Animal Care Committee and conforming to the Canadian Council on Animal Care guidelines. Mice were group housed (maximum of five mice per cage) on 12 L : 12 D cycle (from 7.00), with food and water ad libitum. Female Fmr1 KO mice [78] on a C57BL/6J genetic background (B6.129P2-Fmr1tm1Cgr; RRID:IMSR_JAX:003025) were further backcrossed with male C57BL/6J mice for three generations. To obtain male littermate WT and KO mice, female Fmr1 HET mice were crossed with male Fmr1 WT mice to produce WTHET and KOHET mice (subject genotype maternal genotype). Male non-littermate WTWT mice were produced by breeding female and male Fmr1 WT mice while breeding female Fmr1 KO with male hemizygous KO mice resulted in non-littermate KOKO mice (breeding scheme and notation are summarized in figure 1). Note that breeders in both breeding strategies came from shared grandparents, thereby reducing genetic drift between the two groups. To study the cage effect (the differences, including GxE interactions, between littermate and non-littermate environments), male Fmr1 mice were used and WTWT, KOKO, WTHET and KOHET mice were weaned at three weeks of age and housed with their littermates. All other aspects of the cages (dimensions, bedding and environmental enrichment) were identical between littermates and non-littermates. Mice aged between 8 and 14 weeks, with genotypes interleaved, were used for electrophysiology, CFC and biochemistry experiments. For each type of experiment, the same female researcher identified, handled and studied or collected each mouse (genotypes were not blinded). The number of animals is denoted as N, whereas the number of slices is shown as n.
Figure 1.
Schematic of the breeding paradigm used to generate mice of each experimental genotypematernal genotype and cage background (littermate vs non-littermate). FXS is an X-linked disorder and, therefore, male mice only inherit their X chromosome from their mothers, whereas female mice inherit one X chromosome from each of the parents. A pool of breeders was created by crossing (i) a HET mother and WT father, and (ii) a HET mother with a KO father (shared grandparents). Using parents from the pool of breeders, male WT and hemizygous KO (males have one X chromosome) subject mice (experimental animals) were obtained; their genotype notation is shown together with their mother’s genotype in superscript (subject genotypematernal genotype). Non-littermate WTWT mice were produced by crossing WT female and WT male mice, whereas for the non-littermate KOKO mice, KO female mice were crossed with KO males. To obtain littermate WT and KOs, HET females were crossed with male WTs to produce WTHET and KOHET mice. These WTWT, KOKO, WTHET and KOHET mice were used for electrophysiological, behavioural and Western blotting experiments.
(b). Hippocampal slice preparation
Hippocampal slices were prepared as previously described [79]. Briefly, mice were decapitated under isoflurane anaesthesia and brains were removed and placed in cold (4°C) artificial cerebrospinal fluid (ACSF; all salts purchased from Wisent Inc., Canada) containing in mM: 130 NaCl, 10 d-glucose, 26 NaHCO3, 3.5 KCl, 1.2 NaH2PO4, 2 MgCl2 and 2 CaCl2 bubbled with carbogen (95% O2–5% CO2). Transverse dorsal hippocampal slices were prepared using a McIlwain tissue chopper and allowed to recover in room temperature ACSF for at least 2 h before electrophysiological recordings.
(c). Electrophysiological recordings and data analysis
Hippocampal slices were transferred to a submerged chamber system (Scientifica, Uckfield, United Kingdom) and perfused at 2.5 ml min-1 with ACSF maintained at 30°C. Field excitatory postsynaptic potentials (fEPSPs) were evoked in the stratum radiatum of the CA1 area in response to bi-phasic stimulation (STG 4002; MCS, Multichannel systems, Germany) of Schaffer collateral/commissural (SCC) afferents with a platinum/iridium stimulation electrode (FHC, Bowdoin, ME, USA). Stimulation intensity was set to three times the threshold current required to evoke an fEPSP and baseline fEPSPs were recorded at 0.05 Hz. Recordings were amplified (Axopatch 1D, Molecular Devices, San Jose, CA, USA), digitized at 40 kHz (A/D) and recorded using WinLTP [80,81]. After 30 min of stable baseline, LTP was induced by theta-burst stimulation (TBS), consisting of four pulses at 100 Hz repeated five times at 5 Hz. In experiments with compounds, these were applied for 30 min prior to the induction of LTP.
Synaptic responses were analysed off-line to quantify changes in the initial fEPSP slope. To analyse STP and LTP, individual experiments were then normalized to their pre-induction baseline and fitted with a mono-exponential regression using Platin (a custom-built software package, Morten Skovgaard Jensen, Aarhus University, Denmark). Thus, the extent of LTP (%, the plateau) and amplitude of STP (%, maximum potentiation minus the plateau), and the decay time constant of STP (τ in min) were determined, as previously described [82,83]. It should be noted that STP comprises two mechanistically distinct components, STP1 and STP2, that have different τ-values [83]. However, with the current induction protocol, the level of STP2 is small, making it difficult to fit the datasets with double exponentials. Therefore, a single exponential fit was used as a weighted approximation. Furthermore, the assessment of STP ‘amplitude’ assumes that LTP has reached its plateau value at the time of the measurement. Since LTP may develop slowly [84], this value is likely to be an underestimate. Nevertheless, these measures provide a good approximation for the comparative assessment of synaptic plasticity changes between the groups of mice. Results are presented as averages of 2 min, normalized to baseline and plotted over time (mean values ± s.e.m.). The first minute after induction is excluded to reduce contamination of STP measurement by the short-lived, NMDAR-independent post-tetanic potentiation.
(d). Pharmacology
UBP714 [85] was synthesized at the University of Bristol and was prepared in equimolar concentrations of NaOH to give a stock concentration of 100 mM. It was stored at −20°C and diluted in ACSF to the final concentration. The compound had no effect on baseline fEPSPs.
(e). Contextual fear conditioning
CFC was performed using the Video Fear Conditioning System (Med Associates Inc., no. MED-VFC2-USB-M), and all experiments were performed at the same time of day (commencing at 13.00). Briefly, a mouse was placed in a CFC chamber (wiped with 70% isopropyl alcohol as an olfactory cue) and allowed to move freely for 3 min before receiving a 2 s, 0.75 mA, foot-shock. Each mouse was left in the chamber for a further 30 s and then removed into their home cage. At 24 h after conditioning, the mice were placed back into the CFC chamber with the same olfactory cue. No shocks were delivered and the amount of time the mice were immobile (% freezing) was measured over a 5 min period. The automated VideoFreeze™ Video Fear Conditioning Software (Med Associates Inc., Fairfax, VT) was used to analyse the behavioural data.
(f). Western blotting
Hippocampi were extracted and kept in ice-cold ACSF. Under visual guidance using a stereomicroscope, whole dorsal CA1 chunks were isolated from both hippocampi of each mouse, pooled and placed on dry ice. Samples were then stored at −80°C until further tissue processing. Tissue was homogenized with a hand-held homogenizer in ice-cold radioimmunoprecipitation assay (RIPA) buffer (nos. 20–188, Millipore,) with protease/phosphatase inhibitor cocktail (no. 5872, Cell Signaling Technology). Samples were centrifuged at 15 000 × r.p.m. for 20 min at 4°C and the supernatant was collected. Protein concentrations were determined through the Pierce™ BCA Protein Assay Kit (no. 23228, Thermo Fisher Scientific, Canada) according to the manufacturer’s recommended protocol. Samples were denatured with 4 × Laemmli sodium dodecyl-sulfate (SDS) sample buffer (no. 1610747, BioRad) containing 2.5% β-mercaptoethanol (no. M3148, Sigma).
Protein samples (15 μg) were loaded on 7.5% TGX stain-free polyacrylamide gels (no. 1610181, BioRad) submerged in 1 × tris-glycine (TG)-SDS running buffer (no. 811–570-FL, Wisent). The gels were electrophoresed for 40 min at 200 V, before being activated and imaged using a ChemiDoc MP Imaging System (Bio-Rad Laboratories, Canada). Proteins were transferred to a low fluorescence polyvinylidene fluoride (PVDF) membrane (no. 1620264, Bio-Rad) using a wet transfer protocol in 1 × TG transfer buffer (no. 811–560-FL, Wisent) for 2 h at 35 V. Following transfer, total protein was imaged on the ChemiDoc system. Membranes were blocked using EveryBlot Blocking Buffer (no. 12010020, Bio-Rad) for 30 min at room temperature and incubated overnight with primary antibodies (GluN1: 1 : 5000 no. G8913, MilliporeSigma Canada; GluN2A: 1 : 5000 no. A0924, AbClonal Technology, USA; GluN2B: 1 : 2000, no. 66565-I-IG, Proteintech Group, USA) diluted in EveryBlot. Membranes were washed with 1 × tris-buffered saline Tween- 20 (TBST) and incubated for 1 h at room temperature with secondary antibody (StarBright SB520 no. 12005869, Bio-Rad or StarBright SB700 no. 12004158, Bio-Rad) diluted in EveryBlot at 1 : 2500 and 1 : 1500 dilution depending on the experiment. For multiplexing GluN2A and GluN2B targets, secondary antibodies with fluorochromes of different emission wavelengths were combined. Two-channel multiplex fluorescence images were collected on the ChemiDoc system.
Protein expression analysis was conducted using the Image Lab Software (version 6.1, Bio-Rad). Target protein levels were normalized to total protein, defined as the total adjusted lane volume of proteins between 37 and 250 kDa. The protein levels were expressed as a percentage of the WTWT signal. Whole blots and total protein staining of targeted protein (i.e. GluN1, GluN2A and GluN2B) appear in the electronic supplementary material, figure S1.
(g). Statistics
Statistical analysis was performed using GraphPad Prism. To analyse the influence of the parental genotypes and breeding schemes generating non-littermate and littermate mice, comparisons by two-way ANOVAs were applied to electrophysiology, CFC and Western blot data. A Fisher’s least significant difference (LSD) post hoc test was used for multiple comparisons to test the differences between: (i) WTWT and KOKO, (ii) WTHET and KOHET, (iii) WTWT and WTHET, and (iv) KOKO to KOHET (figures 2–4). The cage effects and compound treatment on synaptic plasticity in the two KO groups were examined using a two-way ANOVA and differences between: (i) KOKO and UBP714, (ii) KOHET and UBP714, (iii) KOKO and KOHET, and (iv) KOKO + UBP714 and KOHET + UBP714 were tested using Fisher’s LSD post hoc test (figure 5). The significance level was set to p < 0.05 and precise statistical values appear either within the text or figure captions, and post hoc results are denoted on the figures as *p < 0.05, **p < 0.01 and ***p < 0.001.
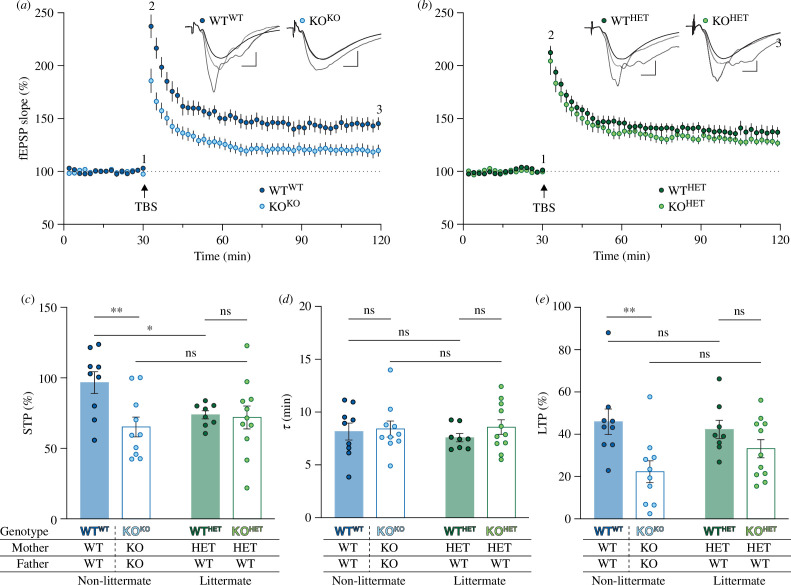
Figure 2.
Breeding in a littermate or non-littermate fashion determines the synaptic plasticity deficit outcome in Fmr1 KO mice. (a) Time course of synaptic responses (fEPSPs) in CA3-CA1 hippocampus synapses from non-littermate WTWT (dark blue circles; n = 9) and KOKO mice (light blue circles; n = 10). (b) Time course of fEPSPs in littermate WTHET (dark green circles; n = 8) and KOHET mice (light green circles; n = 11). Sample fEPSP traces (representative experiment) in the upper right sections of plots (a) and (b) are superimposed from (1) baseline, (2) STP and (3) LTP time points as indicated. Scale bar: 0.5 mV per 5 ms. (c) STP was affected by the genotype (p = 0.046), but not the cage effect (p = 0.269), with a significant interaction between the two variables (p = 0.026). Specifically, the extent of STP was significantly decreased in KOKO and WTHET mice compared with WTWT mice (p = 0.004 and p = 0.039, respectively). There were no significant (ns) differences between slices from littermate KOHET and WTHET mice. (d) Neither genotype (p = 0.777) nor cage effect (p = 0.383; interaction, p = 0.596) influenced the decay time constant ( ) of STP. (e) The genotype (p = 0.003) but not the cage effect (p = 0.475; interaction, p = 0.158) had an effect on the level of LTP induced. Notably, there was only a significant deficit in non-littermate KOKO mice compared with WTWT mice (p = 0.002). Experiments were analysed with a two-way ANOVA with Fisher’s LSD post hoc test.
Figure 3.
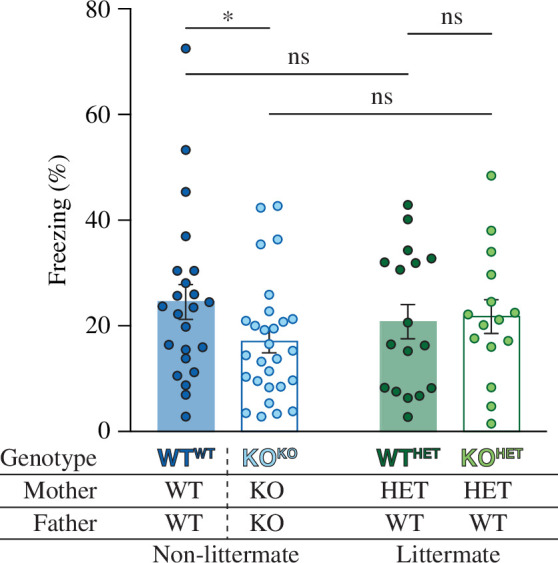
The cage effect determines fear memory deficit in Fmr1 KO mice. At 24 h after contextual fear conditioning, mice were placed back into the conditioning chambers and their immobility (freezing) during the 5 min test was determined. The non-littermate KOKO mice (open blue bar; n = 28) demonstrated deficits in freezing compared with WTWT mice (filled blue bar; n = 23; p = 0.049). However, KOHET mice (open green bar; n = 15) immobility was similar to their littermate WTHET mice (filled green bar; n = 17; p = 0.840). Experiments were analysed with a two-way ANOVA with Fisher’s LSD post hoc test.
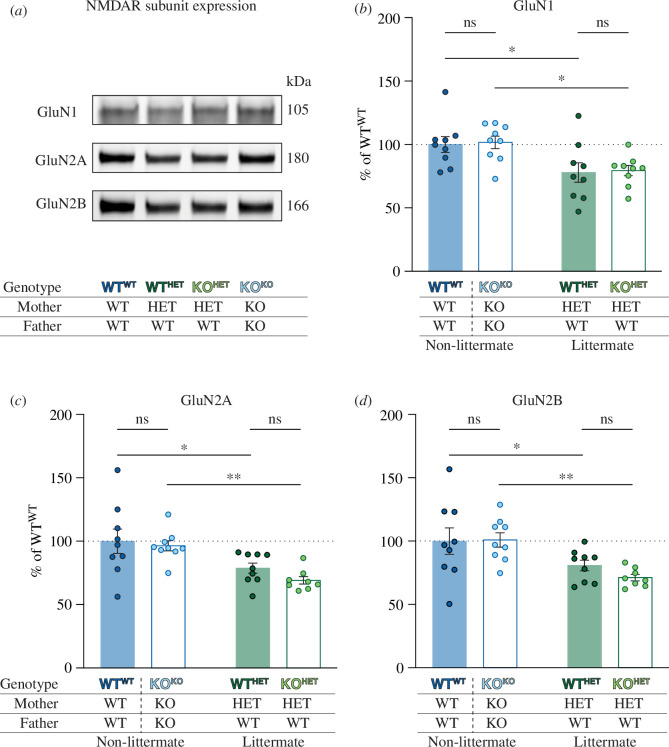
Figure 4.
Differential expression of NMDAR subunits in the CA1 area of the hippocampus from littermate versus non-littermate Fmr1 KO mice. (a) Representative blots of NMDAR GluN1, GluN2A and GluN2B subunits from hippocampus of WTWT, WTHET, KOHET and KOKO mice. Quantification of each targeted protein was normalized to total protein and expressed as a per cent of WTWT (see the electronic supplementary material, figure S1 for full blots). (b) The cage effect (p = 0.001), but not genotype (p = 0.786; interaction, p = 0.983) influenced the expression of GluN1 subunits. WTHET (filled green bar; n = 9) and KOHET mice (open green bar; n = 9) had significantly decreased expression of GluN1 compared with WTWT (filled blue bar; n = 9; p = 0.012) and KOKO mice (open blue bar; n = 9; p = 0.012), respectively. (c) The decreased GluN2A expression in Fmr1 KO mice was a result of the cage effect (p < 0.001) and not genotype (p = 0.269; interaction, p = 0.615). GluN2A expression in WTHET mice (n = 9) was decreased compared with WTWT mice (n = 9; p = 0.015) and expression in KOHET mice (n = 8) was decreased compared with KOKO mice (n = 9; p = 0.003). (d) GluN2B expression was not affected by the genotype (p = 0.515) but was affected by the cage effect (p = 0.001; interaction, p = 0.434). Specifically, expression of GluN2B was reduced in WTHET mice (n = 9) compared with WTWT mice (n = 9; p = 0.043) and in KOHET mice (n = 8) compared with KOKO mice (n = 9; p = 0.004). Experiments were analysed with a two-way ANOVA with Fisher’s LSD post hoc test.
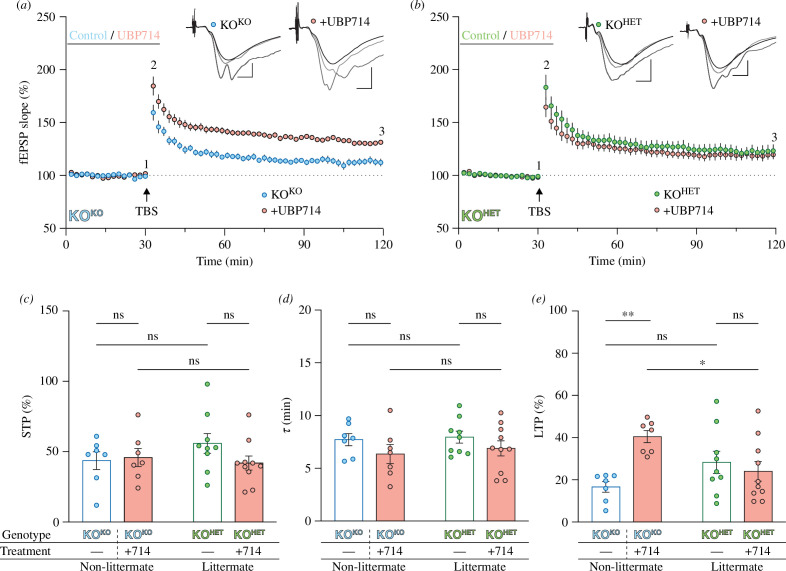
Figure 5.
LTP in non-littermate KOKO but not littermate KOHET, is sensitive to a NMDAR PAM. (a) Time course of fEPSPs in hippocampal slices from KOKO mice, under vehicle (blue circles; n = 7) or UBP714 (100 μM; pink circles; n = 7) conditions. (b) Time course of fEPSPs in KOHET slices, under vehicle (green circles; n = 9) or UBP714 (100 μM; pink circles; n = 10) conditions. Sample fEPSP traces (upper right section of plots in (a) and (b) are superimposed from (1) baseline, (2) STP and (3) LTP periods as shown. Scale bar: 0.5 mV per 5 ms. (c) Neither UBP714 application (p = 0.360), nor cage effect (p = 0.513) influenced STP (interaction, p = 0.211) in the two types of KO mice. (d) Similarly, the τ of STP was unaffected by the cage effect (p = 0.590) or UBP714 application (p = 0.097; interaction: p = 0.832). (e) Treatment with UBP714 had a significant effect on LTP (p = 0.035), with a significant interaction between UBP714 treatment and the cage effect (p = 0.004). The cage effect itself had no significant effect on LTP (p = 0.587). Specifically, the application of UBP714 to non-littermate KOKO hippocampal slices significantly enhanced LTP (p = 0.001), while in the KOHET slices UBP714 had no effect (p = 0.464). Notably, the level of LTP in KOKO + UBP714 was significantly greater than in KOHET + UBP714 slices (p = 0.012). Experiments were analysed with a two-way ANOVA with Fisher’s LSD post hoc test.
3. Results
To examine whether CA1 synaptic plasticity in male Fmr1 KO mice is sensitive to the cage effect (see figure 1 for breeding scheme and nomenclature), TBS was delivered to the SCC to induce STP and LTP (figure 2; for all ANOVA statistics see the electronic supplementary material, table S1). When compared with WTWT mice (96.7 ± 7.7%; n = 9), STP was significantly reduced in the KOKO mice (65.2 ± 7.0%; n = 10; p = 0.004). Conversely, the level of STP in WTHET mice (73.8 ± 2.9%; n = 8) and KOHET mice (72.0 ± 8.1%; n = 11) was similar. The difference in the effects on STP can be attributed to a reduction in STP in the WTHET compared with WTWT mice (p = 0.039). In contrast to the cage effect on STP magnitude, the estimate of STP decay was similar between WTWT (8.2 ± 0.8 min), KOKO (8.4 ± 0.8 min), WTHET (7.6 ± 0.4 min) and KOHET (8.6 ± 0.7 min) mice. With respect to LTP, there was a breeding strategy-dependent effect. Thus, when compared with WTWT mice (45.9 ± 6.0%), LTP was reduced in the KOKO (22.3 ± 5.2%; p = 0.002). However, when compared with WTHET mice (42.3 ± 4.3%), the level of LTP in the KOHET mice (33.2 ± 4.3%) was not significantly different. In summary, with respect to both STP and LTP, there is a clear reduction in the KOKO compared with the WTWT groups but little or no difference between the between the WTHET and KOHET groups. Both the LTP and STP deficits show a cage effect and therefore are influenced by the breeding scheme.
CFC was carried out to test whether contextual fear memory is influenced by the cage effect (figure 3). Compared with WTWT mice (24.6 ± 3.3%; n = 23), there was reduced freezing in the KOKO mice (17.1 ± 2.1%; n = 28; p = 0.049). By contrast, the level of freezing in the WTHET mice (20.8 ± 3.2%; n = 17) was similar to that in the KOHET mice (21.7 ± 3.2%; n = 15). Neither the genotype nor the cage effect alone is sufficient to cause a deficit in contextual fear memory. Both the non-littermate environment and the lack of FMRP are necessary, leading to deficits in KOKO mice compared with WTWT mice.
To determine whether the expression of NMDARs relates to the differences in synaptic plasticity (figure 2) and fear memory results (figure 3), Western blots were carried out on GluN1, GluN2A and GluN2B subunits from CA1 hippocampus (figure 4). Irrespective of the breeding strategy, there were no significant differences between WT and KO mice in the expression of GluN1, GluN2A and GluN2B subunits. Interestingly, the expression of the three subunits was reduced in the littermate mice compared with the non-littermate mice. Thus, GluN1 expression, when compared with WTWT mice (100.0 ± 6.2%; n = 9), was decreased in WTHET mice (77.9 ± 7.7%; n = 9; p = 0.012); meanwhile, compared with KOKO mice (101.7 ± 4.9%; n = 9), GluN1 was reduced in KOHET mice (79.4 ±. 4.0%; n = 9; 0.012). Similarly, GluN2A expression was reduced in WTHET mice (78.8 ± 4.0%; n = 9) compared with WTWT mice (100.0 ± 9.6%; n = 9; p = 0.015) and in KOHET mice (69.2 ± 2.9%; n = 8) compared with KOKO mice (96.4 ± 4.0%; n = 9; p = 0.003). GluN2B subunit expression followed a similar pattern, with decreased expression in WTHET mice (80.7 ± 4.2%; n = 9) compared with WTWT mice (100.0 ± 10.4%; n = 9; p = 0.043), as well as in KOHET mice (71.2 ± 2.6%; n = 8) mice when compared with KOKO mice (100.9 ± 5.6%; n = 9; p = 0.004). As such, the cage effect appears to primarily be responsible for changes in the expression of NMDAR subunits.
Potentiation of NMDAR function using the PAM UBP714 enhances LTP in CA1 slices from WT rats and mice [79]. To examine whether this compound improves synaptic plasticity in the two types of Fmr1 KOs, 100 µM UBP714 was applied for 30 min prior to TBS (figure 5). Treatment with UBP714 had no effect on baseline transmission or the magnitude of STP, with similar levels in KOKO (43.7 ± 6.3%; n = 7), KOKO + UBP714 (45.9 ± 6.4%; n = 7), KOHET (55.9 ± 7.1%; n = 9) and KOHET + UBP714 (42.0 ± 5.0%; n = 10) slices. Similarly, the decay time constant, τ, was similar in KOKO (7.9 ± 0.5 min), KOKO + UBP714 (6.4 ± 0.9 min), KOHET (8.0 ± 0.6 min) and KOHET + UBP714 (6.9 ± 0.7 min) slices. However, UBP714 enhanced LTP in the non-littermate KOKO (from 16.7 ± 2.5% to 40.5 ± 2.9%; p = 0.001) but was ineffective in the littermate KOHET (28.3 ± 5.3% and 24.0 ± 4.6%; p = 0.076). Thus, the effect of a NMDAR-PAM is also dependent on the cage effect.
4. Discussion
The current study investigated whether the cage effect (comprising differences between the environments or G×E interactions) of using male non-littermate, Fmr1 WTWT and KOKO, or littermate, Fmr1 WTHET and KOHET mice (figure 1), influences synaptic plasticity, fear memory, protein expression and neuromodulation by a PAM, UBP714. It was found that all of these measures are sensitive to the cage effect.
(a). Cage effects on long-term potentiation
The KOKO mice had a substantially reduced LTP compared with WTWT mice, while the KOHET mice only showed a trend towards reduction of LTP compared with WTHET mice. This effect was primarily caused by greater LTP in the KOHET mice compared with the KOKO mice. As such, the cage effect may ameliorate some of the LTP deficits observed in Fmr1 KO mice in the CA1 area of the hippocampus. These results are consistent with the majority of studies carried out in the CA1, where studies using non-littermate Fmr1 mice resulted in more frequently reported deficits in LTP than in littermate mice (table 1). It is known that environmental factors can influence LTP. For example, poor maternal care (low grooming by mothers) inhibits the induction of LTP in the offspring [86,87], and social defeat stress has been found to enhance LTP [88]. The majority of studies in other brain areas found a reduction of LTP regardless of whether littermate or non-littermate mice were examined (table 1), suggesting that LTP in area CA1 may be particularly sensitive to the cage effect.
(b). Cage effects on short-term potentiation
Another form of synaptic plasticity, STP, was also examined in this study. STP, like LTP, is an increase in the strength of synaptic transmission following high-frequency stimulation, often delivered in bursts to mimic the endogenous theta rhythm. This increase in synaptic strength is transient and decays to either a stable LTP or back to baseline [89–91] in an activity-dependent manner [79,82]. While the role of LTP in long-term memory is commonly accepted, there is growing interest in STP as the potential mechanism for some forms of short-term memory [82,92–96]. STP has not been systematically examined in Fmr1 KO mice. STP was substantially reduced in the KOKO mice but was unaltered in the KOHET mice when compared with WTWT and WTHET mice, respectively. The STP difference was principally owing to lower STP in WTHET mice compared with WTWT mice. Indeed, STP in WTHET mice is similar to STP in both KOs. Interestingly, this STP pattern is similar to hyperactivity [64] and social avoidance phenotypes [65] in Fmr1 mice, where the cage effect primarily influenced the WTHET mice to be more like the KOs, rather than causing changes in the KOHET mice. In summary, the breeding strategy influences the induction of STP.
(c). Fear memory deficit is influenced by the cage effect
CFC is dependent on the hippocampus and, consistent with the LTP deficit, there was impaired CFC when KOKO mice were compared with WTWT mice but not when the two genotypes born to HET mothers were compared. As with LTP, environmental factors, such as poor maternal care [86] or exposure to social defeat [97], can influence memory in CFC. Studies examining CFC in Fmr1 KO mice have yielded diverse results, with some studies reporting deficits in contextual fear memory [39,54,70–72], while other studies found normal levels of freezing [73–77]. The majority of studies have been carried out using littermate mice, with a relatively even split between those that found a deficit and those that did not. On the other hand, two non-littermate studies both showed impairments (see table 3). Collectively, these studies suggest that contextual fear memory in Fmr1 KO mice is sensitive to the cage effect.
(d). Expression of N-methyl-d-aspartate is sensitive to the cage effect
The cage effect also applied to the expression of NMDAR subunits, with reductions in GluN1, GluN2A and GluN2B subunits in both littermate WTHET and KOHET mice, compared with either WTWT or KOKO mice. KOHET mice showed a trend towards a decrease in the expression of the GluN2 subunits compared with WTHET mice, while WTWT and KOKO were similar in the expression of all three subunits. Previous studies have also reported variable effects on the expression of GluN subunits in FXS model mice (table 2). For example, a reduction in LTP has been associated with both an increase [ 37] and a decrease [31] in the expression of the GluN2A subunit. Studies in non-littermate mice showed either increased [38,66,67] or decreased [50] expression of NMDARs, whereas in littermate mice, the expression of NMDARs was either unaffected [32,68,69] or decreased [32] in the KOHET. As with LTP and CFC, environmental factors have been shown to affect the expression of NMDARs [87].
A surprising observation was that, compared with WTWT mice, KOKO mice had similar levels of expression of the NMDAR subunits even though STP, LTP and CFC were all reduced. Perhaps pertinent to this issue, the expression levels of the NMDAR subunits were measured in whole homogenate, which is a relevant parameter because FMRP is a translation regulator [98]. Total protein may not, however, reflect the synaptic density of these proteins, their functional state, receptor regulation or signalling.
(e). N-methyl-d-aspartate modulation and cage effects
Ultimately, the goal of studying Fmr1 KO mice is to understand the role of FMRP and to develop effective treatment strategies for FXS. Previous studies in Fmr1 KO mice have found that modulation of specific NMDAR subunits could rescue deficits in LTP. Specifically, when increases in GluN2A subunit expression were found, an antagonist was able to reverse deficits in LTP [38], while when a reduction in GluN1 subunits was observed, d-serine and glycine could reverse deficits in LTP [32]. In the current study, the NMDAR was targeted using UBP714, a GluN2A/2B-preferring PAM. UBP714 was able to enhance LTP in the KOKO but not the KOHET mice; this may relate to the reduction in LTP in the KOKO mice relative to the other three groups that showed more robust LTP. Consistent with this possibility, UBP714 was shown to potentiate LTP in WTs when a weak but not strong induction protocol is used [79]. Furthermore, UBP714 had no effect on STP in KOKO and KOHET mice, which is in line with previous results in WT mice [79]. In summary, the present findings demonstrate that environmental factors, such as the cage effect, may influence the effectiveness of neuromodulators in the Fmr1 KO mouse model.
(f). Do gene or environment interactions contribute to the cage effects?
FXS model phenotypes are influenced by the littermate environment. Specifically, littermate Fmr1 WTHET mice (i.e. born to Fmr1 HET mothers) have been shown to be hyperactive and to display social avoidance profiles that are more similar to those found in littermate and non-littermate KOs (KOHET and KOKO, respectively), than to non-littermate WT (WTWT) mice [64,65]. Indeed, the current study considered these cage effects can explain the range of inconsistent phenotypes that exist in the literature (tables 1–3).
It is now well-established that the sex of the experimenter can influence mouse stress, behaviours and responses [99–101], a confound that was minimized herein. To reduce other potential ‘human factors’, the fear memory freezing measures and the majority of the data analyses were automated. The current study was therefore designed to exhaust as many potential confounds as possible, and then to examine whether a range of parameters (synaptic plasticity, fear memory, NMDAR subunit expression and pharmacological rescue with a PAM) are influenced by the cage effect.
In comparisons between studies, the role of other factors, such as the mouse age or strain background, the induction protocol used, or the brain area examined, cannot be discounted. Furthermore, in our systematic study herein, the underlying factors leading to the cage effects on young adult mice are difficult to elucidate; the maternal or paternal care could vary between the breeding cages, there could be maternal programming effects, imprinting differences, the maternal microbiome could be influencing their offspring, or there could be other G×E interactions. Another possibility is the influence WT and KO littermate siblings have on each other. In terms of parental care, Zupan et al. [65] found no changes in grooming, arched back nursing in mothers, and time on nest in fathers between the groups [65]. Cross-fostering the WTHET mice with WT mothers did not correct the social avoidance phenotype in the WTHET mice. Furthermore, when they cross-fostered WTWT mice with Fmr1 HET mothers, they found that the WTWT mice developed the social avoidance phenotype [65]. The authors suggest that both prenatal and postnatal exposure to the maternal HET environment is sufficient to induce changes in social behaviours [65]. As mentioned, another possibility is that the littermate siblings influence the phenotypes in each other. In the Neuroligin3 KO mouse model of autism, the KO siblings influence the sociability phenotype of their WT littermates [102].
(g). Concluding remarks
The current study has demonstrated that the cage effect of using littermate or non-littermate Fmr1 KO mice differentially influences several phenotypes, including synaptic function, receptor expression, contextual fear memory and sensitivity to pharmacological rescue. The cage effect could explain some discrepancies in the literature on these and additional phenotypes. Overall, it is not known to what extent the littermate cage effect may be obscuring or enhancing phenotypes in mouse models of other disorders. Irrespective of the underlying causes, the present findings emphasize the need to carefully consider the type of breeding scheme used when studying FXS model mice and controls.
Acknowledgements
We would like to thank Lijia Wang Zhang, Ashish Kadia and Parisa Tari for their genotyping assistance. We appreciate the invaluable help of our research coordinator, Francesca Fabry.
Contributor Information
Rasa Volianskis, Email: r.volianskis@gmail.com.
Camilla J. Lundbye, Email: camillajlundbye@hotmail.com.
Gillian N. Petroff, Email: gillianpetroff@gmail.com.
David. E. Jane, Email: davidjane@blueyonder.co.uk.
John Georgiou, Email: georgiou@lunenfeld.ca.
Graham L. Collingridge, Email: collingridge@lunenfeld.ca.
Ethics
The research was carried out according to AUP protocol no. 24-0292H which was approved by The Centre for Phenogenomics (TCP; Toronto, Canada) Animal Care Committee.
Data accessibility
Representative samples of Western blots and the raw electrophysiological field responses are included herein.
Supplementary material is available online at [103].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
R.V.: conceptualization, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing; C.J.L.: formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing; G.N.P.: formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing; D.E.J.: conceptualization, methodology, resources, writing—review and editing; J.G.: conceptualization, funding acquisition, project administration, supervision, writing—original draft, writing—review and editing; G.L.C.: conceptualization, funding acquisition, project administration, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
G.L.C. and D.E.J. are on the Scientific Advisory Board of Hello Bio, a supplier of UBP714.
Funding
This research was funded by CIHR (Canadian Institutes of Health Research) Foundation grant no. 154276 (to G.L.C.). G.L.C. is also the holder of the Krembil Family Chair in Alzheimer’s Research. We are grateful for the support of the Lundbeck Foundation (Lundbeckfonden PhD fellowship to C.J.L.).
References
- 1. Hagerman RJ, et al. 2017. Fragile X syndrome. Nat. Rev. Dis. Primers 3 , 17065. ( 10.1038/nrdp.2017.65) [DOI] [PubMed] [Google Scholar]
- 2. Verkerk AJ, et al. 1991. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65 , 905–914. ( 10.1016/0092-8674(91)90397-h) [DOI] [PubMed] [Google Scholar]
- 3. Oberlé I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boué J, Bertheas MF, Mandel JL. 1991. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 252 , 1097–1102. ( 10.1126/science.252.5009.1097) [DOI] [PubMed] [Google Scholar]
- 4. Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. 1991. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66 , 817–822. ( 10.1016/0092-8674(91)90125-i) [DOI] [PubMed] [Google Scholar]
- 5. Naumann A, Hochstein N, Weber S, Fanning E, Doerfler W. 2009. A distinct DNA-methylation boundary in the 5’- upstream sequence of the FMR1 promoter binds nuclear proteins and is lost in fragile X syndrome. Am. J. Hum. Genet. 85 , 606–616. ( 10.1016/j.ajhg.2009.09.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jin P, Warren ST. 2000. Understanding the molecular basis of fragile X syndrome. Hum. Mol. Genet. 9 , 901–908. ( 10.1093/hmg/9.6.901) [DOI] [PubMed] [Google Scholar]
- 7. Coffee B, Zhang F, Warren ST, Reines D. 1999. Acetylated histones are associated with FMR1 in normal but not fragile X-syndrome cells. Nat. Genet. 22 , 98–101. ( 10.1038/8807) [DOI] [PubMed] [Google Scholar]
- 8. Coffee B, Zhang F, Ceman S, Warren ST, Reines D. 2002. Histone modifications depict an aberrantly heterochromatinized FMR1 gene in fragile X syndrome. Am. J. Hum. Genet. 71 , 923–932. ( 10.1086/342931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pietrobono R, et al. 2005. Molecular dissection of the events leading to inactivation of the FMR1 gene. Hum. Mol. Genet. 14 , 267–277. ( 10.1093/hmg/ddi024) [DOI] [PubMed] [Google Scholar]
- 10. Kumari D, Usdin K. 2010. The distribution of repressive histone modifications on silenced FMR1 alleles provides clues to the mechanism of gene silencing in fragile X syndrome. Hum. Mol. Genet. 19 , 4634–4642. ( 10.1093/hmg/ddq394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mor-Shaked H, Eiges R. 2018. Reevaluation of FMR1 hypermethylation timing in fragile X syndrome. Front. Mol. Neurosci. 11 , 31, ( 10.3389/fnmol.2018.00031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J, Pelletier MR, Perez Velazquez J-L, Carlen PL. 2002. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol. Cell. Neurosci. 19 , 138–151. ( 10.1006/mcne.2001.1085) [DOI] [PubMed] [Google Scholar]
- 13. Larson J, Jessen RE, Kim D, Fine AK, du Hoffmann J. 2005. Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking the fragile X mental retardation protein. J. Neurosci. 25 , 9460–9469. ( 10.1523/JNEUROSCI.2638-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao M-G, Toyoda H, Ko SW, Ding H-K, Wu L-J, Zhuo M. 2005. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J. Neurosci. 25 , 7385–7392. ( 10.1523/JNEUROSCI.1520-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lauterborn JC, Rex CS, Kramár E, Chen LY, Pandyarajan V, Lynch G, Gall CM. 2007. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J. Neurosci. 27 , 10685–10694. ( 10.1523/JNEUROSCI.2624-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meredith RM, Holmgren CD, Weidum M, Burnashev N, Mansvelder HD. 2007. Increased threshold for spike-timing-dependent plasticity is caused by unreliable calcium signaling in mice lacking fragile X gene FMR1. Neuron 54 , 627–638. ( 10.1016/j.neuron.2007.04.028) [DOI] [PubMed] [Google Scholar]
- 17. Wilson BM, Cox CL. 2007. Absence of metabotropic glutamate receptor-mediated plasticity in the neocortex of fragile X mice. Proc. Natl Acad. Sci. USA 104 , 2454–2459. ( 10.1073/pnas.0610875104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu H, Qin Y, Bochorishvili G, Zhu Y, van Aelst L, Zhu JJ. 2008. Ras signaling mechanisms underlying impaired GluR1-dependent plasticity associated with fragile X syndrome. J. Neurosci. 28 , 7847–7862. ( 10.1523/JNEUROSCI.1496-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shang Y, Wang H, Mercaldo V, Li X, Chen T, Zhuo M. 2009. Fragile X mental retardation protein is required for chemically-induced long-term potentiation of the hippocampus in adult mice. J. Neurochem. 111 , 635–646. ( 10.1111/j.1471-4159.2009.06314.x) [DOI] [PubMed] [Google Scholar]
- 20. Suvrathan A, Hoeffer CA, Wong H, Klann E, Chattarji S. 2010. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc. Natl Acad. Sci. USA 107 , 11591–11596. ( 10.1073/pnas.1002262107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee HY, Ge WP, Huang W, He Y, Wang GX, Rowson-Baldwin A, Smith SJ, Jan YN, Jan LY. 2011. Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron 72 , 630–642. ( 10.1016/j.neuron.2011.09.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yun SH, Trommer BL. 2011. Fragile X mice: reduced long-term potentiation and N-methyl-D-aspartate receptor-mediated neurotransmission in dentate gyrus. J. Neurosci. Res. 89 , 176–182. ( 10.1002/jnr.22546) [DOI] [PubMed] [Google Scholar]
- 23. Eadie BD, Cushman J, Kannangara TS, Fanselow MS, Christie BR. 2012. NMDA receptor hypofunction in the dentate gyrus and impaired context discrimination in adult Fmr1 knockout mice. Hippocampus 22 , 241–254. ( 10.1002/hipo.20890) [DOI] [PubMed] [Google Scholar]
- 24. Xu ZH, et al. 2012. Group I mGluR antagonist rescues the deficit of D1-induced LTP in a mouse model of fragile X syndrome. Mol. Neurodegener. 7 , 24. ( 10.1186/1750-1326-7-24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu Z-H, Yang Q, Ma L, Liu S-b, Chen G-s, Wu Y-m, Li X-q, Liu G, Zhao M-g. 2012. Deficits in LTP induction by 5-HT2A receptor antagonist in a mouse model for fragile X syndrome. PLoS ONE 7 , e48741. ( 10.1371/journal.pone.0048741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Padmashri R, Reiner BC, Suresh A, Spartz E, Dunaevsky A. 2013. Altered structural and functional synaptic plasticity with motor skill learning in a mouse model of fragile X syndrome. J. Neurosci. 33 , 19715–19723. ( 10.1523/JNEUROSCI.2514-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boda B, Mendez P, Boury-Jamot B, Magara F, Muller D. 2014. Reversal of activity-mediated spine dynamics and learning impairment in a mouse model of fragile X syndrome. Eur. J. Neurosci. 39 , 1130–1137. ( 10.1111/ejn.12488) [DOI] [PubMed] [Google Scholar]
- 28. Chen T, Lu JS, Song Q, Liu MG, Koga K, Descalzi G, Li YQ, Zhuo M. 2014. Pharmacological rescue of cortical synaptic and network potentiation in a mouse model for fragile X syndrome. Neuropsychopharmacology 39 , 1955–1967. ( 10.1038/npp.2014.44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Franklin AV, King MK, Palomo V, Martinez A, McMahon LL, Jope RS. 2014. Glycogen synthase kinase-3 inhibitors reverse deficits in long-term potentiation and cognition in fragile X mice. Biol. Psychiatry 75 , 198–206, ( 10.1016/j.biopsych.2013.08.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Franklin AV, Rusche JR, McMahon LL. 2014. Increased long-term potentiation at medial-perforant path-dentate granule cell synapses induced by selective inhibition of histone deacetylase 3 requires fragile X mental retardation protein. Neurobiol. Learn Mem. 114 , 193–197. ( 10.1016/j.nlm.2014.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang S, Yang S, Park JS, Kirkwood A, Bao S. 2014. Failed stabilization for long-term potentiation in the auditory cortex of FMR1 knockout mice. PLoS ONE 9 , e104691. ( 10.1371/journal.pone.0104691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bostrom CA, Majaess NM, Morch K, White E, Eadie BD, Christie BR. 2015. Rescue of NMDAR-dependent synaptic plasticity in Fmr1 knock-out mice. Cereb. Cortex 25 , 271–279. ( 10.1093/cercor/bht237) [DOI] [PubMed] [Google Scholar]
- 33. Ghilan M, Hryciw BN, Brocardo PS, Bostrom CA, Gil-Mohapel J, Christie BR. 2015. Enhanced corticosteroid signaling alters synaptic plasticity in the dentate gyrus in mice lacking the fragile X mental retardation protein. Neurobiol. Dis. 77 , 26–34, ( 10.1016/j.nbd.2015.01.008) [DOI] [PubMed] [Google Scholar]
- 34. Koga K, Liu MG, Qiu S, Song Q, O’Den G, Chen T, Zhuo M. 2015. Impaired presynaptic long-term potentiation in the anterior cingulate cortex of Fmr1 knock-out mice. J. Neurosci. 35 , 2033–2043. ( 10.1523/JNEUROSCI.2644-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neuhofer D, Henstridge CM, Dudok B, Sepers M, Lassalle O, Katona I, Manzoni OJ. 2015. Functional and structural deficits at accumbens synapses in a mouse model of fragile X. Front. Cell. Neurosci. 9 , 100. ( 10.3389/fncel.2015.00100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martin HGS, Lassalle O, Brown JT, Manzoni OJ. 2016. Age-dependent long-term potentiation deficits in the prefrontal cortex of the Fmr1 Knockout mouse model of fragile X syndrome. Cereb. Cortex 26 , 2084–2092. ( 10.1093/cercor/bhv031) [DOI] [PubMed] [Google Scholar]
- 37. Yau SY, et al. 2016. Impaired bidirectional NMDA receptor dependent synaptic plasticity in the dentate gyrus of adult female Fmr1 heterozygous knockout mice. Neurobiol. Dis. 96 , 261–270. ( 10.1016/j.nbd.2016.09.012) [DOI] [PubMed] [Google Scholar]
- 38. Lundbye CJ, Toft AKH, Banke TG. 2018. Inhibition of GluN2A NMDA receptors ameliorates synaptic plasticity deficits in the Fmr1-/y mouse model. J. Physiol. 596 , 5017–5031. ( 10.1113/JP276304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martinez LA, Tejada-Simon MV. 2018. Increased training intensity induces proper membrane localization of actin remodeling proteins in the hippocampus preventing cognitive deficits: implications for fragile X syndrome. Mol. Neurobiol. 55 , 4529–4542. ( 10.1007/s12035-017-0666-4) [DOI] [PubMed] [Google Scholar]
- 40. Wang W, et al. 2018. Treating a novel plasticity defect rescues episodic memory in fragile X model mice. Mol. Psychiatry 23 , 1798–1806. ( 10.1038/mp.2017.221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Feuge J, Scharkowski F, Michaelsen-Preusse K, Korte M. 2019. FMRP modulates activity-dependent spine plasticity by binding cofilin1 mRNA and regulating localization and local translation. Cereb. Cortex 29 , 5204–5216. ( 10.1093/cercor/bhz059) [DOI] [PubMed] [Google Scholar]
- 42. Banke TG, Barria A. 2020. Transient enhanced GluA2 expression in young hippocampal neurons of a fragile X mouse model. Front. Synaptic Neurosci. 12 , 588295. ( 10.3389/fnsyn.2020.588295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seese RR, Le AA, Wang K, Cox CD, Lynch G, Gall CM. 2020. A TrkB agonist and ampakine rescue synaptic plasticity and multiple forms of memory in a mouse model of intellectual disability. Neurobiol. Dis. 134 , 104604, ( 10.1016/j.nbd.2019.104604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhan X, Asmara H, Cheng N, Sahu G, Sanchez E, Zhang FX, Zamponi GW, Rho JM, Turner RW. 2020. FMRP(1-297)-tat restores ion channel and synaptic function in a model of fragile X syndrome. Nat. Commun. 11 , 2755. ( 10.1038/s41467-020-16250-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ordemann GJ, Apgar CJ, Chitwood RA, Brager DH. 2021. Altered a-type potassium channel function impairs dendritic spike initiation and temporoammonic long-term potentiation in fragile X syndrome. J. Neurosci. 41 , 5947–5962. ( 10.1523/JNEUROSCI.0082-21.2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hwang JY, Monday HR, Yan J, Gompers A, Buxbaum AR, Sawicka KJ, Singer RH, Castillo PE, Zukin RS. 2022. CPEB3-dependent increase in GluA2 subunits impairs excitatory transmission onto inhibitory interneurons in a mouse model of fragile X. Cell Rep. 39 , 110853, ( 10.1016/j.celrep.2022.110853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jeon SJ, Kwon H, Bae HJ, Gonzales EL, Kim J, Chung HJ, Kim DH, Ryu JH, Shin CY. 2022. Agmatine relieves behavioral impairments in fragile X mice model. Neuropharmacology 219 , 109234, ( 10.1016/j.neuropharm.2022.109234) [DOI] [PubMed] [Google Scholar]
- 48. Monday HR, Kharod SC, Yoon YJ, Singer RH, Castillo PE. 2022. Presynaptic FMRP and local protein synthesis support structural and functional plasticity of glutamatergic axon terminals. Neuron 110 , 2588–2606.( 10.1016/j.neuron.2022.05.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Borreca A, De Luca M, Ferrante A, Boussadia Z, Pignataro A, Martire A, Ammassari-Teule M. 2023. Fmr1-KO mice failure to detect object novelty associates with a post-test decrease of structural and synaptic plasticity upstream of the hippocampus. Sci. Rep. 13 , 755. ( 10.1038/s41598-023-27991-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li YJ, Zhang K, Sun T, Guo YY, Yang Q, Liu SB, Wu YM, Zhao MG. 2023. Improvement of learning and memory by elevating brain D-aspartate in a mouse model of fragile X syndrome. Mol. Neurobiol. 60 , 6410–6423. ( 10.1007/s12035-023-03438-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martín R, et al. 2023. The activation of mGluR4 rescues parallel fiber synaptic transmission and LTP, motor learning and social behavior in a mouse model of Fragile X Syndrome. Mol. Autism 14 , 14. ( 10.1186/s13229-023-00547-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ostrovskaya OI, Cao G, Eroglu C, Harris KM. 2020. Developmental onset of enduring long-term potentiation in mouse hippocampus. Hippocampus 30 , 1298–1312. ( 10.1002/hipo.23257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Godfraind JM, Reyniers E, De Boulle K, D’Hooge R, De Deyn PP, Bakker CE, Oostra BA, Kooy RF, Willems PJ. 1996. Long-term potentiation in the hippocampus of fragile X knockout mice. Am. J. Med. Genet. 64 , 246–251. () [DOI] [PubMed] [Google Scholar]
- 54. Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. 1999. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience 94 , 185–192. ( 10.1016/s0306-4522(99)00285-7) [DOI] [PubMed] [Google Scholar]
- 55. Pilpel Y, Kolleker A, Berberich S, Ginger M, Frick A, Mientjes E, Oostra BA, Seeburg PH. 2009. Synaptic ionotropic glutamate receptors and plasticity are developmentally altered in the CA1 field of Fmr1 knockout mice. J. Physiol. 587 , 787–804. ( 10.1113/jphysiol.2008.160929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang J, Hou L, Klann E, Nelson DL. 2009. Altered hippocampal synaptic plasticity in the FMR1 gene family knockout mouse models. J. Neurophysiol. 101 , 2572–2580. ( 10.1152/jn.90558.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Auerbach BD, Bear MF. 2010. Loss of the fragile X mental retardation protein decouples metabotropic glutamate receptor dependent priming of long-term potentiation from protein synthesis. J. Neurophysiol. 104 , 1047–1051. ( 10.1152/jn.00449.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Connor SA, Hoeffer CA, Klann E, Nguyen PV. 2011. Fragile X mental retardation protein regulates heterosynaptic plasticity in the hippocampus. Learn. Mem. 18 , 207–220. ( 10.1101/lm.2043811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Seese RR, Babayan AH, Katz AM, Cox CD, Lauterborn JC, Lynch G, Gall CM. 2012. LTP induction translocates cortactin at distant synapses in wild-type but not Fmr1 knock-out mice. J. Neurosci. 32 , 7403–7413. ( 10.1523/JNEUROSCI.0968-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brager DH, Akhavan AR, Johnston D. 2012. Impaired dendritic expression and plasticity of h-channels in the fmr1(-/y) mouse model of fragile X syndrome. Cell Rep. 1 , 225–233. ( 10.1016/j.celrep.2012.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Routh BN, Johnston D, Brager DH. 2013. Loss of functional A-type potassium channels in the dendrites of CA1 pyramidal neurons from a mouse model of fragile X syndrome. J. Neurosci. 33 , 19442–19450. ( 10.1523/JNEUROSCI.3256-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Holmdahl R, Malissen B. 2012. The need for littermate controls. Eur. J. Immunol. 42 , 45–47. ( 10.1002/eji.201142048) [DOI] [PubMed] [Google Scholar]
- 63. Jiménez JA, Zylka MJ. 2021. Controlling litter effects to enhance rigor and reproducibility with rodent models of neurodevelopmental disorders. J. Neurodev. Disord. 13 , 2. ( 10.1186/s11689-020-09353-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zupan B, Toth M. 2008. Wild-type male offspring of fmr-1+/− mothers exhibit characteristics of the fragile X phenotype. Neuropsychopharmacology 33 , 2667–2675. ( 10.1038/sj.npp.1301651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zupan B, Sharma A, Frazier A, Klein S, Toth M. 2016. Programming social behavior by the maternal fragile X protein. Genes. Brain. Behav. 15 , 578–587. ( 10.1111/gbb.12298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schütt J, Falley K, Richter D, Kreienkamp HJ, Kindler S. 2009. Fragile X mental retardation protein regulates the levels of scaffold proteins and glutamate receptors in postsynaptic densities. J. Biol. Chem. 284 , 25479–25487. ( 10.1074/jbc.M109.042663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Toft AKH, Lundbye CJ, Banke TG. 2016. Dysregulated NMDA-receptor signaling inhibits long-term depression in a mouse model of fragile X syndrome. J. Neurosci. 36 , 9817–9827. ( 10.1523/JNEUROSCI.3038-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chatterjee M, Kurup PK, Lundbye CJ, Hugger Toft AK, Kwon J, Benedict J, Kamceva M, Banke TG, Lombroso PJ. 2018. STEP inhibition reverses behavioral, electrophysiologic, and synaptic abnormalities in Fmr1 KO mice. Neuropharmacology 128 , 43–53. ( 10.1016/j.neuropharm.2017.09.026) [DOI] [PubMed] [Google Scholar]
- 69. Yau SY, Bettio L, Vetrici M, Truesdell A, Chiu C, Chiu J, Truesdell E, Christie BR. 2018. Chronic minocycline treatment improves hippocampal neuronal structure, NMDA receptor function, and memory processing in Fmr1 knockout mice. Neurobiol. Dis. 113 , 11–22, ( 10.1016/j.nbd.2018.01.014) [DOI] [PubMed] [Google Scholar]
- 70. Baker KB, Wray SP, Ritter R, Mason S, Lanthorn TH, Savelieva KV. 2010. Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes. Brain. Behav. 9 , 562–574. ( 10.1111/j.1601-183X.2010.00585.x) [DOI] [PubMed] [Google Scholar]
- 71. Ding Q, Sethna F, Wang H. 2014. Behavioral analysis of male and female Fmr1 knockout mice on C57BL/6 background. Behav. Brain Res. 271 , 72–78, ( 10.1016/j.bbr.2014.05.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Reyes ST, et al. 2021. Effects of the sigma-1 receptor agonist blarcamesine in a murine model of fragile X syndrome: neurobehavioral phenotypes and receptor occupancy. Sci. Rep. 11 , 17150. ( 10.1038/s41598-021-94079-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Van Dam D, D’Hooge R, Hauben E, Reyniers E, Gantois I, Bakker CE, Oostra BA, Kooy RF, De Deyn PP. 2000. Spatial learning, contextual fear conditioning and conditioned emotional response in Fmr1 knockout mice. Behav. Brain Res. 117 , 127–136. ( 10.1016/s0166-4328(00)00296-5) [DOI] [PubMed] [Google Scholar]
- 74. Spencer CM, Serysheva E, Yuva-Paylor LA, Oostra BA, Nelson DL, Paylor R. 2006. Exaggerated behavioral phenotypes in Fmr1/Fxr2 double knockout mice reveal a functional genetic interaction between fragile X-related proteins. Hum. Mol. Genet. 15 , 1984–1994. ( 10.1093/hmg/ddl121) [DOI] [PubMed] [Google Scholar]
- 75. Uutela M, Lindholm J, Louhivuori V, Wei H, Louhivuori LM, Pertovaara A, Akerman K, Castrén E, Castrén ML. 2012. Reduction of BDNF expression in Fmr1 knockout mice worsens cognitive deficits but improves hyperactivity and sensorimotor deficits. Genes. Brain. Behav. 11 , 513–523. ( 10.1111/j.1601-183X.2012.00784.x) [DOI] [PubMed] [Google Scholar]
- 76. Nolan SO, Hodges SL, Binder MS, Smith GD, Okoh JT, Jefferson TS, Escobar B, Lugo JN. 2022. Dietary rescue of adult behavioral deficits in the Fmr1 knockout mouse. PLoS ONE 17 , e0262916. ( 10.1371/journal.pone.0262916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dobkin C, Rabe A, Dumas R, El Idrissi A, Haubenstock H, Brown WT. 2000. Fmr1 knockout mouse has a distinctive strain-specific learning impairment. Neuroscience 100 , 423–429. ( 10.1016/s0306-4522(00)00292-x) [DOI] [PubMed] [Google Scholar]
- 78. Consortium TB, et al. 1994. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell 78 , 23–33. ( 10.1016/0092-8674(94)90569-X) [DOI] [PubMed] [Google Scholar]
- 79. France G, et al. 2022. Differential regulation of STP, LTP and LTD by structurally diverse NMDA receptor subunit-specific positive allosteric modulators. Neuropharmacology 202 , 108840. ( 10.1016/j.neuropharm.2021.108840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Anderson WW, Collingridge GL. 2007. Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J. Neurosci. Methods 162 , 346–356. ( 10.1016/j.jneumeth.2006.12.018) [DOI] [PubMed] [Google Scholar]
- 81. Anderson WW, Fitzjohn SM, Collingridge GL. 2012. Automated multi-slice extracellular and patch-clamp experiments using the WinLTP data acquisition system with automated perfusion control. J. Neurosci. Methods 207 , 148–160. ( 10.1016/j.jneumeth.2012.04.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Volianskis A, Jensen MS. 2003. Transient and sustained types of long-term potentiation in the CA1 area of the rat hippocampus. J. Physiol. (Lond) 550 , 459–492. ( 10.1113/jphysiol.2003.044214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Volianskis A, Bannister N, Collett VJ, Irvine MW, Monaghan DT, Fitzjohn SM, Jensen MS, Jane DE, Collingridge GL. 2013. Different NMDA receptor subtypes mediate induction of long-term potentiation and two forms of short-term potentiation at CA1 synapses in rat hippocampus in vitro. J. Physiol. 591 , 955–972. ( 10.1113/jphysiol.2012.247296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Davies SN, Lester RA, Reymann KG, Collingridge GL. 1989. Temporally distinct pre- and post-synaptic mechanisms maintain long-term potentiation. Nature 338 , 500–503. ( 10.1038/338500a0) [DOI] [PubMed] [Google Scholar]
- 85. Irvine MW, Costa BM, Volianskis A, Fang G, Ceolin L, Collingridge GL, Monaghan DT, Jane DE. 2012. Coumarin-3-carboxylic acid derivatives as potentiators and inhibitors of recombinant and native N-methyl-D-aspartate receptors. Neurochem. Int. 61 , 593–600. ( 10.1016/j.neuint.2011.12.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joëls M. 2009. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol. Learn. Mem. 92 , 292–300. ( 10.1016/j.nlm.2009.03.004) [DOI] [PubMed] [Google Scholar]
- 87. Bagot RC, Tse YC, Nguyen H-B, Wong AS, Meaney MJ, Wong TP. 2012. Maternal care influences hippocampal N-methyl-D-aspartate receptor function and dynamic regulation by corticosterone in adulthood. Biol. Psychiatry 72 , 491–498. ( 10.1016/j.biopsych.2012.03.016) [DOI] [PubMed] [Google Scholar]
- 88. Yang L, et al. 2023. Toward antifragility: social defeat stress enhances learning and memory in young mice via hippocampal synaptosome associated protein 25. Psychol. Sci. 34 , 616–632. ( 10.1177/09567976231160098) [DOI] [PubMed] [Google Scholar]
- 89. McNaughton BL. 1982. Long-term synaptic enhancement and short-term potentiation in rat fascia dentata act through different mechanisms. J. Physiol. 324 , 249–262. ( 10.1113/jphysiol.1982.sp014110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Racine RJ, Milgram NW. 1983. Short-term potentiation phenomena in the rat limbic forebrain. Brain Res. 260 , 201–216. ( 10.1016/0006-8993(83)90675-3) [DOI] [PubMed] [Google Scholar]
- 91. Racine RJ, Milgram NW, Hafner S. 1983. Long-term potentiation phenomena in the rat limbic forebrain. Brain Res. 260 , 217–231. ( 10.1016/0006-8993(83)90676-5) [DOI] [PubMed] [Google Scholar]
- 92. Ingram R, Kang H, Lightman S, Jane DE, Bortolotto ZA, Collingridge GL, Lodge D, Volianskis A. 2018. Some distorted thoughts about ketamine as a psychedelic and a novel hypothesis based on NMDA receptor-mediated synaptic plasticity. Neuropharmacology 142 , 30–40, ( 10.1016/j.neuropharm.2018.06.008) [DOI] [PubMed] [Google Scholar]
- 93. Pradier B, Lanning K, Taljan KT, Feuille CJ, Nagy MA, Kauer JA. 2018. Persistent but labile synaptic plasticity at excitatory synapses. J. Neurosci. 38 , 5750–5758. ( 10.1523/JNEUROSCI.2772-17.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Volianskis A, Collingridge GL, Jensen MS. 2013. The roles of STP and LTP in synaptic encoding. PeerJ 1 , e3. ( 10.7717/peerj.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Erickson MA, Maramara LA, Lisman J. 2010. A single brief burst induces GluR1-dependent associative short-term potentiation: a potential mechanism for short-term memory. J. Cogn. Neurosci. 22 , 2530–2540. ( 10.1162/jocn.2009.21375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lansner A, Fiebig F, Herman P. 2023. Fast Hebbian plasticity and working memory. Curr. Opin. Neurobiol. 83 , 102809, ( 10.1016/j.conb.2023.102809) [DOI] [PubMed] [Google Scholar]
- 97. Novick AM, Mears M, Forster GL, Lei Y, Tejani-Butt SM, Watt MJ. 2016. Adolescent social defeat alters N-methyl-D-aspartic acid receptor expression and impairs fear learning in adulthood. Behav. Brain Res. 304 , 51–59, ( 10.1016/j.bbr.2016.02.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Darnell JC, Klann E. 2013. The translation of translational control by FMRP: therapeutic targets for FXS. Nat. Neurosci. 16 , 1530–1536. ( 10.1038/nn.3379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sorge RE, et al. 2014. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Methods 11 , 629–632. ( 10.1038/nmeth.2935) [DOI] [PubMed] [Google Scholar]
- 100. Georgiou P, et al. 2022. Experimenters’ sex modulates mouse behaviors and neural responses to ketamine via corticotropin releasing factor. Nat. Neurosci. 25 , 1191–1200. ( 10.1038/s41593-022-01146-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chapman CD, Benedict C, Schiöth HB. 2018. Experimenter gender and replicability in science. Sci. Adv. 4 , e1701427. ( 10.1126/sciadv.1701427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kalbassi S, Bachmann SO, Cross E, Roberton VH, Baudouin SJ. 2017. Male and female mice lacking neuroligin-3 modify the behavior of their wild-type littermates. eNeuro 4 , ENEURO.0145-17.2017. ( 10.1523/ENEURO.0145-17.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Volianskis R, Lundbye C, Petroff G, Jane D, Georgiou J, Collingridge G. 2024. Supplementary material from: Cage effects on synaptic plasticity and its modulation in a mouse model of fragile X syndrome. FigShare. ( 10.6084/m9.figshare.c.7202786) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Representative samples of Western blots and the raw electrophysiological field responses are included herein.
Supplementary material is available online at [103].