Abstract
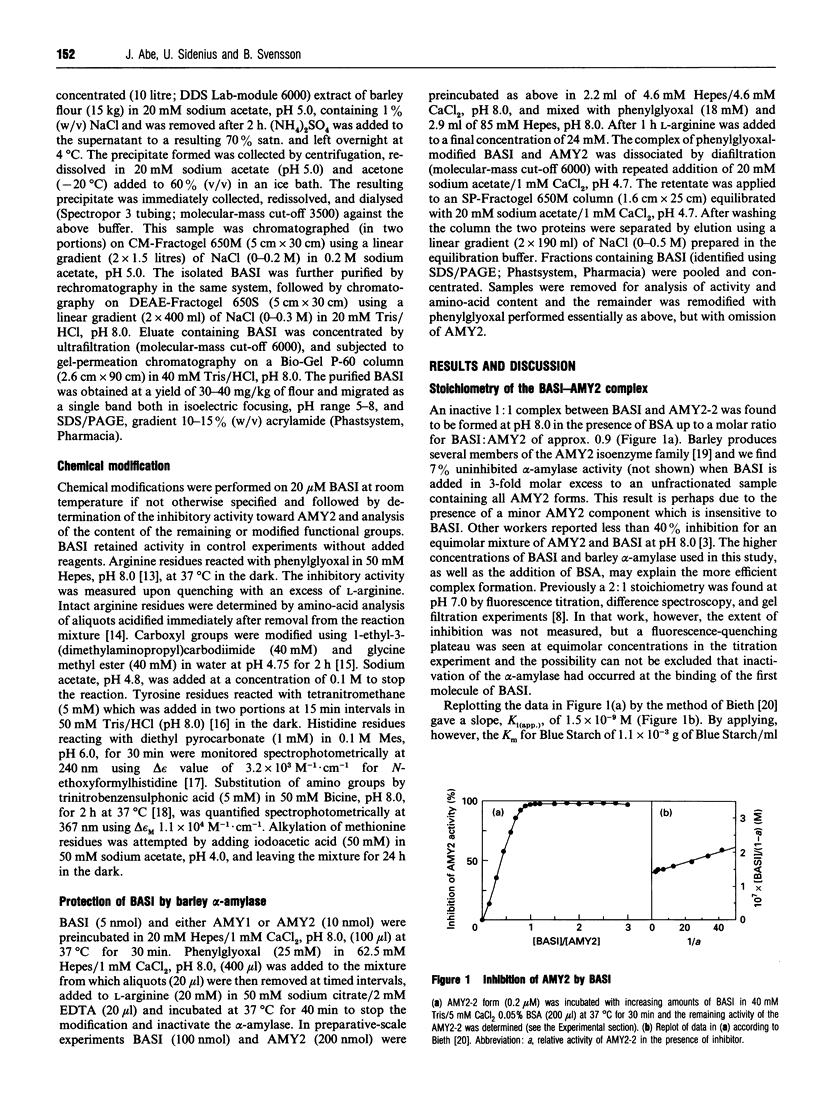
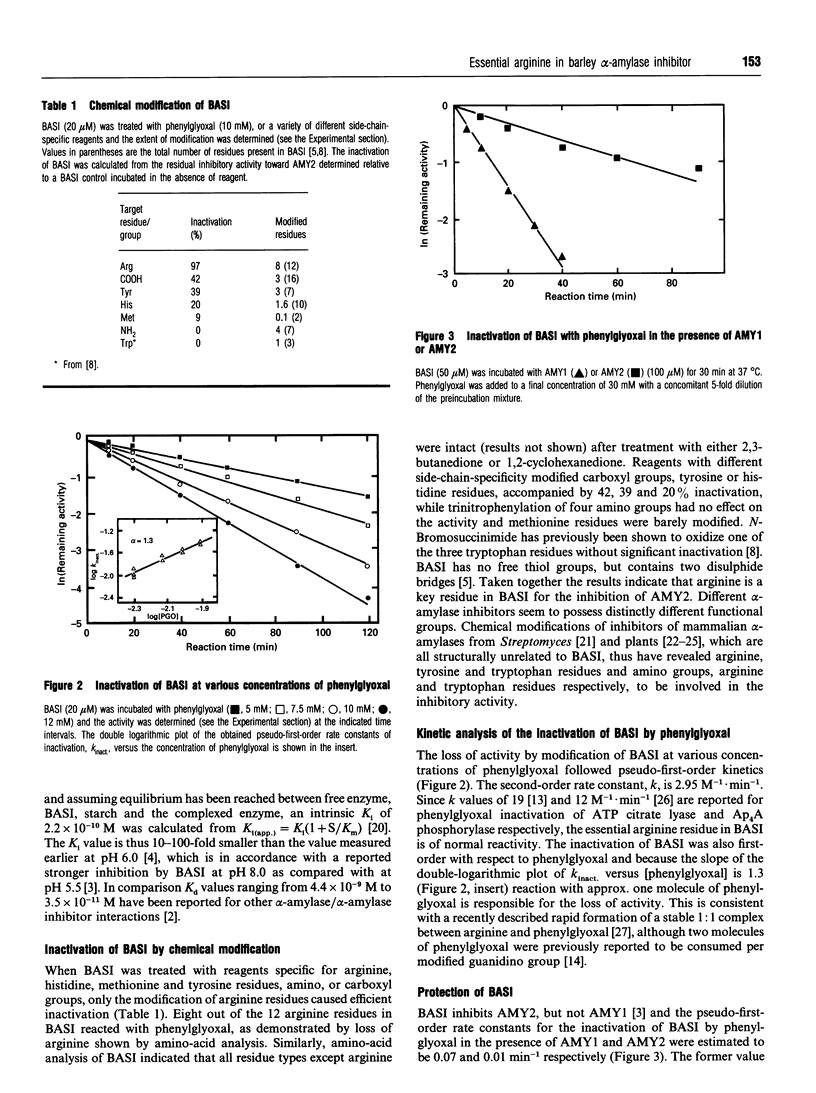
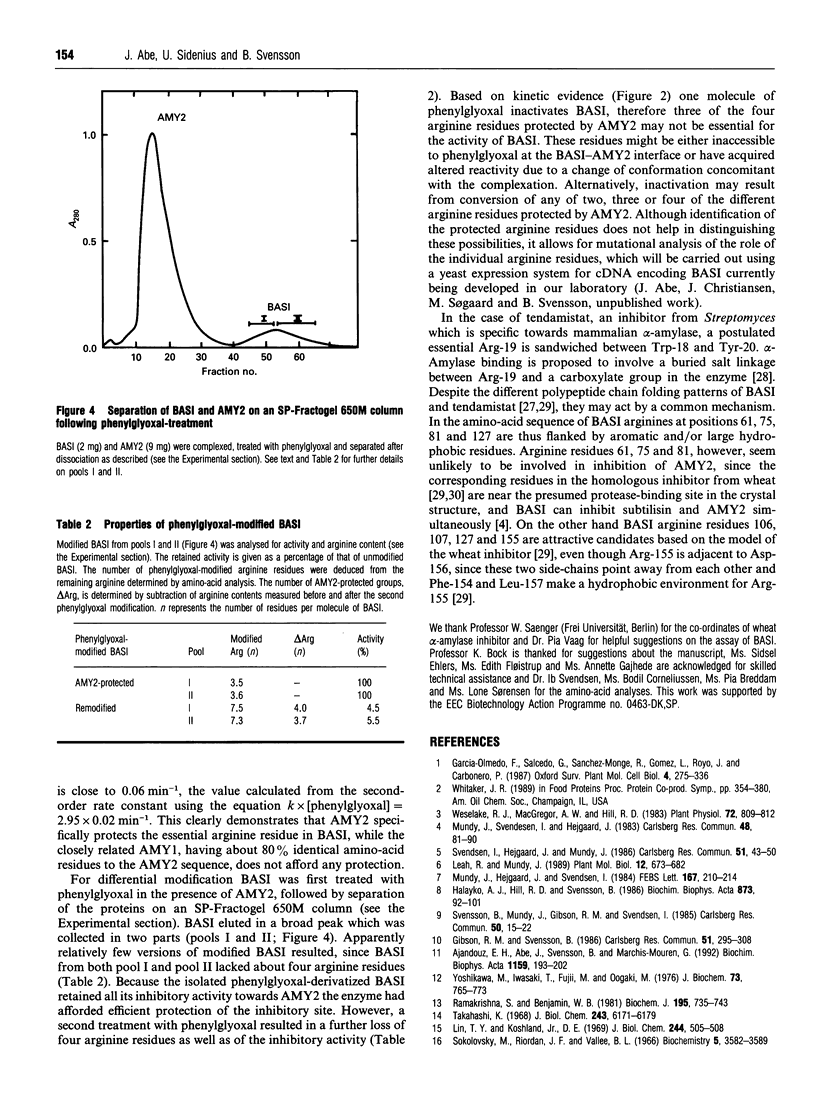
Treatment of barley alpha-amylase/subtilisin inhibitor (BASI) with reagents specific for arginine, histidine, methionine and tyrosine residues and amino and carboxyl groups indicates that an arginine residue(s) is essential for its action on the target enzyme barley alpha-amylase 2. Phenylglyoxal modified eight out of 12 arginine residues in BASI. Kinetic analysis shows that the inactivation of BASI follows a pseudo-first-order reaction and is due to reaction with one molecule of phenylglyoxal; the second-order rate constant is determined to be 2.95 M-1.min-1. At pH 8.0, BASI and barley alpha-amylase 2 form an inactive 1:1 complex. The Ki value of this association is 2.2 x 10(-10) M. The alpha-amylase protects four arginine residues and also the alpha-amylase inhibitory activity of BASI against phenylglyoxal. When BASI from the phenylglyoxal-modified target enzyme-inhibitor complex is isolated and subjected to a second treatment with phenylglyoxal, four additional arginine residues are modified, with concomitant loss of the inhibitory activity. These results are discussed in relation to a three-dimensional model of BASI based on the known structure of the corresponding inhibitor from wheat.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajandouz E. H., Abe J., Svensson B., Marchis-Mouren G. Barley malt-alpha-amylase. Purification, action pattern, and subsite mapping of isozyme 1 and two members of the isozyme 2 subfamily using p-nitrophenylated maltooligosaccharide substrates. Biochim Biophys Acta. 1992 Sep 23;1159(2):193–202. doi: 10.1016/0167-4838(92)90025-9. [DOI] [PubMed] [Google Scholar]
- George A. L., Jr, Borders C. L., Jr Chemical modification of histidyl and lysyl residues in yeast enolase. Biochim Biophys Acta. 1979 Jul 11;569(1):63–69. doi: 10.1016/0005-2744(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Jones R. L., Jacobsen J. V. Regulation of synthesis and transport of secreted proteins in cereal aleurone. Int Rev Cytol. 1991;126:49–88. doi: 10.1016/s0074-7696(08)60682-8. [DOI] [PubMed] [Google Scholar]
- Konishi K., Fujioka M. Chemical modification of a functional arginine residue of rat liver glycine methyltransferase. Biochemistry. 1987 Dec 15;26(25):8496–8502. doi: 10.1021/bi00399a069. [DOI] [PubMed] [Google Scholar]
- Kotaru M., Yeh H. Y., Yoshikawa H., Ikeuchi T., Iwami K., Ibuki F. Activity changes in cranberry bean (Phaseolus vulgaris) alpha-amylase inhibitor by chemical modification and enzymatic digestion. J Nutr Sci Vitaminol (Tokyo) 1989 Feb;35(1):71–80. doi: 10.3177/jnsv.35.71. [DOI] [PubMed] [Google Scholar]
- Kutty A. V., Pattabiraman T. N. Isolation and characterization of an amylase inhibitor from Echinocloa fruneutacea grains. Indian J Biochem Biophys. 1985 Jun;22(3):155–160. [PubMed] [Google Scholar]
- Lin T. Y., Koshland D. E., Jr Carboxyl group modification and the activity of lysozyme. J Biol Chem. 1969 Jan 25;244(2):505–508. [PubMed] [Google Scholar]
- Pflugrath J. W., Wiegand G., Huber R., Vértesy L. Crystal structure determination, refinement and the molecular model of the alpha-amylase inhibitor Hoe-467A. J Mol Biol. 1986 May 20;189(2):383–386. doi: 10.1016/0022-2836(86)90520-6. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Evidence for an essential arginine residue at the active site of ATP citrate lyase from rat liver. Biochem J. 1981 Jun 1;195(3):735–743. doi: 10.1042/bj1950735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. K., Barnes L. D. Chemical modification of a functional arginine residue in diadenosine 5',5'''-P1,P4-tetraphosphate (Ap4A) phosphorylase I from Saccharomyces cerevisiae. Biochem J. 1991 Oct 1;279(Pt 1):135–139. doi: 10.1042/bj2790135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosemont J. L. Reaction of histidine residues in proteins with diethylpyrocarbonate: differential molar absorptivities and reactivities. Anal Biochem. 1978 Jul 15;88(1):314–320. doi: 10.1016/0003-2697(78)90424-4. [DOI] [PubMed] [Google Scholar]
- Shivaraj B., Pattabiraman T. N. Natural plant enzyme inhibitors. Characterization of an unusual alpha-amylase/trypsin inhibitor from ragi (Eleusine coracana Geartn.). Biochem J. 1981 Jan 1;193(1):29–36. doi: 10.1042/bj1930029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovsky M., Riordan J. F., Vallee B. L. Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966 Nov;5(11):3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- Takahashi K. The reaction of phenylglyoxal with arginine residues in proteins. J Biol Chem. 1968 Dec 10;243(23):6171–6179. [PubMed] [Google Scholar]
- Weselake R. J., Macgregor A. W., Hill R. D. An endogenous alpha-amylase inhibitor in barley kernels. Plant Physiol. 1983 Jul;72(3):809–812. doi: 10.1104/pp.72.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M., Iwasaki T., Fujii M., Oogaki M. Isolation and some properties of a subtilisin inhibitor from barley. J Biochem. 1976 Apr;79(4):765–773. doi: 10.1093/oxfordjournals.jbchem.a131129. [DOI] [PubMed] [Google Scholar]
- Zemke K. J., Müller-Fahrnow A., Jany K. D., Pal G. P., Saenger W. The three-dimensional structure of the bifunctional proteinase K/alpha-amylase inhibitor from wheat (PK13) at 2.5 A resolution. FEBS Lett. 1991 Feb 25;279(2):240–242. doi: 10.1016/0014-5793(91)80158-y. [DOI] [PubMed] [Google Scholar]


