Abstract
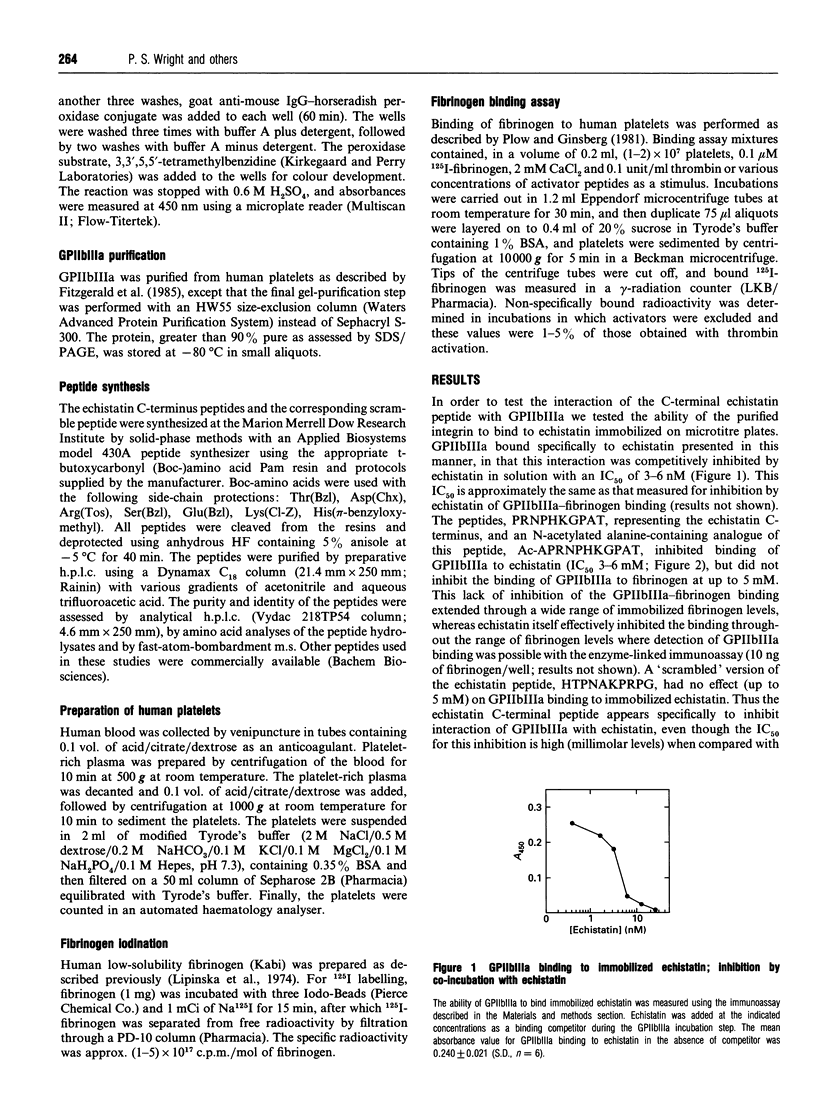
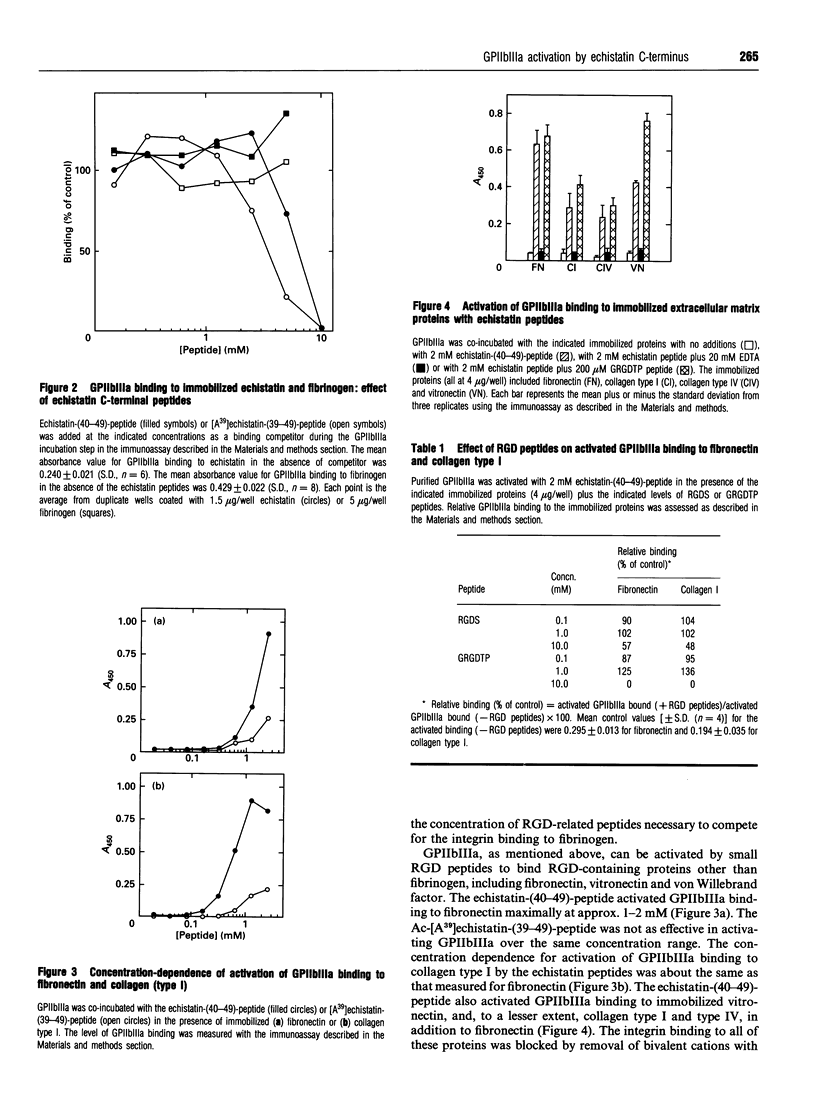
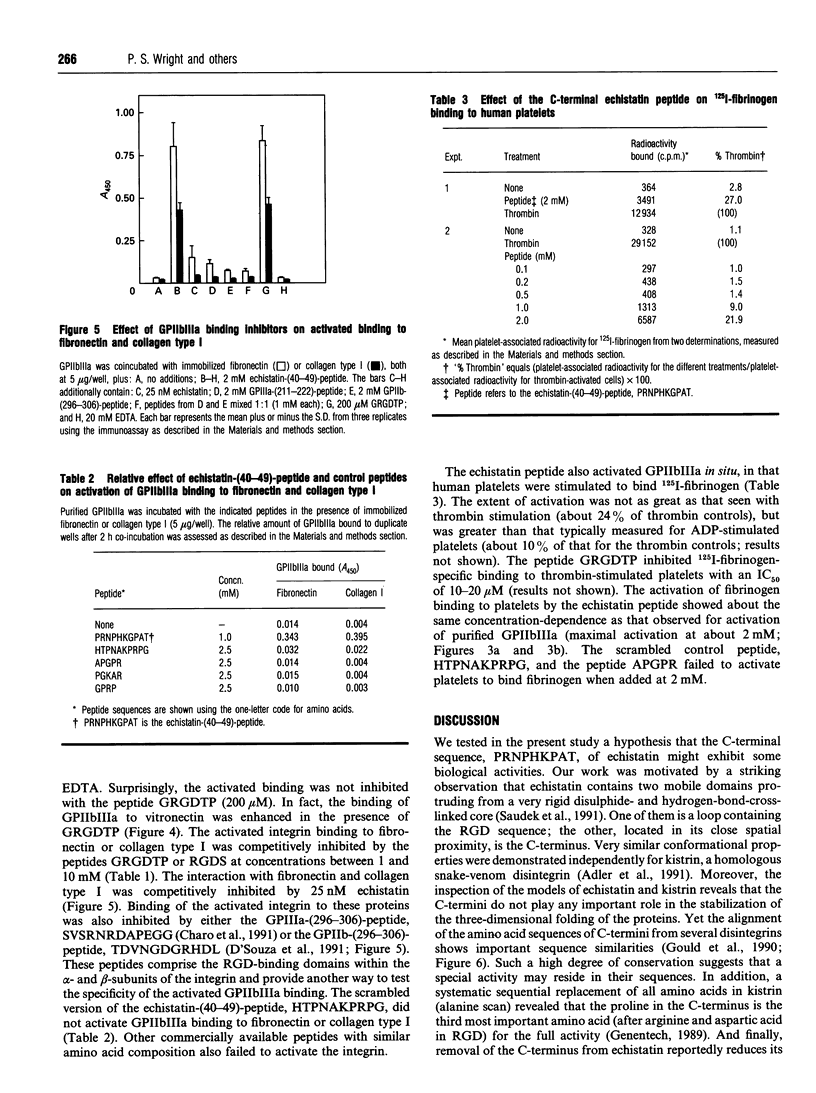
Integrin binding to proteins often involves recognition of domains containing the arginine-glycine-aspartate (RGD) motif. Different binding affinities and specificities of the integrin-ligand protein interactions involve additional protein domains. The n.m.r. structure of the snake-venom protein echistatin suggested that the C-terminal portion of the molecule might be important, in addition to the RGD domain, in binding to the integrin glycoprotein IIbIIIa (GPIIbIIIa) [Saudek, Atkinson and Pelton (1991) Biochem. 30, 7369-7372]. The synthetic C-terminal peptide, echistatin-(40-49), PRNPHKGPAT, (1) inhibited binding of GPIIbIIIa to immobilized echistatin (IC50 3-6 mM), but did not inhibit binding of GPIIbIIIa to immobilized fibrinogen (up to 5 mM peptide), (2) activated GPIIbIIIa binding to fibronectin and vitronectin, usual ligands for the activated integrin, (3) activated binding of GPIIbIIIa to collagen type I and type IV, proteins not usually regarded as ligands for the integrin, and (4) stimulated 125I-fibrinogen binding by human platelets. These findings argue for an interaction of this non-RGD domain in echistatin with GPIIbIIIa, leading to activation of the integrin and extension of the ligand specificity to include immobilized collagen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler M., Lazarus R. A., Dennis M. S., Wagner G. Solution structure of kistrin, a potent platelet aggregation inhibitor and GP IIb-IIIa antagonist. Science. 1991 Jul 26;253(5018):445–448. doi: 10.1126/science.1862345. [DOI] [PubMed] [Google Scholar]
- Agrez M. V., Bates R. C., Boyd A. W., Burns G. F. Arg-Gly-Asp-containing peptides expose novel collagen receptors on fibroblasts: implications for wound healing. Cell Regul. 1991 Dec;2(12):1035–1044. doi: 10.1091/mbc.2.12.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo I. F., Nannizzi L., Phillips D. R., Hsu M. A., Scarborough R. M. Inhibition of fibrinogen binding to GP IIb-IIIa by a GP IIIa peptide. J Biol Chem. 1991 Jan 25;266(3):1415–1421. [PubMed] [Google Scholar]
- Chelberg M. K., McCarthy J. B., Skubitz A. P., Furcht L. T., Tsilibary E. C. Characterization of a synthetic peptide from type IV collagen that promotes melanoma cell adhesion, spreading, and motility. J Cell Biol. 1990 Jul;111(1):261–270. doi: 10.1083/jcb.111.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Matsueda G. R., Plow E. F. A discrete sequence in a platelet integrin is involved in ligand recognition. Nature. 1991 Mar 7;350(6313):66–68. doi: 10.1038/350066a0. [DOI] [PubMed] [Google Scholar]
- Du X. P., Plow E. F., Frelinger A. L., 3rd, O'Toole T. E., Loftus J. C., Ginsberg M. H. Ligands "activate" integrin alpha IIb beta 3 (platelet GPIIb-IIIa). Cell. 1991 May 3;65(3):409–416. doi: 10.1016/0092-8674(91)90458-b. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. A., Leung B., Phillips D. R. A method for purifying the platelet membrane glycoprotein IIb-IIIa complex. Anal Biochem. 1985 Nov 15;151(1):169–177. doi: 10.1016/0003-2697(85)90067-3. [DOI] [PubMed] [Google Scholar]
- Garsky V. M., Lumma P. K., Freidinger R. M., Pitzenberger S. M., Randall W. C., Veber D. F., Gould R. J., Friedman P. A. Chemical synthesis of echistatin, a potent inhibitor of platelet aggregation from Echis carinatus: synthesis and biological activity of selected analogs. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4022–4026. doi: 10.1073/pnas.86.11.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawaz M. P., Loftus J. C., Bajt M. L., Frojmovic M. M., Plow E. F., Ginsberg M. H. Ligand bridging mediates integrin alpha IIb beta 3 (platelet GPIIB-IIIA) dependent homotypic and heterotypic cell-cell interactions. J Clin Invest. 1991 Oct;88(4):1128–1134. doi: 10.1172/JCI115412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R. J., Polokoff M. A., Friedman P. A., Huang T. F., Holt J. C., Cook J. J., Niewiarowski S. Disintegrins: a family of integrin inhibitory proteins from viper venoms. Proc Soc Exp Biol Med. 1990 Nov;195(2):168–171. doi: 10.3181/00379727-195-43129b. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Komoriya A., Akiyama S. K., Olden K., Yamada K. M. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987 May 15;262(14):6886–6892. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Jennings L. K., Phillips D. R. Purification of glycoproteins IIb and III from human platelet plasma membranes and characterization of a calcium-dependent glycoprotein IIb-III complex. J Biol Chem. 1982 Sep 10;257(17):10458–10466. [PubMed] [Google Scholar]
- Komoriya A., Green L. J., Mervic M., Yamada S. S., Yamada K. M., Humphries M. J. The minimal essential sequence for a major cell type-specific adhesion site (CS1) within the alternatively spliced type III connecting segment domain of fibronectin is leucine-aspartic acid-valine. J Biol Chem. 1991 Aug 15;266(23):15075–15079. [PubMed] [Google Scholar]
- Kouns W. C., Wall C. D., White M. M., Fox C. F., Jennings L. K. A conformation-dependent epitope of human platelet glycoprotein IIIa. J Biol Chem. 1990 Nov 25;265(33):20594–20601. [PubMed] [Google Scholar]
- Lipinska I., Lipinski B., Gurewich V. Fibrinogen heterogeneity in human plasma. Electrophoretic demonstration and characterization of two major fibrinogen components. J Lab Clin Med. 1974 Oct;84(4):509–516. [PubMed] [Google Scholar]
- O'Toole T. E., Mandelman D., Forsyth J., Shattil S. J., Plow E. F., Ginsberg M. H. Modulation of the affinity of integrin alpha IIb beta 3 (GPIIb-IIIa) by the cytoplasmic domain of alpha IIb. Science. 1991 Nov 8;254(5033):845–847. doi: 10.1126/science.1948065. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Charo I. F., Parise L. V., Fitzgerald L. A. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988 Apr;71(4):831–843. [PubMed] [Google Scholar]
- Phillips D. R., Charo I. F., Scarborough R. M. GPIIb-IIIa: the responsive integrin. Cell. 1991 May 3;65(3):359–362. doi: 10.1016/0092-8674(91)90451-4. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Ginsberg M. H. Specific and saturable binding of plasma fibronectin to thrombin-stimulated human platelets. J Biol Chem. 1981 Sep 25;256(18):9477–9482. [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Saudek V., Atkinson R. A., Pelton J. T. Three-dimensional structure of echistatin, the smallest active RGD protein. Biochemistry. 1991 Jul 30;30(30):7369–7372. doi: 10.1021/bi00244a003. [DOI] [PubMed] [Google Scholar]
- Savage B., Ruggeri Z. M. Selective recognition of adhesive sites in surface-bound fibrinogen by glycoprotein IIb-IIIa on nonactivated platelets. J Biol Chem. 1991 Jun 15;266(17):11227–11233. [PubMed] [Google Scholar]
- Scarborough R. M., Rose J. W., Hsu M. A., Phillips D. R., Fried V. A., Campbell A. M., Nannizzi L., Charo I. F. Barbourin. A GPIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem. 1991 May 25;266(15):9359–9362. [PubMed] [Google Scholar]
- Williams J., Rucinski B., Holt J., Niewiarowski S. Elegantin and albolabrin purified peptides from viper venoms: homologies with the RGDS domain of fibrinogen and von Willebrand factor. Biochim Biophys Acta. 1990 May 31;1039(1):81–89. doi: 10.1016/0167-4838(90)90229-9. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Adhesive recognition sequences. J Biol Chem. 1991 Jul 15;266(20):12809–12812. [PubMed] [Google Scholar]


