Abstract
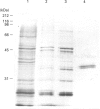
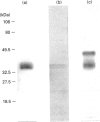
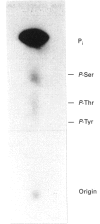
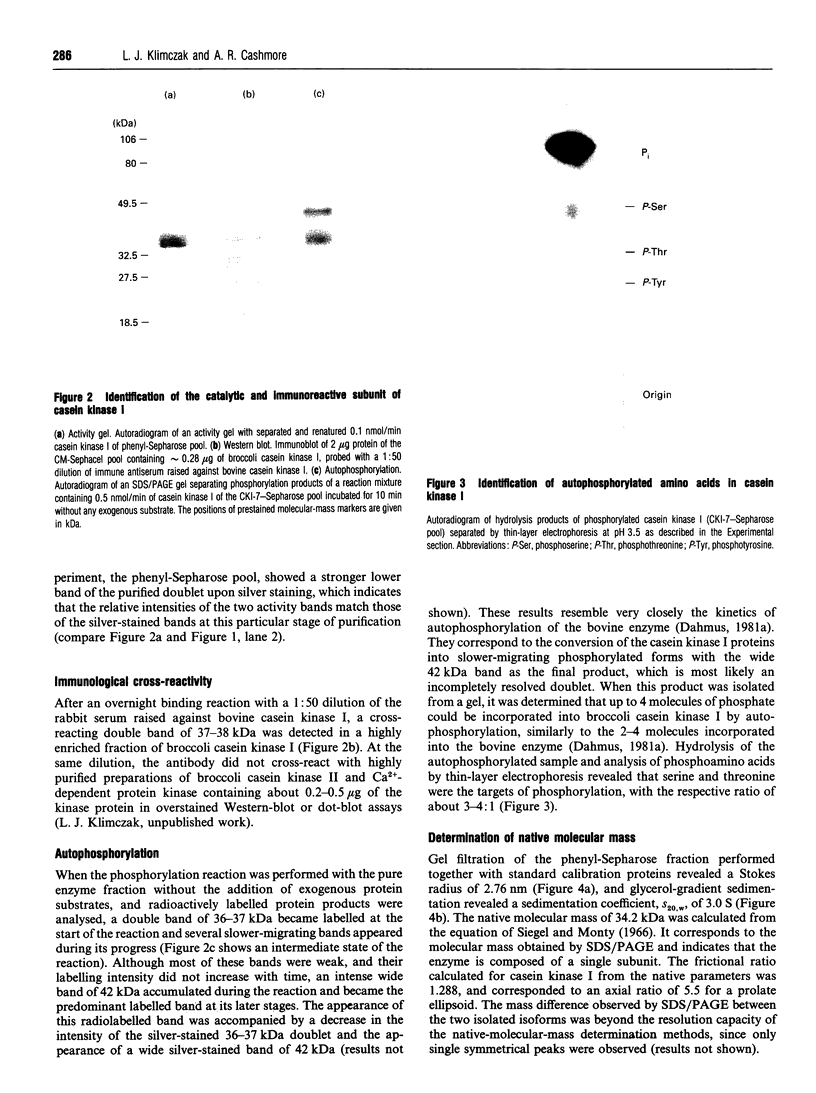
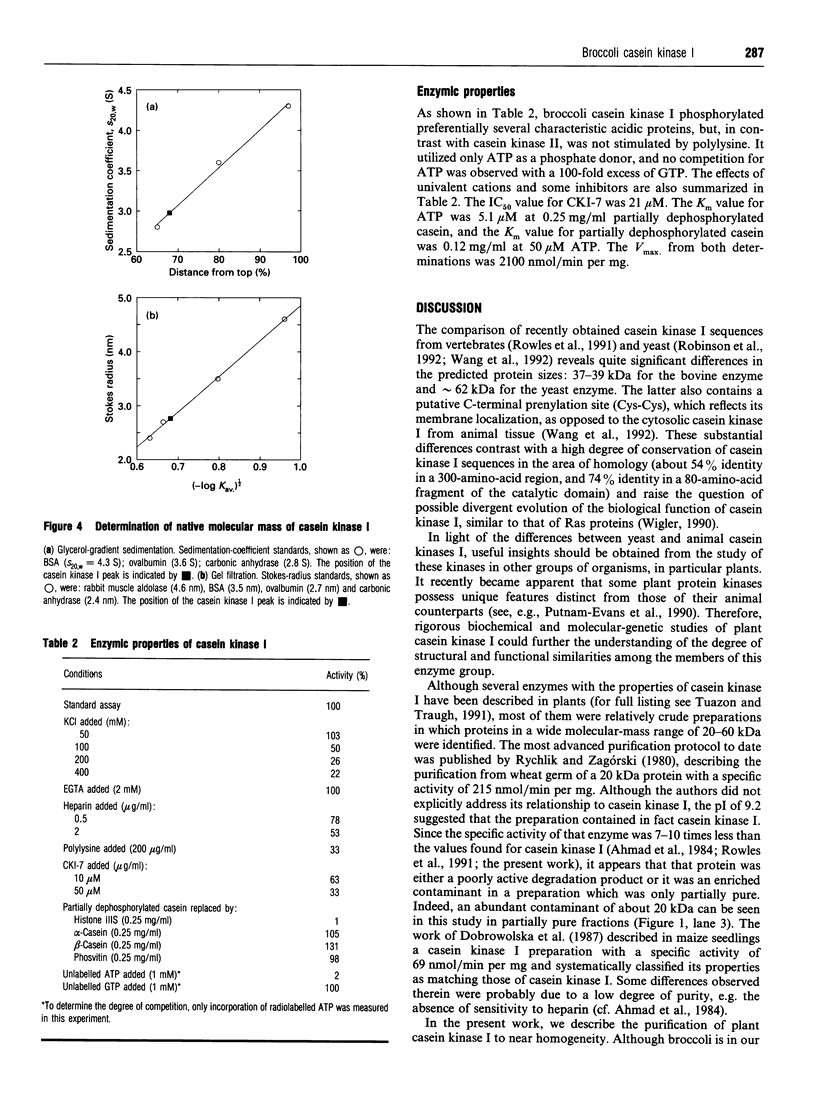
Casein kinase I from broccoli was purified approximately 65,000-fold by chromatography on phosphocellulose, phenyl-Sepharose, CM-Sephacel, and affinity chromatography on N-(2-aminoethyl)-5-chloroisoquinolone-8-sulphonamide (CKI-7)-Sepharose. The catalytic subunit of casein kinase I was identified as a 36-38 kDa polypeptide doublet by using the technique of activity gel assay after SDS/PAGE with casein as a gel-incorporated substrate. A silver-stained polypeptide doublet of the same molecular mass constituted at least 95% of the protein in the final preparation, corresponding to a specific activity of approximately 1800 nmol/min per mg of protein. The enzyme was found to be a monomer by gel filtration and glycerol gradient sedimentation; the native molecular mass was calculated to be 34.2 kDa. These characteristics, as well as other essential features of plant casein kinase I activity, such as substrate specificity and sensitivity to inhibitors, were found to be similar to those established for animal casein kinase I. Broccoli casein kinase I showed weak immunological cross-reactivity with antibodies raised against bovine casein kinase I.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad Z., Camici M., DePaoli-Roach A. A., Roach P. J. Glycogen synthase kinases. Classification of a rabbit liver casein and glycogen synthase kinase (casein kinase-1) as a distinct enzyme. J Biol Chem. 1984 Mar 25;259(6):3420–3428. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chijiwa T., Hagiwara M., Hidaka H. A newly synthesized selective casein kinase I inhibitor, N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide, and affinity purification of casein kinase I from bovine testis. J Biol Chem. 1989 Mar 25;264(9):4924–4927. [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Dahmus M. E. Calf thymus RNA polymerases I and II do not contain subunits structurally related to casein kinases I and II. J Biol Chem. 1981 Nov 10;256(21):11239–11243. [PubMed] [Google Scholar]
- Dahmus M. E. Purification and properties of calf thymus casein kinases I and II. J Biol Chem. 1981 Apr 10;256(7):3319–3325. [PubMed] [Google Scholar]
- Ferrari S., Thomas G. Micro- and macropurification methods for protein kinases. Methods Enzymol. 1991;200:159–169. doi: 10.1016/0076-6879(91)00136-k. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Huang F. L. Purification and analysis of protein kinase C isozymes. Methods Enzymol. 1991;200:241–252. doi: 10.1016/0076-6879(91)00144-l. [DOI] [PubMed] [Google Scholar]
- Jenö P., Thomas G. Affinity purification of protein kinases using adenosine 5'-triphosphate, amino acid, and peptide analogs. Methods Enzymol. 1991;200:178–187. doi: 10.1016/0076-6879(91)00138-m. [DOI] [PubMed] [Google Scholar]
- Klimczak L. J., Hind G. Biochemical Similarities between Soluble and Membrane-Bound Calcium-Dependent Protein Kinases of Barley. Plant Physiol. 1990 Apr;92(4):919–923. doi: 10.1104/pp.92.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Putnam-Evans C. L., Harmon A. C., Cormier M. J. Purification and characterization of a novel calcium-dependent protein kinase from soybean. Biochemistry. 1990 Mar 13;29(10):2488–2495. doi: 10.1021/bi00462a008. [DOI] [PubMed] [Google Scholar]
- Robinson L. C., Hubbard E. J., Graves P. R., DePaoli-Roach A. A., Roach P. J., Kung C., Haas D. W., Hagedorn C. H., Goebl M., Culbertson M. R. Yeast casein kinase I homologues: an essential gene pair. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):28–32. doi: 10.1073/pnas.89.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowles J., Slaughter C., Moomaw C., Hsu J., Cobb M. H. Purification of casein kinase I and isolation of cDNAs encoding multiple casein kinase I-like enzymes. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9548–9552. doi: 10.1073/pnas.88.21.9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychlik W., Zagórski W. Purification and characterisation of adenosine-3',5'-phosphate-independent protein kinase from wheat germ. Eur J Biochem. 1980 May;106(2):653–659. doi: 10.1111/j.1432-1033.1980.tb04613.x. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuazon P. T., Traugh J. A. Casein kinase I and II--multipotential serine protein kinases: structure, function, and regulation. Adv Second Messenger Phosphoprotein Res. 1991;23:123–164. [PubMed] [Google Scholar]
- Wang P. C., Vancura A., Mitcheson T. G., Kuret J. Two genes in Saccharomyces cerevisiae encode a membrane-bound form of casein kinase-1. Mol Biol Cell. 1992 Mar;3(3):275–286. doi: 10.1091/mbc.3.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M. H. Oncoproteins. GAPs in understanding Ras. Nature. 1990 Aug 23;346(6286):696–697. doi: 10.1038/346696a0. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]