Abstract
Cardiovascular sensing and monitoring is a widely used function in cardiovascular devices. Nowadays, achieving desired flexibility, wearability and implantability becomes a major design goal for the advancement of this family of devices. As an emerging technology, nanogenerator (NG) offers an intriguing promise for replacing the battery, an essential obstacle toward tissue-like soft electronics. This article reviews most recent advancements in NG technology for advanced cardiovascular sensing and monitoring. Based on the application targets, the discuss covers implantable NGs on hearts, implantable NGs for blood vessel grafts and patches, and wearable NGs with various sensing functions. The applications of NGs as a power source and as an electromechanical sensing element are both discussed. At the end, current challenges in this direction and future research perspectives are elaborated. This emerging and impactful application direction reviewed in this article is expected to inspire many new research and commercialization opportunities in the field of NG technology.
Keywords: Nanogenerator, Cardiovascular monitoring, Wearable devices, Implantable devices, Self-powering
Introduction
Cardiovascular disease (CVD) has been the leading cause of death in the US and there are over 26 million adults in the United States with cardiovascular diseases nowadays [1–3]. By 2030, CVD is projected to account for 25 million deaths worldwide [4,5], which is a fatal factor for the human health [6]. Common cardiovascular diseases can be divided into two categories. The first category is heart diseases, including heart failure, arrhythmias, myocardial and so on. The other one is vascular diseases, such as peripheral artery disease, carotid artery disease, aortic aneurysms, and venous thromboembolism [7,8]. With the remarkable development of the science and technology in the field of medical devices [9], most of these diseases can be prevented or cured if they are detected and treated at an early stage [10,11]. Nowadays, many portable and even wearable cardiovascular sensors have been developed [12–14]. These sensors have played an important role in early diagnosis and prevention of cardiovascular diseases and have saved millions of people’s lives [15]. They are either applied to capturing vital information from blood and vascular activities, such as pulse sensing [16], blood pressure monitoring [17], and blood analyses [18,19], or directly interfacing with the heart. The later groups of sensors are typically integrated with other cardiac medical systems like pacemakers, cardioverter defibrillators and loop recorders to achieve an autonomous therapeutic function [20,21].
Despite the rapid development of implantable and wearable electronic devices for cardiovascular systems, majority of the devices are still rigid with a relatively large size [22]. While most electronic components and circuits can be made flexible and in the sub-millimeter regime, the powering component is mostly based on a primary battery system [23,24]. The conventional battery technology can provide a continuous and stable electrical power for the devices, satisfying the reliability concern of these critical medical devices. However, they often occupy more than 50 percent of the whole device volume [25,26]. The finite capacity of the batteries also substantially limited the device lifetime, which need to be replaced in a regular base [27]. In addition, to ensure a high safety and reliability, the medical devices, particularly for an implantable system, are typically packaged by a rigid shell (mostly stainless steel), which completely mismatches the mechanical property of the hosting tissues, and increase the potential of complication [28].
Following the technology evolution trend and patient-centered device design concept, cardiovascular sensors see urgent needs of miniaturization, flexibility and tissue compatibility [29]. Of course, sensitivity still remains the most critical factor for the cardiovascular sensors, especially the signals from the cardiovascular system are always faint and subtle [30]. Meanwhile, how to improve the power technology has been a major research concern in recent years, as the battery directly determines the device size, mass, flexibility and lifetime [31]. Among a variety of strategies, the nanogenerator (NG) technology have aroused the greatest interests and have been studied globally by numerous researchers [32]. As a type of electromechanical coupling device, NGs can harvest the low-level mechanical energy form small-scale and irregular movements from various body activities and convert them into electricity [33]. Therefore, NGs are considered as a promising approach to the replacement of battery and enabling self-powered devices [34,35]. Typical NGs rely on either piezoelectric or triboelectric principles, both of which have been extensively studies theoretically and experimentally [34–42]. Numerous energy harvesting devices and systems have been developed using either piezoelectric NGs (PENG) or triboelectric NGs (TENG) from the micrometer scale to the kilometer scale [41,43–51]. The NG technology has also been covered in many comprehensive review articles over years [52–55], and thus we will not discuss the basic principles of this technology in this review articles.
Given its unique electromechanical coupling feature [56], the NG technology has also been introduced to the cardiovascular system to perform various functions [57,58]. The NGs offer advantages of compact sizes, broad materials selections, good flexibility and compatibility, as well as high sensitivity to biomechanical displacements [59–62]. One of the prevailing applications of NGs for cardiovascular devices is to serve as the power source for achieving self-powered pacemakers [27]. The different design principles and recent advancements have been covered in several well-representative review articles [28,55,63–65]. Aside from a power source, NG also naturally provides sensing functions by directly coupling mechanical displacements or pressure changes into electrical signals [66–69]. This behavior enlightened a new way toward real-time and high-accuracy sensing of the activities in cardiovascular systems [70]. This review article therefore focuses on the most recent advancements in NG technology for sensor-related applications in the cardiovascular system. The cardiovascular system has several unique features as a biomechanical energy source in the human body [26]. Specifically, there exists regular periodic motions in the entire system, originated from heartbeats [71]. This includes muscle stretching/expansions directly on heart, from the blood pressure fluctuations inside or at the surface of blood vessels, or remotely through external skin surfaces [72,73]. The unique physical and chemical environment of the cardiovascular system provides different challenges as well as new opportunities for NG devices design and applications [57]. Based on the application conditions, we group our discussions into three major categories, including NG-based sensors for heart activities, on blood vessels, and for remote blood sensing (Figure 1). Existing challenges and perspectives are summarized toward the end of this article. We expect this article will provide a timely overview of the advances of NG technology for cardiovascular sensing and monitoring, and inspire new technology breakthroughs in this exciting NG application direction.
Figure 1.
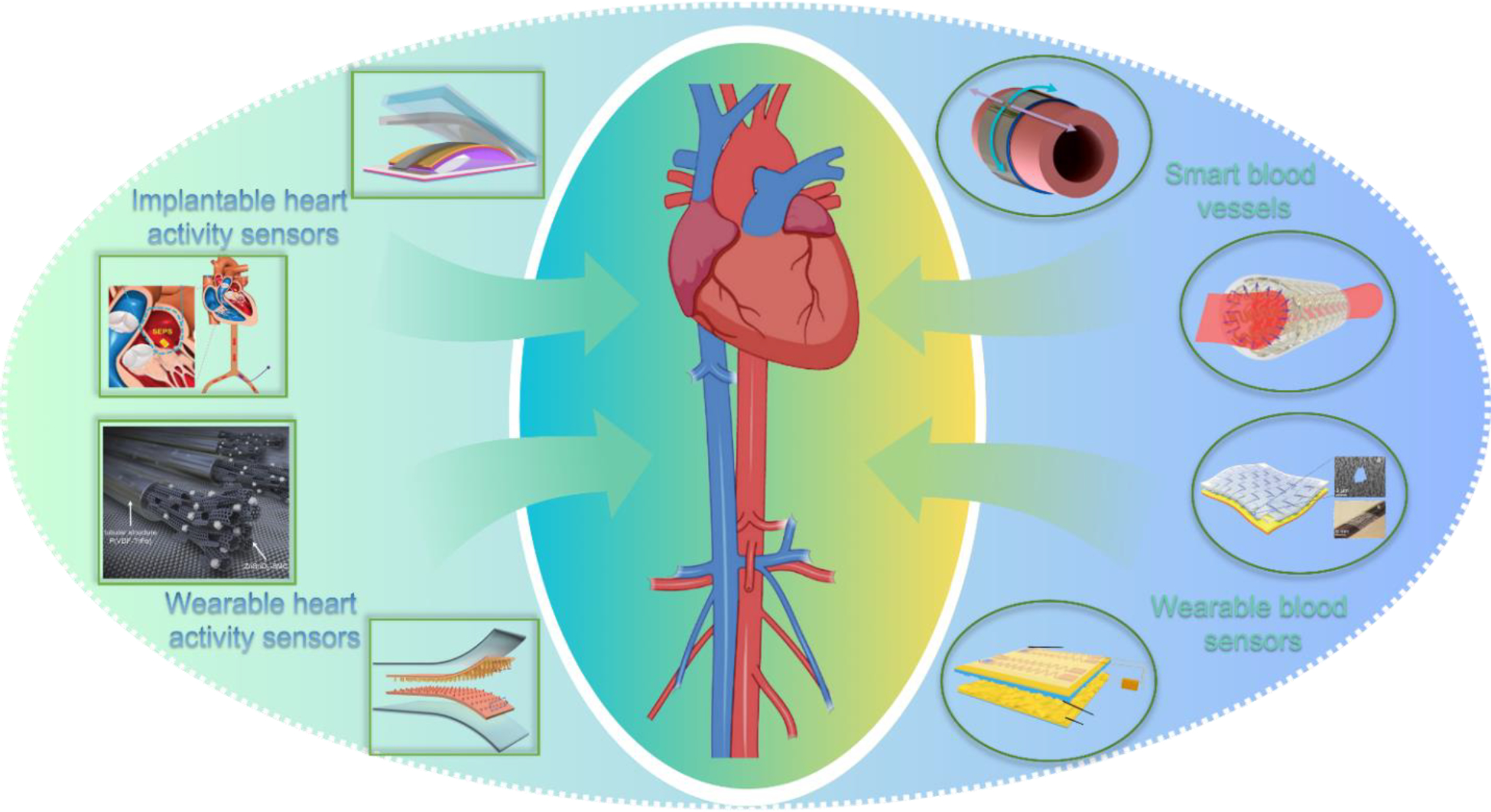
Applications of nanogenerator technology for cardiovascular sensing and monitoring.
Sensors for Heart Activities Monitoring
Heart activities carry a large amount of vital information that can be used to monitor and diagnose various cardiovascular diseases [74]. Clinically, the cardiovascular system is normally examined by electrical signal measurement techniques including electrocardiograph (ECG), phonocardiogram, and sphygmograph [75,76]. This type of measurements is usually done in clinics and require professional operations, due to the sophistication of setup and signal processing [76]. These systems are also bulky and not suitable for mobile or wearable physiological signal monitoring system [77]. Therefore, developing highly sensitive and selective, and miniaturized heart activities sensors are of substantial significance and urgency for realizing daily signal collection from hearts. Following the similar concept of energy harvesting from the hearts, NGs are also well-suited for monitoring the heart activities, via either implanted or wearable strategies.
Implantable NG-based heart activity sensors
Similar to the NGs used for harvesting energy from heartbeats, implantable NG-based sensors have been designed to be responsible for heartbeats. Here the focus of NG technology development is primarily on signal sensitivity rather than the absolute amount of electric energy that can be generated. Given the high energy availability from hearts, the electricity provided from NGs was often used to support the sensing signal transmission or recording, therefore achieving a self-powered operation. In 2016, Zheng et al reported a self-powered wireless cardiac monitoring based on a TENG which was implanted on the heart surface [78] (Figure 2A). The contact-separation TENG device consisted of a nanostructured polytetrafluoroethylene (n-PTFE) film and an Al foil as the two triboelectric layers. A highly resilient titanium strip was introduced as a ‘keel structure’ in the TENG structure to facilitate the contact and separation process. The whole structure was packaged in a PTFE/Polydimethylsiloxane (PDMS)/parylene C trilayer structure. These well-engineered three layers of packaging offered a good protection to the device from its liquid and dynamic implantation environment, as well as improved the device’s stability, corrosion resistance, and biocompatibility. However, such a multilayered packaging structure also impeded the TENG movements and lowered the output amplitude. By implanting the device on different positions of the heart surface of a pig, the highest TENG output was found from the lateral wall of the left ventricular, providing an average output voltage of 14.0V. The output signals well matched the signal obtained from the electrocardiogram (ECG), suggesting an accurate sensing capability. Based on this device, a wireless heart monitoring system was developed by integrating the TENG with a power management unit (PMU) and a wireless transmitter. A capacitor in the PMU was charged by the TENG on-heart, and then powered the wireless transmitter to send the heartbeat information via electromagnetic waves to the external receiving coil. The amplitude of the wireless transmitted signal was directly related to the charging time and the frequency of the applied force. This way, the system enabled a wireless monitoring of heart beating behavior without using a battery. It should be pointed out that in the current design, the PMU was still connected externally. Further development and integration are needed in order to realize a complete implantable system.
Figure 2. Implantable NG-based heart activity sensors.
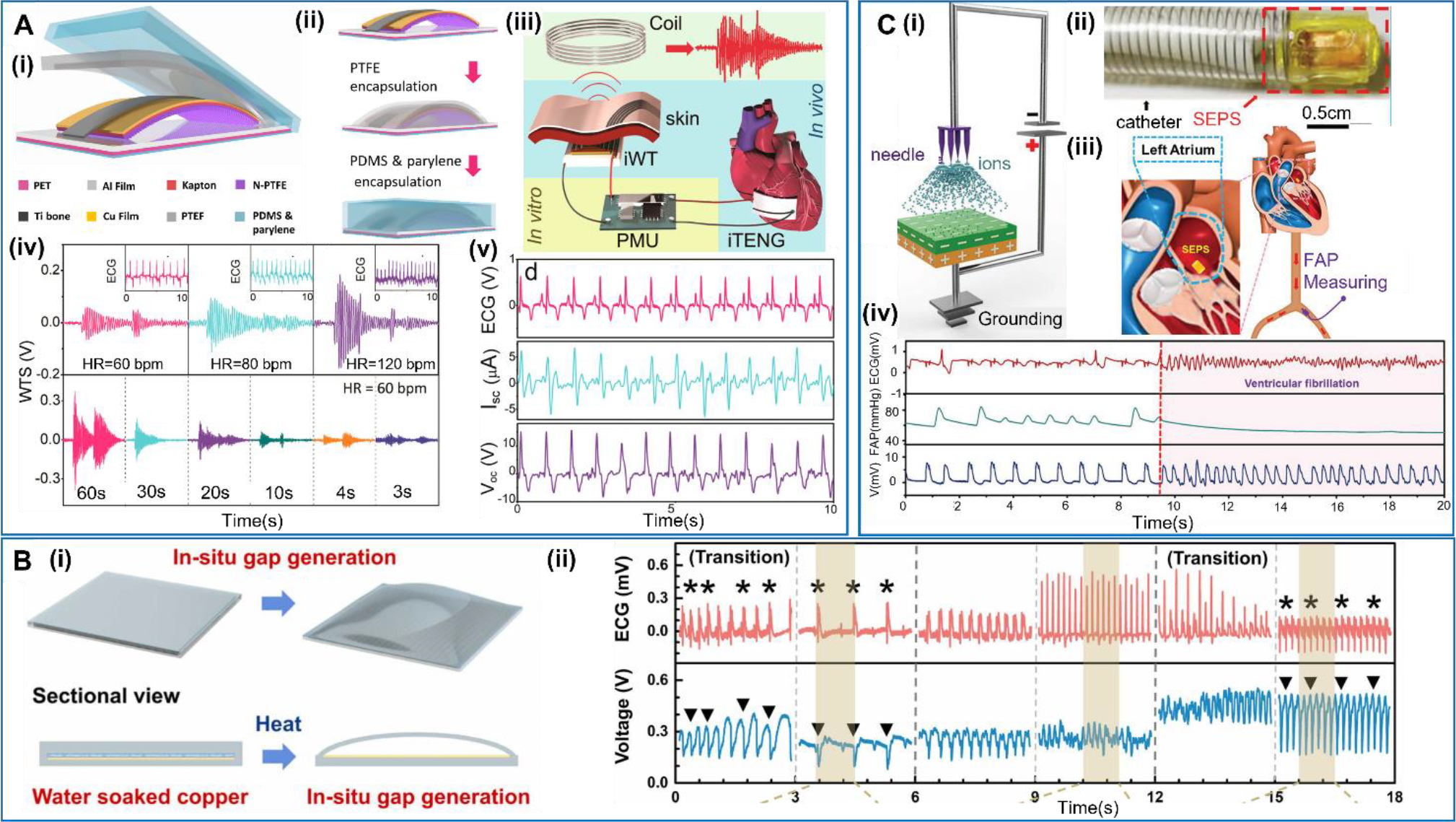
A. Self-powered wireless cardiac activities sensor. (i) Schematic design of the implantable TENG (iTENG). (ii) Encapsulation process of the iTENG. (iii) Schematic diagram of the self-powered wireless transmission system based on the iTENG. (iv) Wireless transmission signals received at different heart rates (charging time = 10 s) and different charging times (HR = 60 bpm). (v) Voc and Isc measured from the iTENG in vivo and simultaneously recorded ECG from the swine. Reproduced with permission [78]. Copyright 2016, American Chemical Society. B. In-situ gap generation of a no-spacer TENG. (i) Schematic diagram of the in-situ gas gap generation in the no-spacer TENG. (ii) The output voltage of the no-spacer TENG at anterior wall and the corresponding ECG of the rat injected with epinephrine. Reproduced with permission [79]. Copyright 2021 Elsevier Ltd. C. Transcatheter self-powered endocardial pressure sensor. (i) Schematic of corona discharge for performance improvement. (ii) Photograph of a device integrated with a surgical delivery system. (iii) Schematic diagram of the SEPS implanted into left atrium and a commercial arterial pressure sensor placed in the right femoral artery. (iv) The comparison of output SEPS signals with and without the ventricular fibrillation. Reproduced with permission [80]. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
A similar implantable TENG-based device was created by Zhao et al. via a more scalable approach [79] (Figure 2B). The device was structured with a copper layer and an outside Ecoflex rubber layer. The gap between the rubber and copper was created in situ by vaporizing distilled water soaking. This process offered a fairly uniform stress/strain distribution in the TENG layers, and thus the TENG could undergo larger displacement compared with the conventional TENG with spacer added. Therefore, this TENG device eliminated the obstacle from spacer, and could sense subtle movements near the spacer. This relatively simple fabrication method was also helpful to improve the device’s biosafety by largely reducing unwanted air contamination in vivo. The output voltage from this device also showed a good consistency to corresponding ECG signals, confirming the potential as a sensing element for monitoring the heart activities.
In addition to the epicardiac sensors that apply on the heart surface, there are also developments on endocardial pressure sensor, which may minimize the implantation invasiveness. Liu et al reported a transcatheter self-powered ultrasensitive endocardial pressure sensor (SEPS) based on the TENG technology [80] (Figure 2C). The device had a multilayered structure, where a nano-PTFE film and an Al foil were employed as the triboelectric layers. Compared to the devices implanted on the surface of the heart, this device had a smaller size of 1cm × 1.5cm × 0.1cm. To improve the output performance and sensitivity of the device, the surface of nano-PTFE was treated with corona discharge to raise the surface charge density. The compact size of the device allowed it to be delivered by a regular polyvinyl chloride (PVC) stretchable catheter, achieving minimally invasive implantation. The device was implanted into the left ventricle and the left atrium of a pig, respectively, and both produced consistent voltage signal following the heart beats. The voltage profile matched well with the ECG and FAP signals. In addition, under simulated ventricular fibrillation conditions, the output signals showed an excellent consistency with heart condition changes. Both proved the feasibility of the endocardial device in monitoring the heart activities. Compared to the epicardiac devices, the endocardial sensor could collect more information from both heart beating and blood pressure change, and may provide more comprehensive data sets for heart function monitoring. It may also be integrated with the new intracardiac pacemaker technology.
Wearable cardiovascular sensors
Compared to the implantable sensors, wearable sensors are much more commonly developed for monitoring the heart activities by sensing the pulsation information [81]. This type of devices has a relatively simple structure, and can potentially be a low-cost and disposable system [82]. Both PENG and TENG have been widely used in this wearable sensor system.
As the first NG technology, PENGs have been widely studied as wearable sensors for heart activities [83]. Among many different material selections, piezoelectric polymers are the first and most common choice due to their high flexibility, good processability, and reasonable biocompatibility [84]. Kang et al reported a flexible nanofibers (NF)-mat design, where the NFs were fabricated using electrospun piezoelectric P(VDF-TrFE) composited with ZnSnO3 nanoparticles-decorated carbon nanotubes (SMC) decorated [85] (Figure 3A). The piezoelectric responses were improved by introducing the highly conductive SMC into the ZnSnO3-P(VDF-TrFE) mixture for charge collection. The piezoelectric NF-mat was sandwiched between two microbead array electrodes (MAE) and two packing films. The microbead structure enabled effective transfer of external mechanical force to the piezoelectric mat for electrical signal generation. The device had a good flexibility and sensitivity, and could provide a continuous and precise reading of the pulses with a voltage output of ~100 mV when wrapping around the wrist.
Figure 3. Wearable PENG-based cardiovascular sensors.
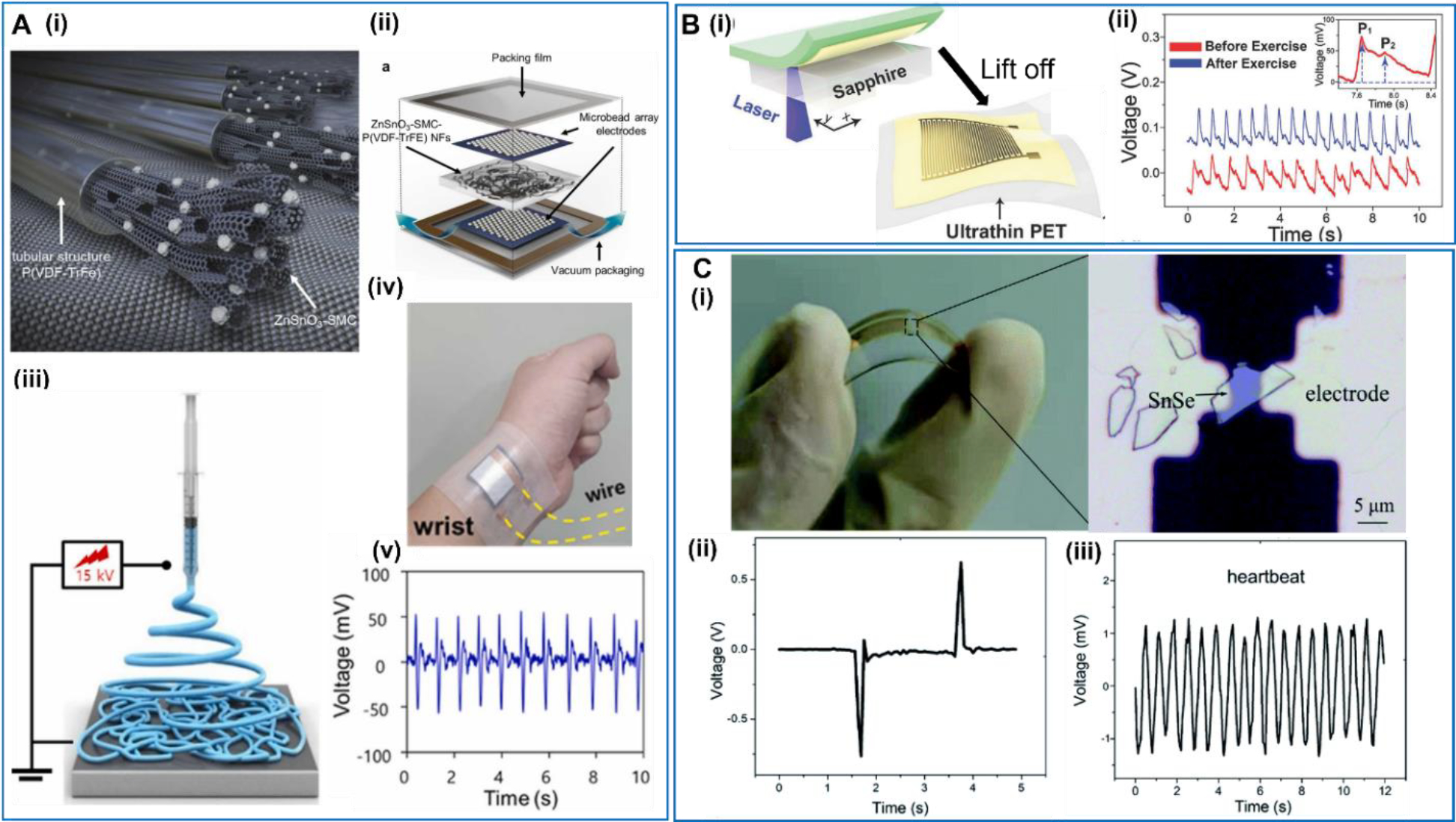
A. Peapod-inspired hierarchical PENG. (i) Conceptual illustration of the piezoelectric ZnSnO3-SMC-P(VDF-TrFE) NFs. (ii) Schematic illustration of the piezoelectric device configuration. (iii) Schematic illustration of the electrospinning process used to prepare the P(VDF-TrFE)-based NFs. (iv) The device attached on the wrist. (v) Real-time output waveforms produced by the piezoelectric device when attached to the wrist. Reproduced with permission [85]. Copyright 2022, Elsevier Ltd. B. Ultrathin epidermal piezoelectric sensor. (i) Schematic illustration of the fabrication process of a self-powered pressure sensor. (ii) Radial artery pulse signals detected by the self-powered pulse sensor. Reproduced with permission [87]. Copyright 2017, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. C. Self-powered 2D-material sensor. (i) Photo and optical microscope image of the SnSe piezoelectric device. (ii) Output voltage signal of a typical SnSe nanogenerator. (iii) Heart rate detection by attaching the SnSe nanogenerator on the chest of the tester. Reproduced with permission [90]. Copyright 2020, Royal Society of Chemistry.
Compared to implantable devices, the wearable sensors have relatively lower requirement on the biocompatibility, and thus allowed more choices of the piezoelectric materials [86], particularly from the group of inorganic piezoelectric ceramics as such Pb[Zrx,Ti1−x]O3 (PZT) and BaTiO3. This group of materials typically has high piezoelectric coefficients, low cost, and good scalability. A self-powered real-time arterial pulse monitoring device was reported by Park et al based on a high-quality PZT thin film [87] (Figure 3B). The PZT film was affixed on an ultrathin PET substrate, with a pair of interdigitated gold electrodes deposited on its surface. The whole device had an extremely small thickness of only 7.8 μm, which offered a very high flexibility. Therefore, the device could achieve conformal contact to the complex human skin topography and thus provided a high sensing ability to pressure changes near the skin surface induced by arterial pulse. In the tests, the wearable pulse sensor was attached to the wrist area and could precisely detect the pulse signals (rate and intensity) change before (red line) and after (blue line) physical exercise (running for 10 min). The enlarged pulse signal clearly revealed the characteristic peaks of peripheral artery waveforms, which carry important biomedical and physiological information such as arterial stiffness, coronary artery disease, and myocardial infraction.
Beyond these traditional piezoelectric materials, more emerging or bio-related materials have also been used to develop PENG sensors, such as spider silk fibers [88], silk [89] and collagen [50]. As a new class of piezoelectric materials, two-dimensional (2D) piezoelectric materials have drawn significant attention in both fundamental and applied research in recent years. Though their overall piezoelectric responses are still generally low, their unique 2D morphology makes them an interesting candidate for sensor applications, particularly toward flexible systems. Li et al investigated the giant in-plane piezoelectric properties of 2D SnSe crystals and discovered a relatively high piezoelectric output voltage of 760 mV from SnSe flakes with an armchair pattern when it was bent [90] (Figure 3C). When attached on the human chest, the SnSe device could successfully sense the mechanical motions from heartbeats, and output electric pulses with an amplitude of ~1.2 mV. The signal frequency was consistent with the heart rate, showing a potential of self-powered wearable monitor for vital signals in healthcare applications.
Compared to the piezoelectric materials, triboelectric materials offer a wider selection for achieving desired electromechanical coupling for the wearable sensor application. TENG based sensors usually offer much high voltage outputs compared to the piezoelectric devices [91]. Ouyang et al reported a Cu-Kapton based TENG which could be integrated in a flexible, self-powered ultrasensitive pulse sensor (SUPS) with excellent output performance, high peak signal-noise ratio, high sensitivity, and high resolution in time [92] (Figure 4A). The Kapton and Cu films both had a nanostructured surface, which largely improved the triboelectric output. The high sensitivity allowed the SUPS to detect weak signals from the heart, and the output voltage profile exhibited an excellent linearity with the ECG readings by analyzing the R–R intervals. This demonstration validated the potential of accurate heart rate monitoring in clinical applications. Given its high temporal resolution, the SUPS could provide an accurate measurement of the pulse wave velocity (PWV), which was accepted as the robust, simple, and reproducible method to determine the regional arterial stiffness. PWV was determined from the signals obtained from two sensors placed 4.5 cm apart on the radial artery of the wrist. The ability of regional artery PWV measurement could be more convenient and patient-friendly than the commonly used ankle-brachial PWV and carotid\-femoral PWV in clinical. Liu et al presented a simple and low-cost strategy to achieve high sensitivity and flexibility for pressure sensing based on TENGs created by synergizing PDMS and thermally expandable microspheres [93] (Figure 4B). A large-area patterned triboelectrification thin layer was fabricated by spin-coating a mixture of thermally expandable microspheres and PDMS on a flat substrate. When heated, the microspheres expanded and protruded out of the flat PDMS surface. When a constant force/pressure was applied, the upper FEP film came into contact with and then deformed the soft microspheres. Different pressures yielded different microsphere deformations resulting in different contact areas, and thereby produced different amount of triboelectric surface charges serving as the pressure sensor signals. The ultra-softness of the microsphere structure offered excellent sensitivity to surface pressure. When fixed on the wrist, the pulse rate could be precisely recorded. Moreover, the percussion wave (P-wave), the valley, and the diastolic wave (D-wave) could also be detected clearly, providing vital information about the systolic blood pressure, the diastolic blood pressure, and the ventricular pressure. In addition to enlarging the contact area of the triboelectric layers, Zhang et al proposed another strategy to enhance the output signal by using a lever-inspired contact-separation TENG (Li-TENG) [94] (Figure 4C). The top electrode of TENG was fixed on the lever bar, while the bottom electrode was fixed on a stage. The contact and separation distance and speed between the two electrodes was thus modulated by the lever ratio, which can be tuned to amplify the output amplitudes without changing the amount of charge transfer. The Vp-p could reach up to 18V when the device was fixed on the wrist to measure the pulse signal from the right radial artery.
Figure 4. Wearable TENG-based cardiovascular sensors.
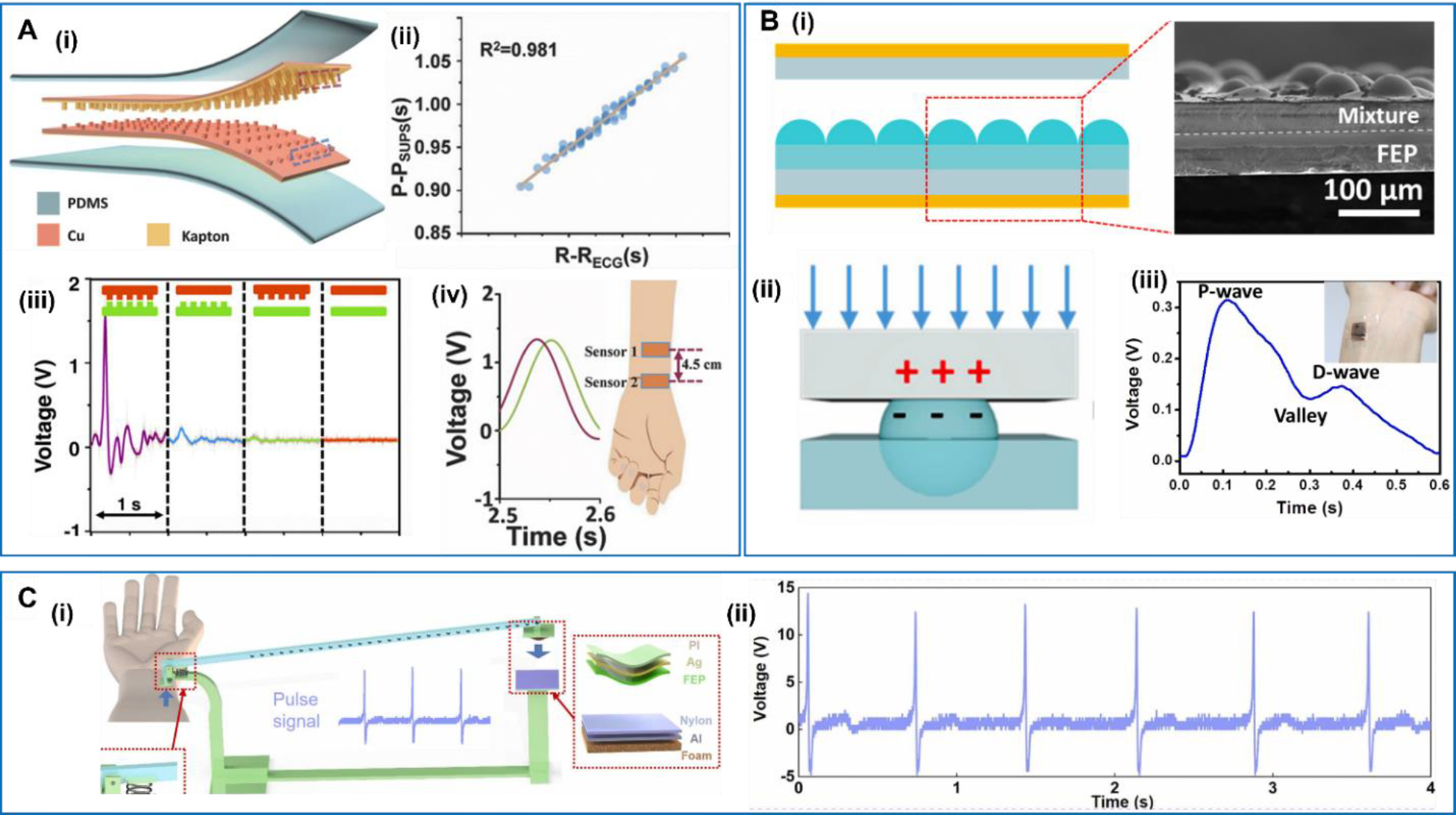
A. Flexible and self-powered ultrasensitive pulse sensor (SUPS). (i) Schematic diagram of TENG. (ii) The linear relationship of SUPS with ECG through the analysis of R–R interval. (iii) The output voltage of SUPS with different structure of friction layers. (iv) The signal output of two SUPS pressed on the radial artery with the distance of 4.5 cm. Reproduced with permission [92]. Copyright 2017, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. B. Expandable microsphere-based TENG. (i) Cross-sectional view of the PDMS-microsphere layer. (ii) Deformation process of one unit block when pressure is applied. (iii) Voltage signals recorded during the pulse rate monitoring test. Reproduced with permission [93]. Copyright 2019, Elsevier Ltd. C. Lever-inspired TENG. (i) Schematic diagram of Li-TENG. (ii) Signal diagrams of pulse measurement with Li-TENG. Reproduced with permission [94]. Copyright 2022, Elsevier Ltd.
Sensors and Monitors for Blood Vessels
In addition to the heart, blood vessel is another essential organ in the cardiovascular system. Vascular replacement is a prevalent surgical intervention for the restoration of obstructed or nonfunctional blood vessels in the management of coronary artery disease, cerebrovascular accidents, and critical limb ischemia, [95]. Approximately 450,000 prosthetic vascular graft implantations are performed annually in the United States [96]. Nonetheless, vascular implant failure can transpire without substantial warning signs, with 40–60% of vascular grafts failing within the first-year post-implantation, leading to increased morbidity and mortality rates [97,98]. It is therefore crucial to monitor associated local physiological parameters, such as hemodynamic pressure, graft flow velocity, and alterations continuously and promptly in luminal dimensions, to identify significant lesions at an early stage [99]. At present, surveillance of implanted blood vessels necessitates periodic clinical assessments using advanced diagnostic tools, including ultrasound imaging devices, sphygmomanometers, and contrast-enhanced computed tomography (CT) scans [100,101]. However, these methods have proven to be insufficient and ineffective for the prompt detection of complications and prevention of graft failure [102]. Consequently, there is a pressing need for the development of real-time monitoring systems for implanted vascular grafts to accurately identify complications and facilitate early intervention through less invasive and safer approaches [103]. Comparing to intermittent measurements, real-time monitoring can provide timely detection of sudden changes from blood vessels and allow early diagnosis of health problems. However, the reliance to traditional batteries as the power supply limits the lifetime of real-time monitoring systems as well as impedes their application in vivo. As a type of electromechanical coupling device with a broad range of configuration and material selections, NG technology showed a unique potential to realize self-powering capability and real-time monitoring of blood vessels [104]. Generally, there are two ways to realize the real-time monitoring: smart blood vessels with self-monitoring functions and functional patches that can be wrapped around the blood vessels.
Smart blood vessels
In 2018, Hu et al reported a multifunctional piezoelectric elastomer composite which could be used for artificial arteries [105] (Figure 5A). The elastomer was composed of PDMS, PVDF and carbon nanotubes (CNT). The introduction of the PVDF enhanced the mechanical properties such as strength, instantaneous modulus, and Young’s modulus. Adding CNT could improve the dynamic mechanical properties of viscoelasticity. Therefore, the composite could endure more mechanical stress and deliver higher electric output under a fixed pressure. The composite was used as the functional layer in a smart artery, which had a five-layer structure with the other layers being electrodes and packaging. The pressure applied on the internal wall of the artificial artery could effectively deform of the PVDF-PDMS-CNT layer and drove an alternative voltage pattern. The peak-to-peak voltage reached up to 0.28 V under simulated blood flow at an inner pressure of ~1.1 kPa, showing the capability of capturing the blood pressure in vivo and consistently monitoring the heartbeats. Wang et al reported a different double-layered vascular graft based on TENG technology using poly(3-hydroxybutyrate (PHB) and expanded polytetrafluoroethylene (ePTFE) membranes as the positive and negative materials, respectively [106] (Figure 5B). The output voltage of this artificial artery reached more than 300 V and it was able to charge a 100 μF capacitor to 7.5 V in just 100 s under a simulated 30 N force. When driven by a dynamic peristaltic pump flow, the output signals by the TENG-based blood vessel showed obviously different patterns between the obstructed and unobstructed blood flow conditions, indicating the potential of monitoring endothelial/vascular tissue growth.
Figure 5. Multi-layered smart blood vessels.
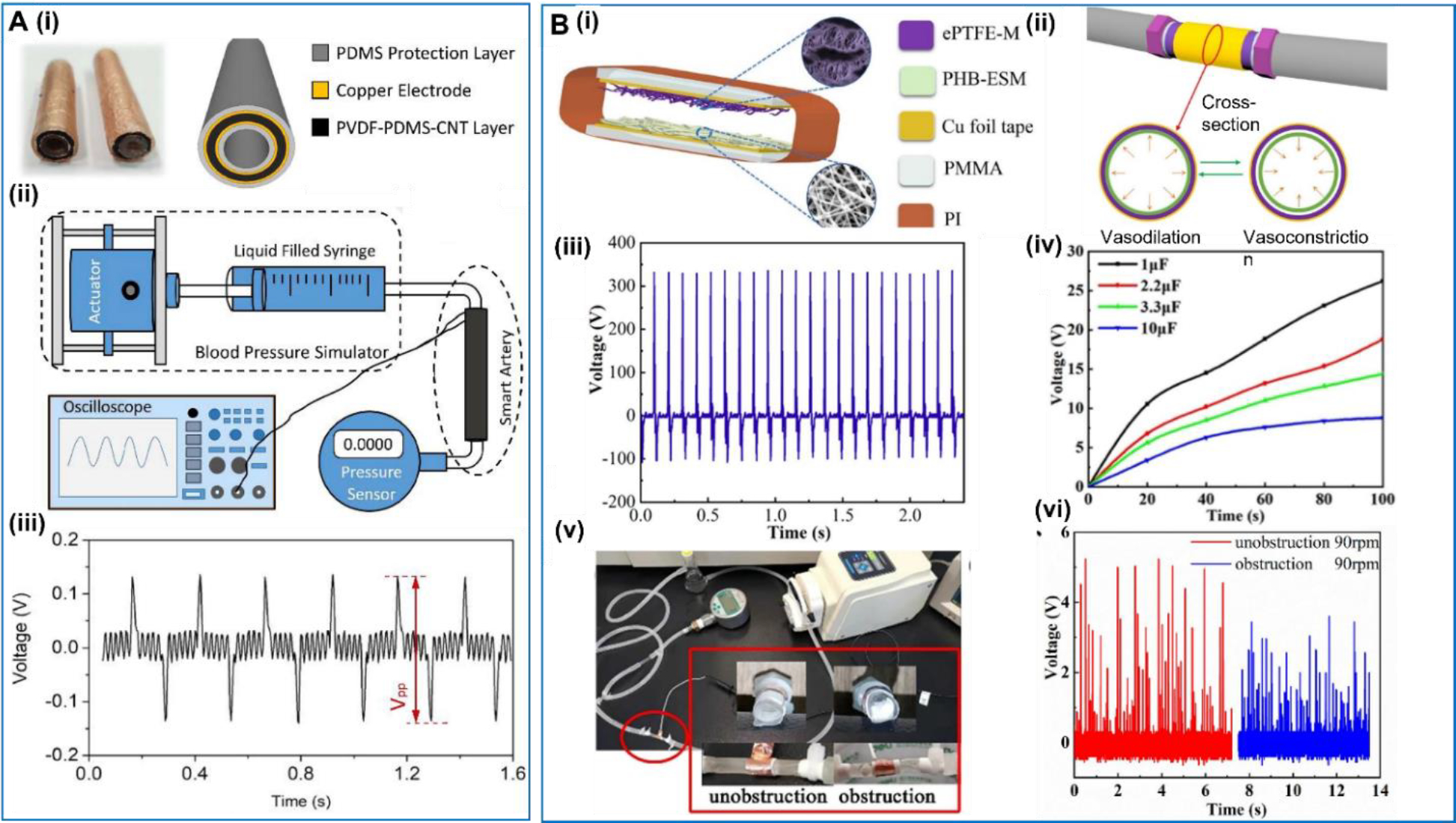
A. Multifunctional piezoelectric elastomer composite. (i) Structure of the smart artery. (ii) The schematic diagram of the experimental system. (iii) Electric output in voltage from the smart artery. Reproduced with permission [105]. Copyright 2018, Elsevier Ltd. B. Multi-layered vascular graft based on TENG. (i) and (ii) Structure of ePTFE/PHB TENG. (iii) Open circuit voltage of the ePTFE/PHB TENGs. (iv) Charging capacity under different capacitor capacities (1–10 μF). (v) Set-up of the blood flow experiment. (vi) Output signals of the ePTFE/PHB TENG under unobstructed and obstructed blood flow conditions. Reproduced with permission [106]. Copyright 2022, Elsevier B.V.
In 2020, Li et al introduced 3D printing technology for fabricating multifunctional artificial artery, in which an electric field was applied to achieve in situ poling of the ferroelectric printing materials [107] (Figure 6A). In their work, a ferroelectric composite consisting of 35vol% functionalized sodium potassium niobate (KNN) piezoceramic particles and 65vol% PVDF polymer was used as the printing material. KNN-based materials are a group of high-performance lead-free ferroelectric ceramics that are also biocompatible. PVDF was selected as it is a soft thermoplastic polymer with excellent printability, flexibility, and acceptable piezoelectricity. The 3D-printed sinusoidal lattice with a thickness of 0.2mm served as the piezoelectric backbone, which was encapsulated by PDMS to form the artificial artery. An artificial circulation system was used to demonstrate the self-powered blood pressure sensing capability of the artificial artery. The piezoelectric voltage output was proportional to the exerting pressure in the artificial artery and a good monotonic relationship was identified within the normal human blood pressure range from 1.5kPa to 30 kPa. The complete 3D piezoelectric lattice also allowed precise determination of the blood vessels movements, allowing early detection of thrombosis. When the blood vessel was only blocked by a small percentage (e.g. 20%), blood pressure change was indistinguishable. However, the slight asymmetric artery movement because of the different blood flows/pressures pattern at two sides of the thrombosis could be clearly captured by the artificial artery with an obvious double-peak characteristic. Besides, Cafarelli et al proposed a nanocomposite graft by combining the poly(ether)urethane and PDMS with BaTiO3 nanoparticles [108] (Figure 6B). The graft had an internal diameter of 6 mm with the wall thickness of ~500 μm. With the addition of BaTiO3 nanoparticles, the average d33 of the device was raised to 1.91 pm/V, which was more than two times of that of the control group without doping.
Figure 6. Smart blood vessels based on new fabrication methods.
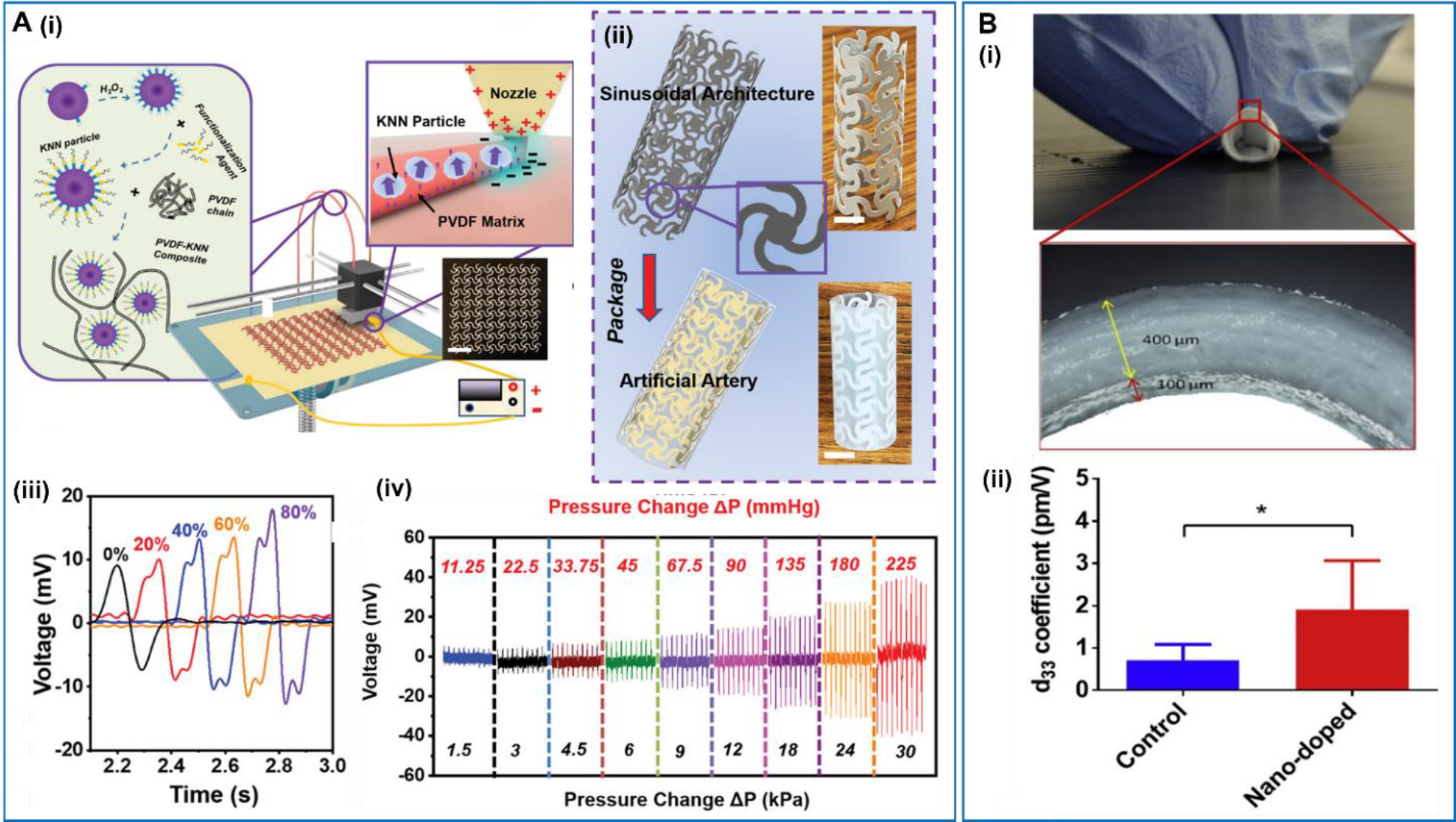
A. Multifunctional artificial artery from 3D printing. (i) Schematic illustration of the electric field-assisted 3D printing system. (ii) Schematic illustration of artificial artery consisting of printed piezoelectric tube with sinusoidal lattice and PDMS package. (iii) Comparison of voltage envelopes under different levels of occlusion. (iv) Voltage response of the artery as a function of pressure change. Reproduced with permission [107]. Copyright 2020, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. B. Small-caliber vascular graft. (i) Photos of the vascular graft (top) and optical microscope images (bottom) of its external surface and its cross-section. (ii) Average d33 values for the control and the nano-doped samples. Reproduced with permission [108]. Copyright 2019, Elsevier Ltd.
Blood vessel patches
Rather than developing the blood vessel completely, some studies focused on designing a piezoelectric patch that can be wrapped on a regular graft to monitor the pressure inside. In a representative example, D’Ambrogio et al reported a NaNbO3 fibers/PDMS piezoelectric composite patch, which could realize real-time monitoring of the blood pressure and anomalies related to the thrombus [109] (Figure 7A). The patch had a simple multi-layered structure with a piezoelectric composite mash, two electrodes and an epoxy base. The patch was wrapped on a graft connecting two tubes. To simulate the vascular stenosis caused by a thrombus, two clips were integrated on the tubes, one before and one after the sensor. The output voltage of the patch exhibited a linear increase from 0.92V to 1.29V, when the simulated occlusion was built up from 0% to 25% by the after-clip. When the occlusion was introduced by the before-clip, an opposite voltage output change was obtained. This work demonstrated that the position and the degree of the occlusion could be determined based on the position of the patch and the output signal variation.
Figure 7. Smart blood vessel patches based on the PENG.
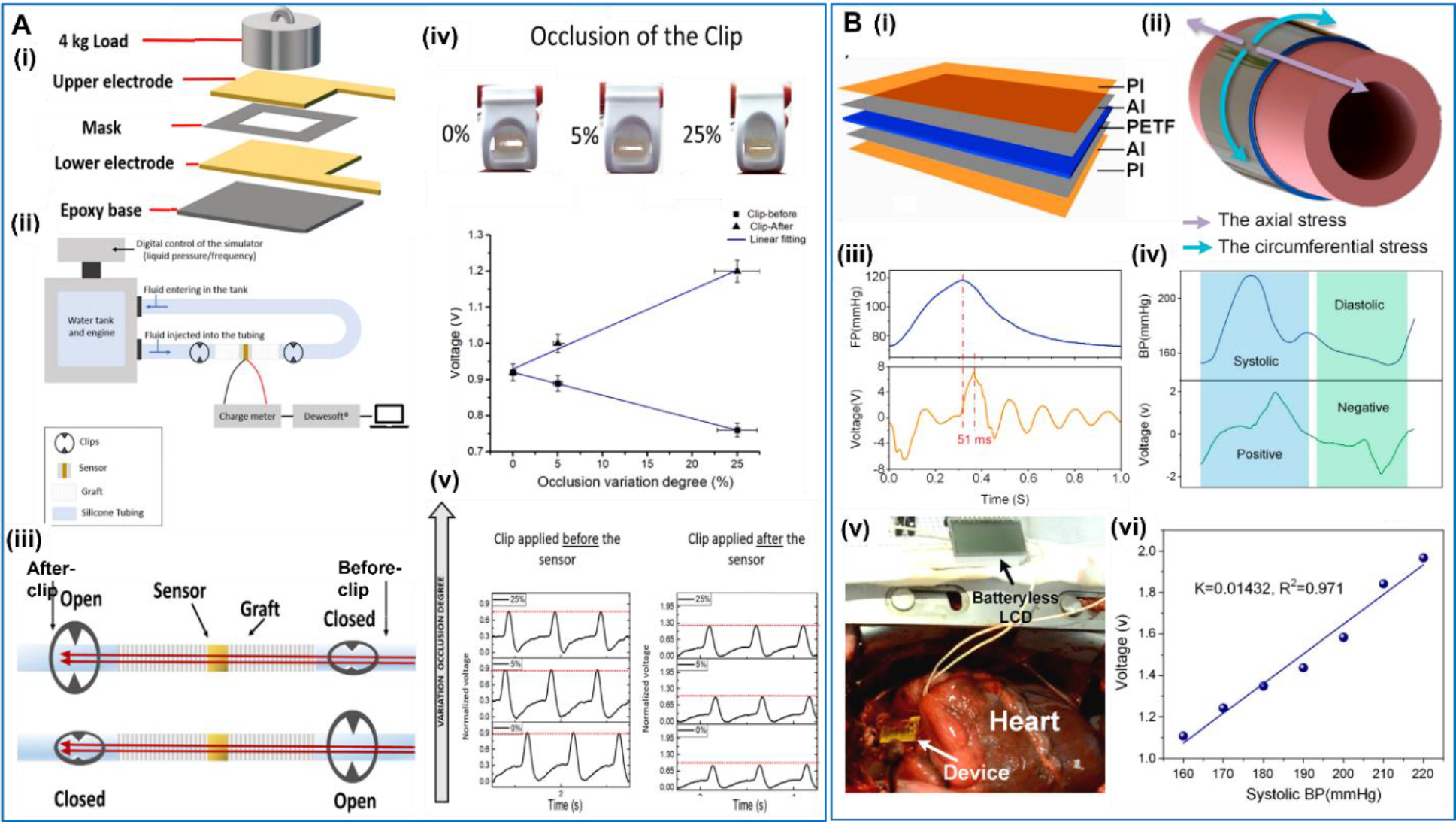
A. Piezoelectric biosensor patch. (i) Setup for fabricating the piezoelectric composites. (ii) Sketch of the setup simulating the cardiovascular system. (iii) Schematic of the smart graft integrated sensor, where two clips are applied prior and after the graft and individually closed to simulate the presence of a clot occlusion. (iv) Voltage amplitude change versus occlusion variation for the before-clip and after-clip. (v) A change in the pressure sensed by the graft when different occlusion applied before and after the sensor. Reproduced with permission [109]. Copyright 2022, Elsevier Ltd. B. Implantable and self-powered blood pressure monitoring patch. (i) Exploded view of the device showing the multilayer thin film structure. (ii) The circumferential stress in expanded aorta wall. (iii) Waveform comparison of the blood pressure with output voltage of the device. (iv) The waveforms of the output voltage and filling pressure (FP) in a single cycle. (v) Photograph of the implanted patch in vivo. (vi) The measured peak output voltage at different systolic blood pressure (BP) and linear fits of the results. Reproduced with permission [110]. Copyright 2016, ElsevierLtd.
Due to the relatively simple design of patch, many different kinds of piezoelectric materials have been applied to the development of piezoelectric patches for blood pressure and vessel monitoring, such as PVDF, PVDF-TrFE, and even piezoelectric ceramics (e.g. AlN). They all showed high sensitivity and reliable mechanical performance. Cheng et al reported an implantable and self-powered blood pressure monitor based on a piezoelectric thin film [110] (Figure 7B). The device was fabricated with a 200 μm piezoelectric thin film of polarized PVDF which was sandwiched by compliant aluminum thin layers as electrodes, and the whole device was encapsulated in two polyimide films. The device was designed to be wrapped on the surface of the aorta and could output alternating current continuously in an external circuit. In the in vivo test, the voltage output responded well to the blood pressure fluctuation, and the systolic signal and diastolic signal leaded to the positive and negative voltage pulses, respectively. The delay time of the voltage was only ~51 ms, showing a great responsibility of the sensor.
For transient applications, bioresorbable materials have been explored as an implantable sensor that only functions for a designed time period, and then degrades and is absorbed locally. Ouyang et al proposed a kind of implantable bioresorbable self-powered sensor based on the triboelectric effect (BTS) [111] (Figure 8A). One poly(lactic acid)–(chitosan 4%) (PLA/C) film with a nanostructured surface was used as the triboelectric layer. Mg was deposited on the other PLA/C film serving as the electrode. All these materials are biodegradable and bioresorbable. The sensor was attached to the vascular wall, and the ambulatory blood pressure signal obtained by the device showed a good consistency with commercial blood pressure sensors. Abnormal vascular occlusion situations could also be detected by the device by identifying abnormal drop of blood pressure. This device has a potential of monitoring the prognosis during cardiovascular surgery. Tan et al. also presented a design of a perforated piezoelectric tube which might be applied as a self-powered smart stent for real-time blood pressure monitoring [112] (Figure 8B). The device was made from a PVDF film with a perforation design to improve its mechanical compatibility. Both sides of the PVDF film were covered by a submicron-thick metal film as electrodes, which gave a total thickness of 100 μm. The ultrathin structure allowed the device being able to wrap the artery, and harvest energy from the blood pressure changes in the artery.
Figure 8. Blood vessel patches with novel designs.
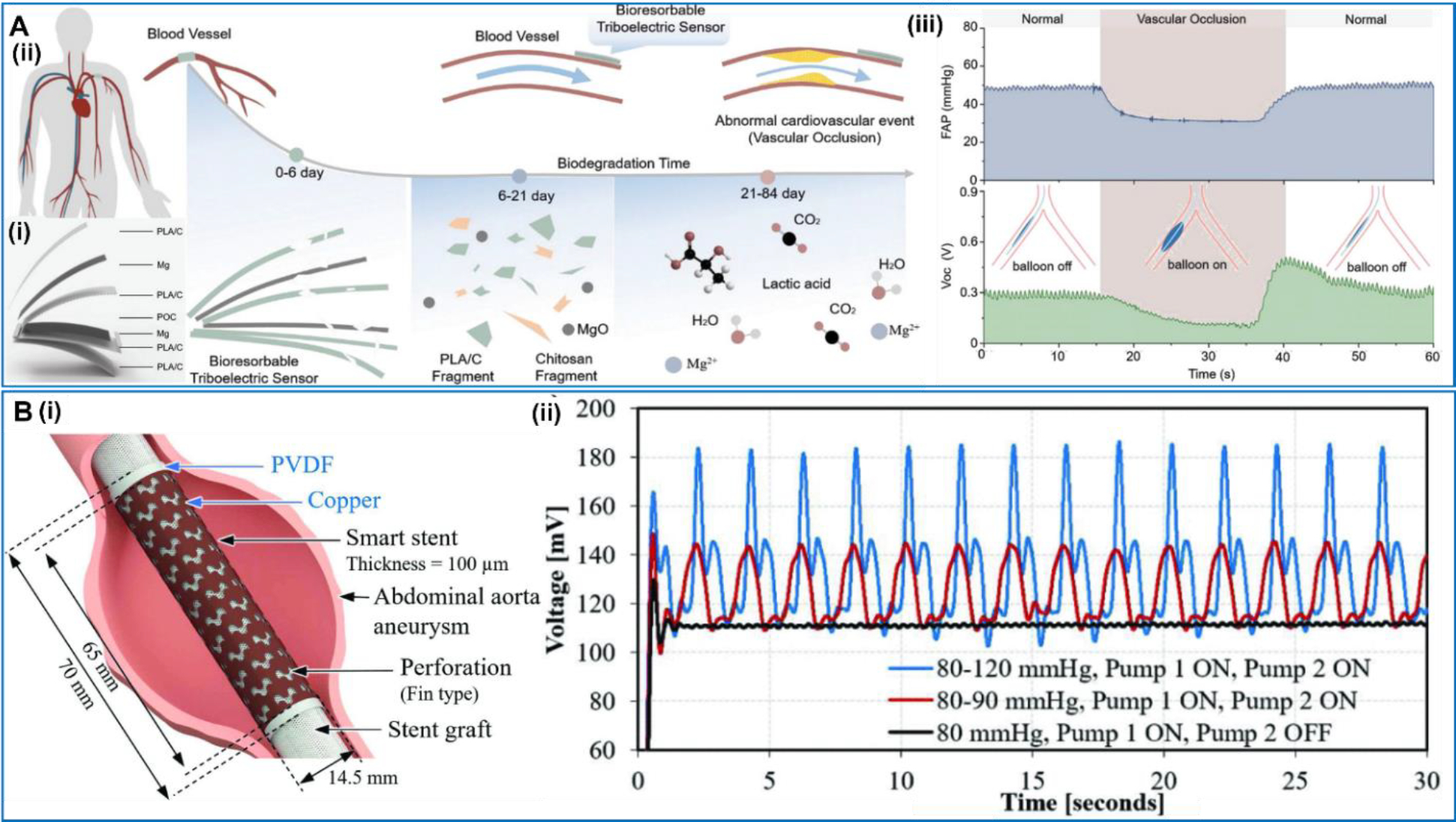
A. Bioresorbable dynamic pressure sensor patch. (i) Structure of the BTS. (ii) Illustration of the BTS. (iii) Simulated abnormal vascular occlusion events are detected by the BTS. Reproduced with permission [111]. Copyright 2021, Wiley-VCH GmbH. B. Smart patch based on a perforation design. (i) Conceptual drawing of the smart patch mounted on a graft in an abdominal aorta aneurysm. (ii) 30-s continuous measurement of the patch at different cumulative pressure. Reproduced with permission [112]. Copyright 2023, IEEE.
In general, both the smart blood vessels and patches are able to provide the real-time monitoring of the blood vessel conditions and diagnosis of the diseases. The patches have a simpler structure and are used directly on healthy blood vessels. Their implementation may be relatively simple and application situations can be broad. However, the patch attachment may influence the movements of the vessels and may cause complications over a long term. Compared to the patches, smart vessels are a substitute of the real vessels, which have more specific medical situations to be used for. As a complete replacement, the smart vessels have a more sophisticated structure and working mechanism. They also need to satisfy other requirements to meet a normal vessel function, such as mechanical properties. Therefore, a more comprehensive and longer time development before clinical trials can be expected for smart vessels. Despite the relatively straightforward structure and functionality, both artificial blood vessel and patches are mainly studied in vitro so far. This is possibly due to the high requirements of in vivo experiments for blood vessel grafting. While most current studies are primarily focusing on the functionality, the equally important parameters that require in-depth studies include biocompatibility, flexibility and long-term stability. Nevertheless, it is unequivocal that multifunctional artificial blood vessels hold significant promises for future biomedical engineering solutions treating cardiovascular diseases.
Wearable Blood Sensors.
As an essential component in the cardiovascular systems, blood carry many vital signals that can reflect the health conditions to a large extent [113]. As mentioned earlier, blood pressure is one of the critical health parameters that need to be monitored regularly [114]. High blood pressure is the lead risk factor for arteriosclerosis, cerebrovascular disease, heart disease, retinal damage, and renal failure [115]. In addition, blood oxygen, glucose levels and many other parameters from blood need to be regularly monitored particularly for patients with chronic diseases, such as diabetes [116,117]. These health factors are often sensed externally from the body, where a wearable device is becoming a prevailing strategy for achieving daily monitoring. As a unique self-powering technique, NG also found broad application potentials in this group of wearable sensors, enabling battery-free and self-sustainable device designs.
Wearable blood pressure sensors.
NG-based wearable blood pressure sensors devices have been widely studied recent years as a representative self-powered wearable medical device. In 2018, a weaving constructed self-powered pressure sensor for continuous and cuffless measurement of blood pressure was reported by Meng et al [118] (Figure 9A). The device was based on a contact-separation TENG and held a multilayer structure which was inspired the common textile for clothes with traditional woven patterns. A layer of PTFE strips with interlaced woven structure was placed above a PET substrate, acting as an electrification layer. The weaving structure was designed to offer a high sensitivity, and the device could respond to small pressure changes in no more than 5 ms. The device was integrated with a signal management circuits for real-time blood pressure measurements. Two sensor devices were placed at the fingertip and behind the ear respectively to detect human pulse waves, and the collected signals could be used to calculate the systolic and diastolic blood pressure. The measurement results showed a good consistency with a cuff-based electronic sphygmomanometer, evidencing the potential for practical applications. Other than the weaving configuration, the conical or pyramid microstructure was also commonly used for pressure sensing in TENG design. Chen et al reported a hierarchical elastomer microstructure (HEM)-based TENG by using a two-stage conical PDMS configuration [119] (Figure 9B). The HEM was designed to serve as supporting structure to generate a highly compressible and tunable gap for achieving a high sensing performance. The ultrathin fluorinated ethylene propylene (FEP)/Ag film served as the other triboelectric layer. The ultrasmall thickness together with the microstructure-supported airgap enabled a conformal attachment of the device to the largely-curved skin surface at the wrist. Its high sensitivity allowed a continuous monitoring of the arterial pulse waveform caused by the faint motions of vascular vessels, which could reflect the artery and heart condition. Based on the measured pulse waveforms, the diastolic blood pressure (DBP) and systolic blood pressure (SBP) could be calculated by the oscillometric blood pressure measurement or the pulse transient time (PTT) analysis. The calculated blood pressure values matched well with the values measured by commercial sphygmomanometer.
Figure 9. Wearable blood pressure sensors.
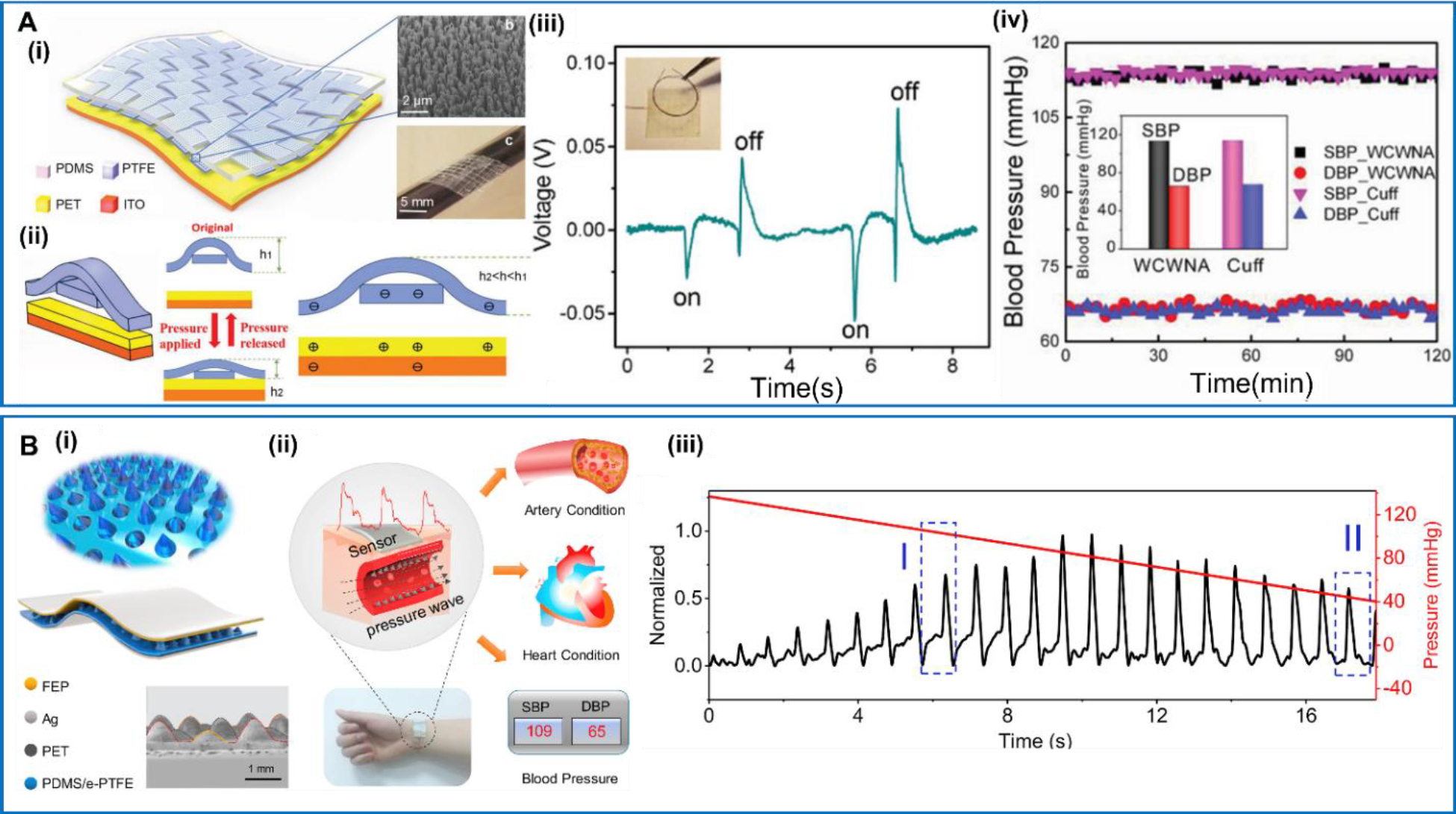
A. Flexible weaving constructed self-powered pressure sensor. (i) Schematic of the pressure sensor and its weaving pattern. (ii) Schematic of the cross-sectional view and the electrical signal generation process. (iii) The response of the sensor under tiny pressures. (iv) Systolic blood pressure and diastolic blood pressure measurements. Reproduced with permission [118]. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. B. Hierarchical elastomer tuned self-powered pressure sensor (HSPS). (i) Schematic structure of HSPS. (ii) The HSPS for cardiovascular motoring. (iii) The pulse waveform, relative pulse wave amplitude and corresponding cuff pressure during deflating process. Reproduced with permission [119]. Copyright 2020, Elsevier Ltd.
Glucose sensors.
Real-time monitoring of the blood glucose level is a critical need for diabetic patents, and there are quite a few wearable glucose sensors becoming commercially available recently with limited lifetime. Alleviating from battery reliance would be promise to further improve the devices’ lifetime and compatibility. In 2016, Xue et al provided a strategy to fabricate a self-powered electronic skin for detecting glucose level in body fluid [120] (Figure 10A). The e-skin includes a graphene oxidase (GOx) @ZnO nanowire array as the sensing functional material. Ti and Al layers were used as the two electrodes supported by elastic PDMS layers. The whole device was supported by a Kapton film. The sensing was realized by the piezo-enzymatic-reaction coupling effect on the GOx@ZnO nanowire arrays. By immersing the nanowires in the glucose aqueous solution, the enzyme reaction between GOx and glucose would lead to the production of the H+ carriers and electrons. These charged species on the nanowire surface would screen the piezoelectric effect from the ZnO nanowires when they were under a certain applied force. Therefore, the piezoelectric voltage output could be reduced as the glucose concentration increases in the fluid, so that the blood glucose level could be determined. Based on similar principle, Zhang et al implanted a self-powered implantable skin-like glucometer into rats’ abdomen to monitor the blood glucose level [121] (Figure 10B). The device was assembled by positioning parallel ZnO nanowires (along the same direction) onto Ti interdigitated electrodes on a soft Kapton substrate. In the in vivo test, a constant force of 4 N was applied to drive the TENG when the rats were in anesthesia. Under a constant strain, the piezoelectric voltage output obviously decreased from 0.16 V to 0.075 V after injection of glucose solution, suggesting the feasibility of NG-based glucometer. Using a ZnO nanowire-based TENG as the glucose sensor, Yang et al designed a self-powered closed-loop brain-machine-interface system, which could realize real-time detecting and rapidly adjusting blood glucose concentration [122] (Figure 10C). The flexible glucose sensor was made of enzyme/ZnO nanowire arrays and self-powered by the body motion. The output could be treated as the glucose-detecting signal through the biosensing-piezoelectric coupling effect, which allowed active monitoring of the saliva glucose concentration in real time. Another TENG used in the system to power a micro control unit and a brain stimulator, which analyzes glucose concentration and transmits the stimulation current to the electrode of the brain stimulator, and thereby adjust the blood glucose concentration. This study demonstrated a new path to integrate the NG technology into a sophisticated multifunctional system to realize complex functionalities.
Figure 10. Self-powered real-time glucose sensors.
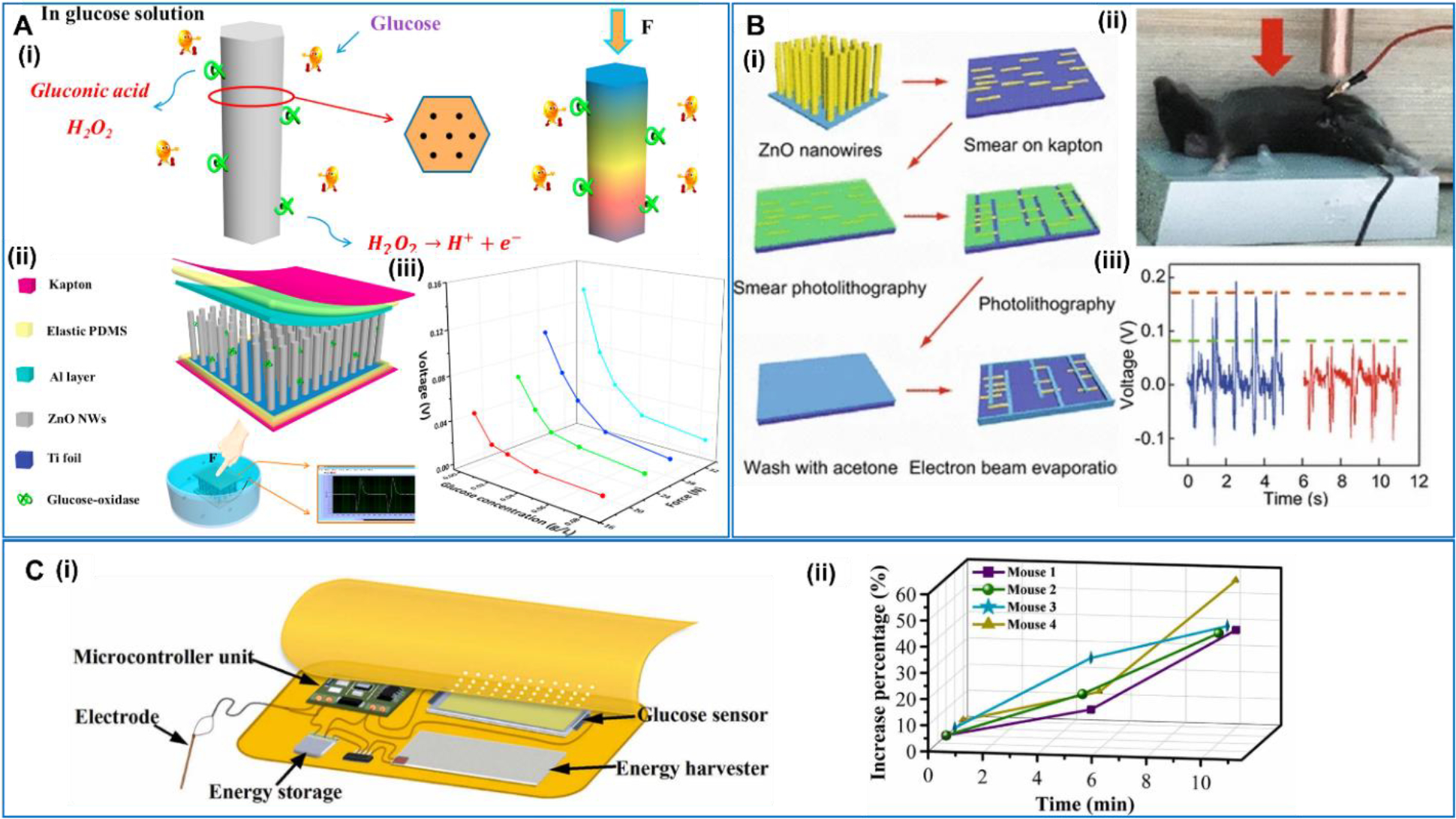
A. Self-powered electronic-skin for detecting glucose level. (i) ZnO nanowire in glucose aqueous solution and the piezoelectric output under a compressive force. (ii) Structural design of the self-powered e-skin. (iii) The relationship between the outputting piezoelectric voltage of the e-skin and glucose concentration under different forces. Reproduced with permission [120]. Copyright 2016, Elsevier Ltd. B. Self-powered implantable skin-like glucometer. (i) Device architecture, material system, and fabrication procedure. (ii) Implanting the device into the mouse and the measurement setup. (iii) The outputting piezoelectric voltage changes before and after injection of glucose solution. Reproduced with permission [121]. Copyright 2018, Springer. C. Self-powered closed-loop brain-machine-interface system. (i) The components of the system. (ii) The increase percentage of blood glucose concentration with electrical stimulation. Reproduced with permission [122]. Copyright 2021, Elsevier Ltd.
Blood oxygen sensors.
While the blood oxygen level is usually monitored optically, the powering part could also be replaced by the NG technology to achieve a self-powered operation. In 2019, Chen et al reported a TENG-based self-powered blood oxygen monitor system [123] (Figure 11A). The system was composed of a flexible TENG, an energy storage unit, and a blood oxygen detector unit. The TENG device was comprised of a crumpled Au electrode and a PDMS triboelectric layer. The rough microstructured surfaces of the electrode and PDMS enhanced the output power, which could charge the battery to 2.0 V from 1.9 V in ~3 h. The battery was connected to red and IR LEDs, serving as the blood oxygen detector. The whole device was supported on a thumbnail PI substrate. Supported by the TENG, a stable photoplethysmography (PPG) signal was achieved from the LEDs to calculate the oxyhemoglobin saturation (SpO2). The SpO2 is defined as the ratio between the concentration of hemoglobin (HbO2) over the total concentration of HbO2 and deoxyhemoglobin (Hb) using the light absorption intensities via the Lambert–Beer law [124]. In 2021, Chen et al designed another TENG-based device for the blood oxygen monitoring [125] (Figure 11B). The stretchable TENG was built on a crumpled poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS)/porous carbon structure. The PEDOT: PSS/porous carbon was spin-coated onto a pre-stretched Ecoflex film acting as the stretchable TENG electrode. The TENG was fixed on the finger pulp and converted the finger movements into electricity to power a supercapacitor, which was connected to the LEDs for blood oxygen measurement. In general, the integration of TENG makes self-powering possible. Nevertheless, the use of LEDs typically requires higher energy compared to other electronic sensor systems. More applicable demonstrates would still require higher power output, which could drive the power source to the usable energy level within a shorter time to realize a meaningful real-time monitoring.
Figure 11. TENG-based blood oxygen sensors.
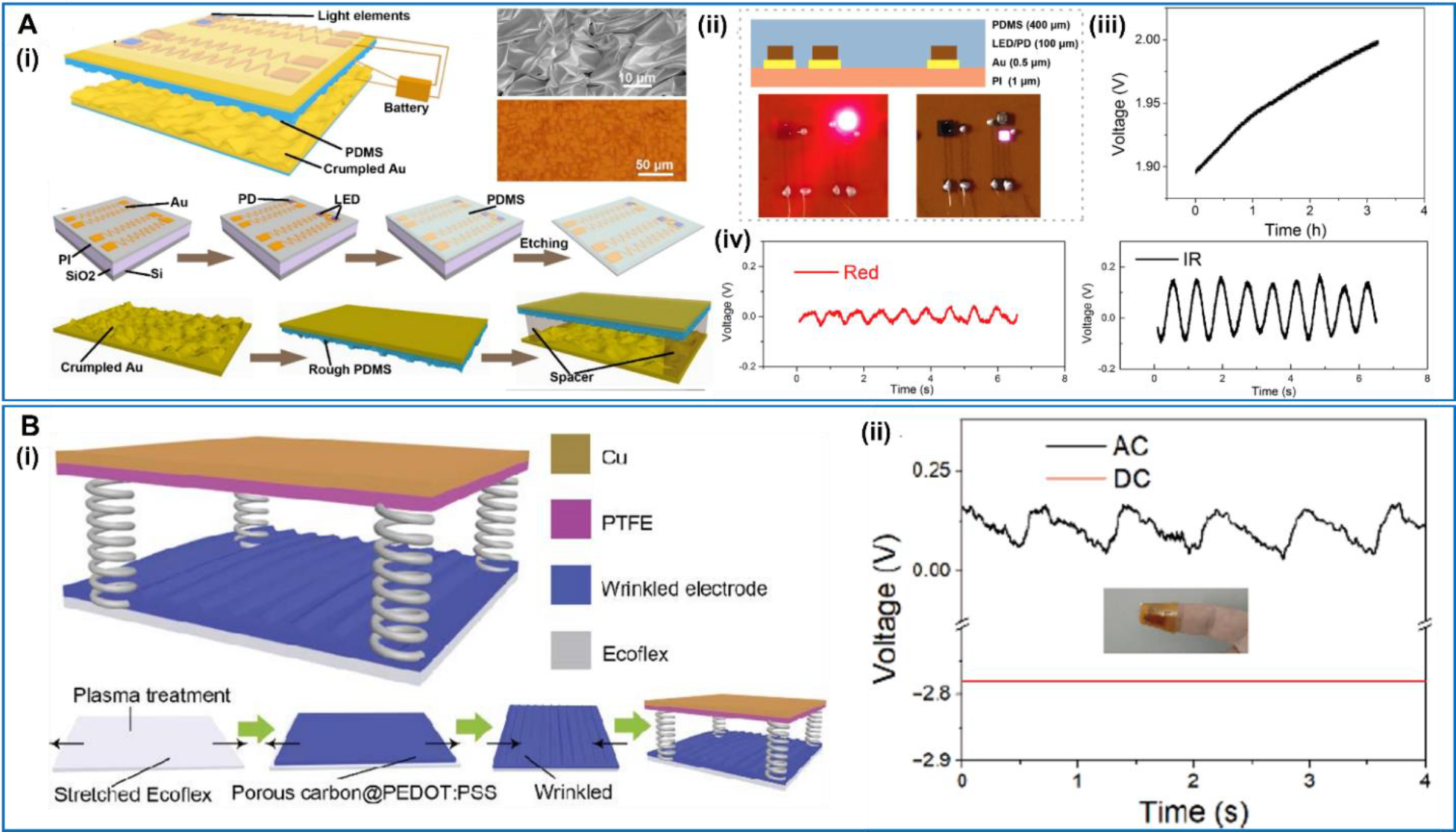
A. Self-Powered flexible blood oxygen monitoring system. (i) The schematic illustration, SEM image and the fabrication process of the self-powered flexible blood oxygen monitoring system. (ii) The image of the flexible device structure. (iii) The charging curve of the battery charged by the TENG. (iv) The blood oxygen signal of the red LED and the IR LED. Reproduced with permission [123]. Copyright 2019, by the authors. Licensee MDPI, Basel, Switzerland. B. Wearable SpO2 monitoring system. (i) The schematic device structure and the fabrication process of TENG. (ii) The PPG signal measured by the red LED. Reproduced with permission [124]. Copyright 2021, Tsinghua University Press and Springer-Verlag GmbH Germany, part of Springer Nature.
Prospective and Conclusion
As one of the most promising candidates for replacing the batteries in future electronics, NG technology has experienced tremendous developments in the last decade. Applications of NGs in medical devices have shown particular promises for enabling self-powered sensing and care-free real-time monitoring. In order to bring this intriguing concept to a practical system, there are still a few critical challenges need to be addressed in future technology development.
Biocompatibility.
As a kind of devices which are implanted in vivo or directly contact the skin, NG-based cardiovascular sensors and monitors require high biocompatibility to prevent inflammations and complications. Most of current researches indeed focused on using flexible, biocompatible and even biodegradable materials for device developments. However, in order to support the long-term in vivo test and particularly clinical translation, more rigorous tests are still needed. Currently, most of the biocompatibility tests were conducted in the controlled lab environment, which may be oversimplified and ignore the complex condition in vivo. Sometimes, the biocompatibility of the materials does not necessarily support the in vivo compatibility of the device, as more fabrication and processing processes may involve additional contaminations. In addition, long-term biocompatibility tests, spinning months to years, are conspicuously lacking in almost all researches [126]. While such a long-term test is very time consuming, it may become necessary to standardize an accelerated approach to expedite the biocompatibility test in a faster pace for device developments. In general, biocompatibility remains a critical aspect that must be proved prior to the commercialization of the implantable devices. Along this direction, it’s also favorable to discover more biocompatible materials. Some nature-derived biomaterials, such as amino acids, peptides, collagens, and silks, have now been increasingly used in NG designs to leverage their piezoelectric or triboelectric properties. Besides, we envision that the packaging materials may provide an ultimate solution to biocompatibility matter, because it is needed by almost all devices. So far, research efforts in the developments of biocompatible packaging materials are rather slim. Further endeavors are required to strike a balance between device output performance and biocompatibility in the complete device assembly and packaging [127].
Electrical energy output enhancement.
Though the energy requirements of most sensor devices are relatively small and can be satisfied by many NG designs, the electrical energy output is still the top priority for NGs that are developed for sensor applications. This is because in order to achieve the complete function of sensing and monitoring, the sensor elements usually need to be associated with complex electronic systems for data and signal processing and transmission. Therefore, the power requirement of a fully self-powered monitoring system can be much more beyond a single sensing element would require. While the majority of current research on NG-based sensor device is still focused on single sensor element, the trend is moving toward more complete sensor or monitoring systems, which may include components for power management, data storage, wireless transmission and adaptive control. Accordingly, to serve these comprehensive purposes, NGs will need to be more powerful to support the operation of all electronics. This falls into the general strategies for NG output power improvements, which have been reviewed in many other articles [30,34,128–132] Considering the sensors are interfaced with human body or organs, materials development is particularly important. In addition to the high electromechanical conversion efficiency, the materials and device structures should be specifically designed according the motion patterns, rates and amplitudes of the interfacing body parts. Besides, the power management of the NG system is also important. Given the outputs of human-driven NGs are typically pulsatile, small and unstable [133], specially designed electronic systems are needed to efficiently convert and store the electrical energy with minimal losses. Meanwhile, they still need to keep the overall flexibility and wearability or implantability. It is good to see some development of power management systems have been investigated for NGs in general. However, more improvements or complete redesigns may still be required in order to fit the wearable and implantable devices as discussed above.
Miniaturization.
Similar to all implantable or wearable devices, size and mass is a critical issue to be considered for NGs used for self-powered sensor and monitor systems. As these NG devices are directly interacting with the body or tissue movements, their size and mass should be specifically designed to fit the placement conditions and do not disturb the normal movements or function of the interfacing or surrounding tissues or organs. For example, for NGs placed on tissue surfaces, such as hearts and blood vessels, a flat geometry is preferred and the thickness should ideally be in the range of a few millimeters. For wearable devices, the state-of-the-art sensor technology only has a few grams in weight for the entire device [134], which introduces minimal influences to the body. Considering the power output of a NG is directly related to the volume (piezoelectric) or surface area (triboelectric), how to achieve high energy density within the limited size and mass frames will be the key direction of future development. In this regard, advances in materials science, flexible microelectronic technology, and micro- or nano-processing strategies may provide many opportunities to develop smaller, lighter, and more powerful NGs for next generation self-powered cardiovascular monitoring devices.
Matching mechanical property.
While current wearable and implantable NG devices are aimed at flexible and stretchable systems, their mechanical moduli are mostly at the level of sub-GPa [135], [136]. This is still a few orders of magnitude higher than the moduli of human tissues and organs (such as muscles, blood vessels, skins, hearts, diaphragms, stomach, etc.) [137]. The large difference inhibits the formation of an intimate biotic-abiotic interface, and effective transfer of mechanical motions from tissue to the device. Minimizing this mechanical discrepancy at the interface between the NG and the host organism may alleviate deleterious immune reactions during long-term implantation periods, as well as achieve more efficient mechanical-to-electric energy conversion efficiency. To further bringing the mechanical modulus of NG devices down to the tissue level, new strategies need to be implemented beyond the general materials and structure engineering approaches being practiced nowadays. For example, the pursuit of further structural engineering at the micro- to nano-scale may further optimize directional mechanical properties without jeopardizing the electromechanical coupling performance. It is also of great scientific significance to design, synthesis and fabrication of new piezoelectric and triboelectric materials that offer tunable mechanical properties with high electromechanical coupling behavior. These discoveries should be driven by new understandings of molecular and atomic interactions from large molecules, bio molecules or organic/inorganic hybrid systems. Researchers would largely rely on current powerful molecular/ atomic simulations and machine learning techniques to facilitate the materials innovation and engineering.
Packaging.
Packaging, or encapsulation is equally important as the mechanical property tuning, as it also determines the device overall performance and stability. In most cases, it is the packaging material that directly interfaces the tissue surface and transfers the mechanical displacement. The packaging material is also an inevitable component for implantable or wearable medical devices, because the operation environment is full of fluids or with high humidity, which is fatal for the operation of NGs and electronics. Same as the core components, the packaging material also needs to be flexible and light-weighted, in addition to its desired waterproof capability. Therefore, the size and mechanical property constrains discussed above also apply to the packaging materials. More importantly, different from other implantable or wearable devices, the operation of NGs requires constant movements together with the hosting tissue (e.g. stretching and bending). This introduces an additional constrain to the packaging materials, i.e. their primary waterproof and electrical insulating function should remain stable under the large dynamic straining conditions over lifetime. So far, the main selections of the packaging materials are still conventional polymers like EcoFlex, PDMS and Parylene [138,139]. These materials are either too rigid compared to the tissues, or has a poor water resistivity when strained. A recent study revealed a polymer composite that can satisfy both mechanical property and water resistance[135]. This new materials development suggested a path to meet the new requirements of packaging materials for implantable and wearable NG-based systems. It is expected to see more materials innovations along this highly-underdeveloped direction in the near future.
In conclusion, the emergence of NG and self-powered technology aligns seamlessly with the development of wearable and implantable cardiovascular sensors and monitors. These devices are now undergoing an incessant transformation, with the latest generation of self-powered CEDs exhibiting enhanced outputs, improved safety, increased durability, and heightened functional complexity compared to their predecessors. These advancements offer the potential for achieving greater lifespan, enhanced miniaturization, improved human biomechanical adaptability, increased sensitivity, and mobile data processing. Adapting the NG technology for self-powered electronic medical devices can be in three stages. In the first stage, nanogenerators can be as a complementary power source of traditional batteries. As the outputs enhance, the nanogenerators can replace the batteries completely at the second stage. And at the third stage, nanogenerators can be the power source as well as the sensor at the same time. Looking forward, it is highly plausible that self-powered implantable or wearable medical sensors and electronic devices will become predominant modalities in the field of cardiovascular diagnosis and treatment.
Table 1.
Sensors for heart activities monitoring
| Function | Device Name | Technical Highlights | Citation |
|---|---|---|---|
| Implantable NG-based heart activities sensors | Implantable TENG cardiac monitor | Real-time wireless remote cardiac monitoring | 78 |
| Self-powered endocardial pressure sensor | Ultrahigh sensitivity; High mechanical stability | 79 | |
| No-spacer TENG-based monitor | No spacer; More uniform stress/strain distributions | 80 | |
| Wearable NG-based heart activities sensors | ZnSnO3-SMC-P(VDF-TrFE)-based pulse sensor | Surface-modified carbon nanotubes; Significant enhancement output | 85 |
| Self-powered flexible pulse sensor | Ultra-thin; Conformal contact with the skin | 87 | |
| Self-powered sensor unit | 2D materials | 90 | |
| Self-powered ultrasensitive pulse sensor | Excellent long-term performance; Real-time monitoring; High peak signal-noise ratio | 92 | |
| Expandable microsphere-based nanogenerators | Low-cost and simple fabrication process; Ultrahigh sensitivity | 93 | |
| Lever-inspired contact separation TENG | Controllable output voltage | 94 |
Table 2.
Sensors and monitors for blood vessels
| Function | Device Name | Technical Highlights | Citation |
|---|---|---|---|
| Smart blood vessels | Multifunctional elastomer composites | Good mechanical properties; Multi-layered structure | 105 |
| ePTFE/PHB TENG- based vascular graft | Excellent pressure sensitivity; Supporting tissue regeneration; Detecting hemodynamic conditions | 106 | |
| 3D-printing in situ-poled ferroelectric artificial arteries | 3D printing technology; Superb piezoelectric performance; Desirable mechanical modulus | 107 | |
| Small-caliber vascular graft | Spray deposition technology; Great mechanical properties | 108 | |
| Blood vessel patches | Self-monitoring biosensor for artificial graft | High piezoelectric sensitivity; Low elastic modulus | 109 |
| PETF-based implantable and self-powered monitor | High sensitivity; High output performance in vivo; Monitoring the hypertension status | 110 | |
| Bioresorbable triboelectric sensor | Antibacterial bioresorbable material; Identifying abnormal vascular occlusion; Good durability | 111 | |
| Perforation-designed smart stent | Unique perforation design | 112 |
Table 3.
Wearable blood sensors
| Function | Device name | Technical highlights | Citation |
|---|---|---|---|
| Wearable blood pressure sensors | Weaving constructed self-powered pressure sensor | Ultrafast response; Long-term stability; Low power consumption | 118 |
| Flexible hierarchical elastomer tuned pressure sensor | Wide pressure range; Hierarchical microstructures; High signal noise ratio | 119 | |
| Glucose sensors | Self-powered electronic skin | Nanowire arrays; Conformal contact with the skin | 120 |
| Self-powered implantable skin-like glucometer | Real-time detection; In vivo test in the mouse | 121 | |
| Self-powered closed-loop brain-machine-interface system | Adjusting blood glucose concentration | 122 | |
| Blood oxygen sensors | Self-powered blood oxygen monitoring system | High flexibility; Detecting PPG signal | 123 |
| Performance-enhanced wearable SpO2 monitor | Stretchable electrodes; Simple and cost-effective fabrication method | 124 |
Acknowledgement:
This work is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL157077. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reference
- [1].Okwuosa IS, Lewsey SC, Adesiyun T, Blumenthal RS, Yancy CW, Worldwide disparities in cardiovascular disease: Challenges and solutions. Int. J. Cardiol. 202 (2016) 433–440. https://www.ncbi.nlm.nih.gov/pubmed/26433167. [DOI] [PubMed] [Google Scholar]
- [2].Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. 141 (2020) e139–e596. 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- [3].Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang N-Y, Yaffe K, Martin SS, Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. 145 (2022) e153–e639. 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- [4].Kaminsky LA, German C, Imboden M, Ozemek C, Peterman JE, Brubaker PH, The importance of healthy lifestyle behaviors in the prevention of cardiovascular disease. Progress in Cardiovascular Diseases 70 (2022) 8–15. [DOI] [PubMed] [Google Scholar]
- [5].Li J, Wang X, Research Update: Materials design of implantable nanogenerators for biomechanical energy harvesting. APL Mater 5 (2017). https://www.ncbi.nlm.nih.gov/pubmed/29270331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Global C Burden of Cardiovascular Diseases, Roth GA, Johnson CO, Abate KH, Abd-Allah F, Ahmed M, Alam K, Alam T, Alvis-Guzman N, Ansari H, Arnlov J, Atey TM, Awasthi A, Awoke T, Barac A, Barnighausen T, Bedi N, Bennett D, Bensenor I, Biadgilign S, Castaneda-Orjuela C, Catala-Lopez F, Davletov K, Dharmaratne S, Ding EL, Dubey M, Faraon EJA, Farid T, Farvid MS, Feigin V, Fernandes J, Frostad J, Gebru A, Geleijnse JM, Gona PN, Griswold M, Hailu GB, Hankey GJ, Hassen HY, Havmoeller R, Hay S, Heckbert SR, Irvine CMS, James SL, Jara D, Kasaeian A, Khan AR, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Lal D, Larsson A, Linn S, Lotufo PA, Magdy Abd El Razek H, Mazidi M, Meier T, Mendoza W, Mensah GA, Meretoja A, Mezgebe HB, Mirrakhimov E, Mohammed S, Moran AE, Nguyen G, Nguyen M,Ong KL, Owolabi M, Pletcher M, Pourmalek F, Purcell CA, Qorbani M, Rahman M, Rai RK, Ram U, Reitsma MB, Renzaho AMN, Rios-Blancas MJ, Safiri S, Salomon JA, Sartorius B, Sepanlou SG, Shaikh MA, Silva D, Stranges S, Tabares-Seisdedos R, Tadele Atnafu N, Thakur JS, Topor-Madry R, Truelsen T, Tuzcu EM, Tyrovolas S, Ukwaja KN, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Weintraub R, Wolfe C, Workicho A, Xu G, Yadgir S, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Zaidi Z, Zaki MES, Zipkin B, Afshin A, Gakidou E, Lim SS, Mokdad AH, Naghavi M, Vos T, Murray CJL, The Burden of Cardiovascular Diseases Among US States, 1990–2016. JAMA Cardiol 3 (2018) 375–389. https://www.ncbi.nlm.nih.gov/pubmed/29641820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC, Virani SS, Williams KA, Yeboah J, Ziaeian B, 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. 140 (2019) e596–e646. 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Choi H, Lee D-K, Han M-K, Janani G, Surendran S, Kim JH, Kim JK, Cho H, Sim U, Review—Non-Noble Metal-Based Single-Atom Catalysts for Efficient Electrochemical CO2 Reduction Reaction. Journal of The Electrochemical Society 167 (2020) 164503. 10.1149/1945-7111/abc593. [DOI] [Google Scholar]
- [9].Bitkina OV, Kim HK, Park J, Usability and user experience of medical devices: An overview of the current state, analysis methodologies, and future challenges. International Journal of Industrial Ergonomics 76 (2020) 102932. https://www.sciencedirect.com/science/article/pii/S0169814118305316 https://www.sciencedirect.com/science/article/pii/S0169814118305316?via%3Dihub. [Google Scholar]
- [10].Laslett Lawrence J, Alagona P, Clark Bernard A, Drozda Joseph P, Saldivar F, Wilson Sean R, Poe C, Hart M, The Worldwide Environment of Cardiovascular Disease: Prevalence, Diagnosis, Therapy, and Policy Issues. J. Am. Coll. Cardiol. 60 (2012) S1–S49. 10.1016/j.jacc.2012.11.002 https://www.sciencedirect.com/science/article/pii/S0735109712053715?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- [11].Ricotta JJ, Pagan J, Xenos M, Alemu Y, Einav S, Bluestein D, Cardiovascular disease management: the need for better diagnostics. Med Biol Eng Comput 46 (2008) 1059–1068. 10.1007/s11517-008-0416-x . [DOI] [PubMed] [Google Scholar]
- [12].Chen S, Qi J, Fan S, Qiao Z, Yeo JC, Lim CT, Flexible Wearable Sensors for Cardiovascular Health Monitoring. Advanced Healthcare Materials 10 (2021) 2100116. 10.1002/adhm.202100116 . [DOI] [PubMed] [Google Scholar]
- [13].Bayoumy K, Gaber M, Elshafeey A, Mhaimeed O, Dineen EH, Marvel FA, Martin SS, Muse ED, Turakhia MP, Tarakji KG, Elshazly MB, Smart wearable devices in cardiovascular care: where we are and how to move forward. Nature Reviews Cardiology 18 (2021) 581–599. 10.1038/s41569-021-00522-7 https://www.nature.com/articles/s41569-021-00522-7.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rawat J, Sajwan D, Garimella SV, Sharma H, Dwivedi C, Boron Nitride quantum dots: A rising star in sensing applications. Nano Trends 2 (2023) 100008. https://www.sciencedirect.com/science/article/pii/S2666978123000065. [Google Scholar]
- [15].Baig MM, GholamHosseini H, Moqeem AA, Mirza F, Lindén M, A Systematic Review of Wearable Patient Monitoring Systems – Current Challenges and Opportunities for Clinical Adoption. J. Med. Syst. 41 (2017) 115. 10.1007/s10916-017-0760-1. [DOI] [PubMed] [Google Scholar]
- [16].Baek S, Lee Y, Baek J, Kwon J, Kim S, Lee S, Strunk K-P, Stehlin S, Melzer C, Park S-M, Ko H, Jung S, Spatiotemporal Measurement of Arterial Pulse Waves Enabled by Wearable Active-Matrix Pressure Sensor Arrays. ACS Nano 16 (2022) 368–377. 10.1021/acsnano.1c06695. [DOI] [PubMed] [Google Scholar]
- [17].Mishra S, Mohanty S, Ramadoss A, Functionality of Flexible Pressure Sensors in Cardiovascular Health Monitoring: A Review. ACS Sensors 7 (2022) 2495–2520. 10.1021/acssensors.2c00942. [DOI] [PubMed] [Google Scholar]
- [18].Guo Y, Liu X, Peng S, Jiang X, Xu K, Chen C, Wang Z, Dai C, Chen W, A review of wearable and unobtrusive sensing technologies for chronic disease management. Computers in Biology and Medicine 129 (2021) 104163. https://www.sciencedirect.com/science/article/pii/S0010482520304947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huang J-D, Wang J, Ramsey E, Leavey G, Chico TJA, Condell J. Applying Artificial Intelligence to Wearable Sensor Data to Diagnose and Predict Cardiovascular Disease: A Review Sensors [Online], 2022. [DOI] [PMC free article] [PubMed]
- [20].Kaszala K, Ellenbogen KA, Device Sensing. Circulation 122 (2010) 1328–1340. 10.1161/CIRCULATIONAHA.109.919704. [DOI] [PubMed] [Google Scholar]
- [21].Sideris S, Archontakis S, Dilaveris P, Gatzoulis KA, Trachanas K, Sotiropoulos I, Arsenos P, Tousoulis D, Kallikazaros I, Leadless Cardiac Pacemakers: Current status of a modern approach in pacing. Hellenic Journal of Cardiology 58 (2017) 403–410. https://www.sciencedirect.com/science/article/pii/S1109966617300635. [DOI] [PubMed] [Google Scholar]
- [22].Deng J, Sun X, Peng H, Power supplies for cardiovascular implantable electronic devices. EcoMat 5 (2023) e12343. 10.1002/eom2.12343. [DOI] [Google Scholar]
- [23].Prieto-Avalos G, Cruz-Ramos NA, Alor-Hernández G, Sánchez-Cervantes JL, Rodríguez-Mazahua L, Guarneros-Nolasco LR. Wearable Devices for Physical Monitoring of Heart: A Review Biosensors [Online], 2022. 10.3390/bios12050292. [DOI] [PMC free article] [PubMed]
- [24].Lou Z, Wang L, Jiang K, Wei Z, Shen G, Reviews of wearable healthcare systems: Materials, devices and system integration. Materials Science and Engineering: R: Reports 140 (2020) 100523. https://www.sciencedirect.com/science/article/pii/S0927796X19301251. [Google Scholar]
- [25].Shi B, Li Z, Fan Y, Implantable Energy-Harvesting Devices. Adv. Mater. 30 (2018) e1801511. https://www.ncbi.nlm.nih.gov/pubmed/30043422. [DOI] [PubMed] [Google Scholar]
- [26].Mariello M, Heart Energy Harvesting and Cardiac Bioelectronics: Technologies and Perspectives. Nanoenergy Advances 2 (2022) 344–385. 10.3390/nanoenergyadv2040018. [DOI] [Google Scholar]
- [27].Zheng Q, Tang Q, Wang ZL, Li Z, Self-powered cardiovascular electronic devices and systems. Nat. Rev. Cardiol. 18 (2021) 7–21. https://www.ncbi.nlm.nih.gov/pubmed/32895536. [DOI] [PubMed] [Google Scholar]
- [28].Parvez Mahmud MA, Huda N, Farjana SH, Asadnia M, Lang C, Recent Advances in Nanogenerator-Driven Self-Powered Implantable Biomedical Devices. Advanced Energy Materials 8 (2018). 10.1002/aenm.201701210. [DOI] [Google Scholar]
- [29].Xia X, Liu Q, Zhu Y, Zi Y, Recent advances of triboelectric nanogenerator based applications in biomedical systems. EcoMat 2 (2020). 10.1002/eom2.12049. [DOI] [Google Scholar]
- [30].Dong X, Liu F, Wang L, Xu L, Pan H, Qi J, Nanogenerators for biomedical applications. Materials Today Communications 35 (2023). 10.1016/j.mtcomm.2023.105493. [DOI] [Google Scholar]
- [31].Yoon H-J, Kim S-W, Nanogenerators to Power Implantable Medical Systems. Joule 4 (2020) 1398–1407. 10.1016/j.joule.2020.05.003. [DOI] [Google Scholar]
- [32].Feng H, Zhao C, Tan P, Liu R, Chen X, Li Z, Nanogenerator for Biomedical Applications. Adv Healthc Mater 7 (2018) e1701298. https://www.ncbi.nlm.nih.gov/pubmed/29388350. [DOI] [PubMed] [Google Scholar]
- [33].Feng H, Zhao C, Tan P, Liu R, Chen X, Li Z, Nanogenerator for Biomedical Applications. Advanced Healthcare Materials 7 (2018) 1701298. 10.1002/adhm.201701298. [DOI] [PubMed] [Google Scholar]
- [34].Che Z, O’Donovan S, Xiao X, Wan X, Chen G, Zhao X, Zhou Y, Yin J, Chen J, Implantable Triboelectric Nanogenerators for Self-Powered Cardiovascular Healthcare. Small (2023) e2207600. https://www.ncbi.nlm.nih.gov/pubmed/36759957. [DOI] [PubMed]
- [35].Panda S, Hajra S, Mistewicz K, In-na P, Sahu M, Rajaitha PM, Kim HJ, Piezoelectric energy harvesting systems for biomedical applications. Nano Energy 100 (2022). 10.1016/j.nanoen.2022.107514. [DOI] [Google Scholar]
- [36].Wang ZL, Zhu G, Yang Y, Wang S, Pan C, Progress in nanogenerators for portable electronics. Mater. Today 15 (2012) 532–543. https://www.sciencedirect.com/science/article/pii/S1369702113700117. [Google Scholar]
- [37].Li J, Long Y, Wang X, Polymer-based Nanogenerator for Biomedical Applications. Chem. Res. Chin. Univ. 36 (2020) 41–54. 10.1007/s40242-020-9085-6. [DOI] [Google Scholar]
- [38].Cheng T, Gao Q, Wang ZL, The Current Development and Future Outlook of Triboelectric Nanogenerators: A Survey of Literature. Advanced Materials Technologies 4 (2019). 10.1002/admt.201800588. [DOI] [Google Scholar]
- [39].Fan F-R, Tian Z-Q, Lin Wang Z, Flexible triboelectric generator. Nano Energy 1 (2012) 328–334. 10.1016/j.nanoen.2012.01.004. [DOI] [Google Scholar]
- [40].Shen J, Li B, Yang Y, Yang Z, Liu X, Lim KC, Chen J, Ji L, Lin ZH, Cheng J, Application, challenge and perspective of triboelectric nanogenerator as micro-nano energy and self-powered biosystem. Biosens Bioelectron 216 (2022) 114595. https://www.ncbi.nlm.nih.gov/pubmed/35973278. [DOI] [PubMed] [Google Scholar]
- [41].Wu C, Wang AC, Ding W, Guo H, Wang ZL, Triboelectric Nanogenerator: A Foundation of the Energy for the New Era. Advanced Energy Materials 9 (2019) 1802906. 10.1002/aenm.201802906. [DOI] [Google Scholar]
- [42].Zhang C, Fan W, Wang S, Wang Q, Zhang Y, Dong K, Recent Progress of Wearable Piezoelectric Nanogenerators. ACS Applied Electronic Materials 3 (2021) 2449–2467. 10.1021/acsaelm.1c00165. [DOI] [Google Scholar]
- [43].Lu L, Ding W, Liu J, Yang B, Flexible PVDF based piezoelectric nanogenerators. Nano Energy 78 (2020) 105251. https://www.sciencedirect.com/science/article/pii/S2211285520308296. [Google Scholar]
- [44].Wang X, Piezoelectric nanogenerators—Harvesting ambient mechanical energy at the nanometer scale. Nano Energy 1 (2012) 13–24. https://www.sciencedirect.com/science/article/pii/S2211285511000085. [Google Scholar]
- [45].Zhang D, Wang D, Xu Z, Zhang X, Yang Y, Guo J, Zhang B, Zhao W, Diversiform sensors and sensing systems driven by triboelectric and piezoelectric nanogenerators. Coord. Chem. Rev. 427 (2021) 213597. https://www.sciencedirect.com/science/article/pii/S0010854520305725. [Google Scholar]
- [46].Hu Y, Wang ZL, Recent progress in piezoelectric nanogenerators as a sustainable power source in self-powered systems and active sensors. Nano Energy 14 (2015) 3–14. https://www.sciencedirect.com/science/article/pii/S2211285514002390. [Google Scholar]
- [47].Zhu G, Peng B, Chen J, Jing Q, Lin Wang Z, Triboelectric nanogenerators as a new energy technology: From fundamentals, devices, to applications. Nano Energy 14 (2015) 126–138. https://www.sciencedirect.com/science/article/pii/S2211285514002584. [Google Scholar]
- [48].Wang ZL, Triboelectric Nanogenerator (TENG)—Sparking an Energy and Sensor Revolution. Advanced Energy Materials 10 (2020) 2000137. 10.1002/aenm.202000137. [DOI] [Google Scholar]
- [49].Luo J, Gao W, Wang ZL, The Triboelectric Nanogenerator as an Innovative Technology toward Intelligent Sports. Adv. Mater. 33 (2021) 2004178. 10.1002/adma.202004178. [DOI] [PubMed] [Google Scholar]
- [50].Denning D, Kilpatrick JI, Fukada E, Zhang N, Habelitz S, Fertala A, Gilchrist MD, Zhang Y, Tofail SAM, Rodriguez BJ, Piezoelectric Tensor of Collagen Fibrils Determined at the Nanoscale. ACS Biomaterials Science & Engineering 3 (2017) 929–935. 10.1021/acsbiomaterials.7b00183. [DOI] [PubMed] [Google Scholar]
- [51].Qu X, Yang Z, Cheng J, Li Z, Ji L, Development and application of nanogenerators in humanoid robotics. Nano Trends 3 (2023) 100013. https://www.sciencedirect.com/science/article/pii/S2666978123000119. [Google Scholar]
- [52].Niu S, Wang ZL, Theoretical systems of triboelectric nanogenerators. Nano Energy 14 (2015) 161–192. https://www.sciencedirect.com/science/article/pii/S2211285514002353. [Google Scholar]
- [53].Cheng T, Gao Q, Wang ZL, The Current Development and Future Outlook of Triboelectric Nanogenerators: A Survey of Literature. Advanced Materials Technologies 4 (2019) 1800588. 10.1002/admt.201800588. [DOI] [Google Scholar]
- [54].Cao X, Xiong Y, Sun J, Zhu X, Sun Q, Wang ZL, Piezoelectric Nanogenerators Derived Self-Powered Sensors for Multifunctional Applications and Artificial Intelligence. Adv. Funct. Mater. 31 (2021) 2102983. 10.1002/adfm.202102983. [DOI] [Google Scholar]
- [55].Liu H, Zhong J, Lee C, Lee S-W, Lin L, A comprehensive review on piezoelectric energy harvesting technology: Materials, mechanisms, and applications. Applied Physics Reviews 5 (2018) 041306. 10.1063/1.5074184. [DOI] [Google Scholar]
- [56].Kim W-G, Kim D-W, Tcho I-W, Kim J-K, Kim M-S, Choi Y-K, Triboelectric Nanogenerator: Structure, Mechanism, and Applications. ACS Nano 15 (2021) 258–287. 10.1021/acsnano.0c09803. [DOI] [PubMed] [Google Scholar]
- [57].Sun M, Li Z, Yang C, Lv Y, Yuan L, Shang C, Liang S, Guo B, Liu Y, Li Z, Luo D, Nanogenerator-based devices for biomedical applications. Nano Energy 89 (2021) 106461. https://www.sciencedirect.com/science/article/pii/S2211285521007163. [Google Scholar]
- [58].Al-Suhaimi EA, Aljafary MA, Alfareed TM, Alshuyeh HA, Alhamid GM, Sonbol B, Almofleh A, Alkulaifi FM, Altwayan RK, Alharbi JN, Binmahfooz NM, Alhasani ES, Tombuloglu H, Rasdan AS, lardhi AA, Baykal A, Homeida AM, Nanogenerator-Based Sensors for Energy Harvesting From Cardiac Contraction. Frontiers in Energy Research 10 (2022). 10.3389/fenrg.2022.900534. [DOI] [Google Scholar]
- [59].Yi F, Zhang Z, Kang Z, Liao Q, Zhang Y, Recent Advances in Triboelectric Nanogenerator-Based Health Monitoring. Adv. Funct. Mater. 29 (2019) 1808849. 10.1002/adfm.201808849. [DOI] [Google Scholar]
- [60].Wang H, Cheng J, Wang Z, Ji L, Wang ZL, Triboelectric nanogenerators for human-health care. Sci Bull (Beijing) 66 (2021) 490–511. https://www.ncbi.nlm.nih.gov/pubmed/36654185. [DOI] [PubMed] [Google Scholar]
- [61].Wang C, Shi Q, Lee C, Advanced Implantable Biomedical Devices Enabled by Triboelectric Nanogenerators. Nanomaterials (Basel) 12 (2022). https://www.ncbi.nlm.nih.gov/pubmed/35458075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wang R, Sui J, Wang X, Natural Piezoelectric Biomaterials: A Biocompatible and Sustainable Building Block for Biomedical Devices. ACS Nano 16 (2022) 17708–17728. 10.1021/acsnano.2c08164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Parvez Mahmud MA, Huda N, Farjana SH, Asadnia M, Lang C, Recent Advances in Nanogenerator-Driven Self-Powered Implantable Biomedical Devices. Advanced Energy Materials 8 (2018) 1701210. 10.1002/aenm.201701210. [DOI] [Google Scholar]
- [64].Zhao L, Wong SY, Sim JY, Zhou J, Li X, Wang C, Triboelectric nanogenerators and piezoelectric nanogenerators for preventing and treating heart diseases. BMEMat 1 (2023) e12020. 10.1002/bmm2.12020. [DOI] [Google Scholar]
- [65].Quan Y, Wu X, Zhu S, Zeng X, Zeng Z, Zheng Q, Triboelectric nanogenerators for clinical diagnosis and therapy: A report of recent progress. Medicine in Novel Technology and Devices 16 (2022) 100195. https://www.sciencedirect.com/science/article/pii/S2590093522000820. [Google Scholar]
- [66].Li Z, Zheng Q, Wang ZL, Li Z, Nanogenerator-Based Self-Powered Sensors for Wearable and Implantable Electronics. Research (Wash D C) 2020 (2020) 8710686. https://www.ncbi.nlm.nih.gov/pubmed/32259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lu Y, Mi Y, Wu T, Cao X, Wang N, From Triboelectric Nanogenerator to Polymer-Based Biosensor: A Review. Biosensors (Basel) 12 (2022). https://www.ncbi.nlm.nih.gov/pubmed/35624624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mathew AA, Chandrasekhar A, Vivekanandan S, A review on real-time implantable and wearable health monitoring sensors based on triboelectric nanogenerator approach. Nano Energy 80 (2021). 10.1016/j.nanoen.2020.105566 [DOI] [Google Scholar]
- [69].Wang R, Mu L, Bao Y, Lin H, Ji T, Shi Y, Zhu J, Wu W, Holistically Engineered Polymer–Polymer and Polymer–Ion Interactions in Biocompatible Polyvinyl Alcohol Blends for High-Performance Triboelectric Devices in Self-Powered Wearable Cardiovascular Monitorings. Adv. Mater. 32 (2020) 2002878. 10.1002/adma.202002878. [DOI] [PubMed] [Google Scholar]
- [70].Wang X, Liu Z, Zhang T, Flexible Sensing Electronics for Wearable/Attachable Health Monitoring. Small 13 (2017) 1602790. 10.1002/smll.201602790. [DOI] [PubMed] [Google Scholar]
- [71].Nakatani S, Left Ventricular Rotation and Twist: Why Should We Learn? jcu 19 (2011) 1–6. 10.4250/jcu.2011.19.1.1 http://www.e-sciencecentral.org/articles/?scid=1018174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fernandez de Canete J, Pimentel V, Barbancho J, Luque A, System dynamics modelling approach in Health Sciences. Application to the regulation of the cardiovascular function. Informatics in Medicine Unlocked 15 (2019) 100164. https://www.sciencedirect.com/science/article/pii/S2352914818302545 https://www.sciencedirect.com/science/article/pii/S2352914818302545?via%3Dihub. [Google Scholar]
- [73].Iaizzo PA, General Features of the Cardiovascular System. In Handbook of Cardiac Anatomy, Physiology, and Devices, Iaizzo PA, Ed. Springer International Publishing: Cham, 2015; pp 3–12. 10.1007/978-3-319-19464-6_1. [DOI] [Google Scholar]
- [74].Makivić B, Nikić Djordjević M, Willis MS, Heart Rate Variability (HRV) as a tool for diagnostic and monitoring performance in sport and physical activities. J. Exerc. Physiol. Online 16 (2013). https://www.researchgate.net/profile/Bojan_Makivic/publication/285968540_Heart_Rate_Variability_HRV_as_a_Tool_for_Diagnostic_and_Monitoring_Performance_in_Sport_and_Physical_Activities/links/566f019e08ae52dd6c12d205.pdf. [Google Scholar]
- [75].Uemura E, Nomura M, Uehara K, Sawa Y, Nakaya Y, Ito S, Relationship between pulse wave velocity and circulatory hemodynamics during upper gastrointestinal endoscopy. Dig. Endosc. 17 (2005) 73–80. 10.1111/j.1443-1661.2005.00457.x. [DOI] [Google Scholar]
- [76].Gattawar S, Analysis of radial arterial pulse waveform. M. Tech. dissertation, Supervisor: Prof. PC Pandey, BME Dept, IIT Bombay (2005). https://www.ee.iitb.ac.in/~spilab/mtech_thesis/mtp2005_sarika_gattawar_arterial_pulse.pdf
- [77].Prieto-Avalos G, Cruz-Ramos NA, Alor-Hernández G, Sánchez-Cervantes JL, Rodríguez-Mazahua L, Guarneros-Nolasco LR, Wearable devices for physical monitoring of heart: a review. Biosensors 12 (2022) 292. https://mdpi-res.com/d_attachment/biosensors/biosensors-12-00292/article_deploy/biosensors-12-00292.pdf?version=1651490740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zheng Q, Zhang H, Shi B, Xue X, Liu Z, Jin Y, Ma Y, Zou Y, Wang X, An Z, Tang W, Zhang W, Yang F, Liu Y, Lang X, Xu Z, Li Z, Wang ZL, In Vivo Self-Powered Wireless Cardiac Monitoring via Implantable Triboelectric Nanogenerator. ACS Nano 10 (2016) 6510–6518. https://www.ncbi.nlm.nih.gov/pubmed/27253430. [DOI] [PubMed] [Google Scholar]
- [79].Zhao D, Zhuo J, Chen Z, Wu J, Ma R, Zhang X, Zhang Y, Wang X, Wei X, Liu L, Pan C, Wang J, Yang J, Yi F, Yang G, Eco-friendly in-situ gap generation of no-spacer triboelectric nanogenerator for monitoring cardiovascular activities. Nano Energy 90 (2021) 106580. 10.1016/j.nanoen.2021.106580. [DOI] [Google Scholar]
- [80].Liu Z, Ma Y, Ouyang H, Shi B, Li N, Jiang D, Xie F, Qu D, Zou Y, Huang Y, Li H, Zhao C, Tan P, Yu M, Fan Y, Zhang H, Wang ZL, Li Z, Transcatheter Self-Powered Ultrasensitive Endocardial Pressure Sensor. Adv. Funct. Mater. 29 (2019). 10.1002/adfm.201807560. [DOI] [Google Scholar]
- [81].Song P, Yang G, Lang T, Yong K-T, Nanogenerators for wearable bioelectronics and biodevices. J. Phys. D: Appl. Phys. 52 (2018) 023002. 10.1088/1361-6463/aae44d. [DOI] [Google Scholar]
- [82].Dassanayaka DG, Alves TM, Wanasekara ND, Dharmasena IG, Ventura J, Recent progresses in wearable triboelectric nanogenerators. Adv. Funct. Mater. 32 (2022) 2205438. 10.1002/adfm.202205438. [DOI] [Google Scholar]
- [83].Zhao Z, Dai Y, Dou SX, Liang J, Flexible nanogenerators for wearable electronic applications based on piezoelectric materials. Materials Today Energy 20 (2021) 100690. 10.1016/j.mtener.2021.100690. [DOI] [Google Scholar]
- [84].Wu Y, Ma Y, Zheng H, Ramakrishna S, Piezoelectric materials for flexible and wearable electronics: A review. Materials & Design 211 (2021) 110164. 10.1016/j.matdes.2021.110164. [DOI] [Google Scholar]
- [85].Kang S, Kim SH, Lee HB, Mhin S, Ryu JH, Kim YW, Jones JL, Son Y, Lee NK, Lee K, Kim Y, Jung KH, Han H, Park SH, Kim KM, High-power energy harvesting and imperceptible pulse sensing through peapod-inspired hierarchically designed piezoelectric nanofibers. Nano Energy 99 (2022). 10.1016/j.nanoen.2022.107386. [DOI] [Google Scholar]
- [86].Li Z, Zheng Q, Wang ZL, Li Z, Nanogenerator-based self-powered sensors for wearable and implantable electronics. Research 2020 (2020). 10.34133/2020/8710686. [DOI] [PMC free article] [PubMed]
- [87].Park DY, Joe DJ, Kim DH, Park H, Han JH, Jeong CK, Park H, Park JG, Joung B, Lee KJ, Self-Powered Real-Time Arterial Pulse Monitoring Using Ultrathin Epidermal Piezoelectric Sensors. Adv. Mater. 29 (2017). https://www.ncbi.nlm.nih.gov/pubmed/28714239. [DOI] [PubMed] [Google Scholar]
- [88].Karan SK, Maiti S, Kwon O, Paria S, Maitra A, Si SK, Kim Y, Kim JK, Khatua BB, Nature driven spider silk as high energy conversion efficient bio-piezoelectric nanogenerator. Nano Energy 49 (2018) 655–666. 10.1016/j.nanoen.2018.05.014 [DOI] [Google Scholar]
- [89].Li J, Long Y, Yang F, Wang X, Degradable piezoelectric biomaterials for wearable and implantable bioelectronics. Curr. Opin. Solid State Mater. Sci. 24 (2020) 100806. https://www.sciencedirect.com/science/article/pii/S1359028620300036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Li P, Zhang Z, Shen W, Hu C, Shen W, Zhang D, A self-powered 2D-material sensor unit driven by a SnSe piezoelectric nanogenerator. Journal of Materials Chemistry A 9 (2021) 4716–4723. https://pubs.rsc.org/en/content/articlehtml/2020/ta/d0ta10457d [Google Scholar]
- [91].Wang H, Han M, Song Y, Zhang H, Design, manufacturing and applications of wearable triboelectric nanogenerators. Nano Energy 81 (2021) 105627. https://www.sciencedirect.com/science/article/pii/S2211285520312003 [Google Scholar]
- [92].Ouyang H, Tian J, Sun G, Zou Y, Liu Z, Li H, Zhao L, Shi B, Fan Y, Fan Y, Wang ZL, Li Z, Self-Powered Pulse Sensor for Antidiastole of Cardiovascular Disease. Adv. Mater. 29 (2017). https://www.ncbi.nlm.nih.gov/pubmed/28863247. [DOI] [PubMed] [Google Scholar]
- [93].Liu Z, Zhao Z, Zeng X, Fu X, Hu Y, Expandable microsphere-based triboelectric nanogenerators as ultrasensitive pressure sensors for respiratory and pulse monitoring. Nano Energy 59 (2019) 295–301. 10.1016/j.nanoen.2019.02.057. [DOI] [Google Scholar]
- [94].Zhang M, Zhu W, Zhang T, Su Y, Yang Y, Lever-inspired triboelectric nanogenerator with ultra-high output for pulse monitoring. Nano Energy 97 (2022). 10.1016/j.nanoen.2022.107159. [DOI] [Google Scholar]
- [95].Naegeli KM, Kural MH, Li Y, Wang J, Hugentobler EA, Niklason LE, Bioengineering human tissues and the future of vascular replacement. Circulation Research 131 (2022) 109–126. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9213087/pdf/res-131-109.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wilson WR, Bower TC, Creager MA, Amin-Hanjani S, O’Gara PT, Lockhart PB, Darouiche RO, Ramlawi B, Derdeyn CP, Bolger AF, Vascular graft infections, mycotic aneurysms, and endovascular infections: a scientific statement from the American Heart Association. Circulation 134 (2016) e412–e460. 10.1161/CIR.0000000000000457?download=true. [DOI] [PubMed] [Google Scholar]
- [97].Sardar MR, Goldsweig AM, Abbott JD, Sharaf BL, Gordon PC, Ehsan A, Aronow HD, Vascular complications associated with transcatheter aortic valve replacement. Vasc. Med. 22 (2017) 234–244. . [DOI] [PubMed] [Google Scholar]
- [98].de Valence S, Tille J-C, Mugnai D, Mrowczynski W, Gurny R, Möller M, Walpoth BH, Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials 33 (2012) 38–47. https://www.sciencedirect.com/science/article/pii/S0142961211010659?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- [99].Gupta SK, Dietzek AM, Veith FJ, Torres M, Kram HB, Wengerter KR, Use of a piezoelectric film sensor for monitoring vascular grafts. The American Journal of Surgery 160 (1990) 182–186. https://www.sciencedirect.com/science/article/pii/S0002961005803034?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- [100].Hong YJ, Jeong H, Cho KW, Lu N, Kim DH, Wearable and implantable devices for cardiovascular healthcare: from monitoring to therapy based on flexible and stretchable electronics. Adv. Funct. Mater. 29 (2019) 1808247. 10.1002/adfm.201808247. [DOI] [Google Scholar]
- [101].Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV, Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury 42 (2011) S3–S15. https://www.sciencedirect.com/science/article/pii/S002013831100252X?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- [102].Sun H, Deng M, Chen W, Liu M, Dai H, Wang C, Graft dysfunction and rejection of lung transplant, a review on diagnosis and management. The Clinical Respiratory Journal 16 (2022) 5–12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9060084/pdf/CRJ-16-5.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yi F, Zhang Z, Kang Z, Liao Q, Zhang Y, Recent Advances in Triboelectric Nanogenerator-Based Health Monitoring. Advanced Functional Materials 29 (2019). 10.1002/adfm.201808849. [DOI] [Google Scholar]
- [104].Al-Suhaimi EA, Aljafary MA, Alfareed TM, Alshuyeh HA, Alhamid GM, Sonbol B, Almofleh A, Alkulaifi FM, Altwayan RK, Alharbi JN, Nanogenerator-Based Sensors for Energy Harvesting From Cardiac Contraction. Frontiers in Energy Research 10 (2022) 579. 10.3389/fenrg.2022.900534. [DOI] [Google Scholar]
- [105].Hu S, Shi Z, Zhao W, Wang L, Yang G, Multifunctional piezoelectric elastomer composites for smart biomedical or wearable electronics. Composites Part B: Engineering 160 (2019) 595–604. 10.1016/j.compositesb.2018.12.077. [DOI] [Google Scholar]
- [106].Wang D, Wang C, Bi Z, Zhou B, Wang L, Li Q, Turng L-S, Expanded polytetrafluoroethylene/poly(3-hydroxybutyrate) (ePTFE/PHB) triboelectric nanogenerators and their potential applications as self-powered and sensing vascular grafts. Chem. Eng. J. 455 (2023). 10.1016/j.cej.2022.140494. [DOI] [Google Scholar]
- [107].Li J, Long Y, Yang F, Wei H, Zhang Z, Wang Y, Wang J, Li C, Carlos C, Dong Y, Wu Y, Cai W, Wang X, Multifunctional Artificial Artery from Direct 3D Printing with Built-In Ferroelectricity and Tissue-Matching Modulus for Real-Time Sensing and Occlusion Monitoring. Adv. Funct. Mater. 30 (2020). https://www.ncbi.nlm.nih.gov/pubmed/33679279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Cafarelli A, Losi P, Salgarella AR, Barsotti MC, Di Cioccio IB, Foffa I, Vannozzi L, Pingue P, Soldani G, Ricotti L, Small-caliber vascular grafts based on a piezoelectric nanocomposite elastomer: Mechanical properties and biocompatibility. J. Mech. Behav. Biomed. Mater. 97 (2019) 138–148. https://www.ncbi.nlm.nih.gov/pubmed/31121432. [DOI] [PubMed] [Google Scholar]
- [109].D’Ambrogio G, Zahhaf O, Le M-Q, Bordet M, Lermusiaux P, Della Schiava N, Liang R, Cottinet P-J, Capsal J-F, Piezoelectric biosensor for smart cardiovascular grafts based on NaNbO3 fibers/PDMS structured composite. Materials & Design 223 (2022). 10.1016/j.matdes.2022.111195. [DOI] [Google Scholar]
- [110].Cheng X, Xue X, Ma Y, Han M, Zhang W, Xu Z, Zhang H, Zhang H, Implantable and self-powered blood pressure monitoring based on a piezoelectric thinfilm: Simulated, in vitro and in vivo studies. Nano Energy 22 (2016) 453–460. 10.1016/j.nanoen.2016.02.037. [DOI] [Google Scholar]
- [111].Ouyang H, Li Z, Gu M, Hu Y, Xu L, Jiang D, Cheng S, Zou Y, Deng Y, Shi B, Hua W, Fan Y, Li Z, Wang Z, A Bioresorbable Dynamic Pressure Sensor for Cardiovascular Postoperative Care. Adv. Mater. 33 (2021) e2102302. https://www.ncbi.nlm.nih.gov/pubmed/34369023. [DOI] [PubMed] [Google Scholar]
- [112].Tan JY, Islam S, Li Y, Kim A, Kim JJ, An Optimization of Perforation Design on a Piezoelectric-Based Smart Stent for Blood Pressure Monitoring and Low-Frequency Vibrational Energy Harvesting In 2023 IEEE 36th International Conference on Micro Electro Mechanical Systems (MEMS), 2023; pp 396–399. 10.1109/mems49605.2023.10052623. [DOI] [Google Scholar]
- [113].Ogedegbe G, Pickering T, Principles and techniques of blood pressure measurement. Cardiol. Clin. 28 (2010) 571–586. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3639494/pdf/nihms462201.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Handler J, The importance of accurate blood pressure measurement. The Permanente Journal 13 (2009) 51. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2911816/pdf/prjl.13.3.051.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Weiss NS, Relation of high blood pressure to headache, epistaxis, and selected other symptoms: The United States Health Examination Survey of Adults. New England Journal of Medicine 287 (1972) 631–633. 10.1056/NEJM197209282871303?articleTools=true. [DOI] [PubMed] [Google Scholar]
- [116].Blockley NP, Griffeth VEM, Simon AB, Buxton RB, A review of calibrated blood oxygenation level-dependent (BOLD) methods for the measurement of task-induced changes in brain oxygen metabolism. NMR Biomed. 26 (2013) 987–1003. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3639302/pdf/nihms436203.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Bonora E, Muggeo M, Postprandial blood glucose as a risk factor for cardiovascular disease in type II diabetes: the epidemiological evidence. Diabetologia 44 (2001) 2107–2114. 10.1007/s001250100020.pdf. [DOI] [PubMed] [Google Scholar]
- [118].Meng K, Chen J, Li X, Wu Y, Fan W, Zhou Z, He Q, Wang X, Fan X, Zhang Y, Yang J, Wang ZL, Flexible Weaving Constructed Self-Powered Pressure Sensor Enabling Continuous Diagnosis of Cardiovascular Disease and Measurement of Cuffless Blood Pressure. Adv. Funct. Mater. (2018). 10.1002/adfm.201806388. [DOI]
- [119].Chen S, Wu N, Lin S, Duan J, Xu Z, Pan Y, Zhang H, Xu Z, Huang L, Hu B, Zhou J, Hierarchical elastomer tuned self-powered pressure sensor for wearable multifunctional cardiovascular electronics. Nano Energy 70 (2020). 10.1016/j.nanoen.2020.104460. [DOI] [Google Scholar]
- [120].Xue X, Qu Z, Fu Y, Yu B, Xing L, Zhang Y, Self-powered electronic-skin for detecting glucose level in body fluid basing on piezo-enzymatic-reaction coupling process. Nano Energy 26 (2016) 148–156. 10.1016/j.nanoen.2016.05.021. [DOI] [Google Scholar]
- [121].Zhang W, Zhang L, Gao H, Yang W, Wang S, Xing L, Xue X, Self-Powered Implantable Skin-Like Glucometer for Real-Time Detection of Blood Glucose Level In Vivo. Nanomicro Lett 10 (2018) 32. https://www.ncbi.nlm.nih.gov/pubmed/30393681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Yang G, Tang Y, Lin T, Zhong T, Fan Y, Zhang Y, Xing L, Xue X, Zhan Y, A self-powered closed-loop brain-machine-interface system for real-time detecting and rapidly adjusting blood glucose concentration. Nano Energy 93 (2022). 10.1016/j.nanoen.2021.106817. [DOI] [Google Scholar]
- [123].Chen H, Xu Y, Zhang J, Wu W, Song G, Self-Powered Flexible Blood Oxygen Monitoring System Based on a Triboelectric Nanogenerator. Nanomaterials (Basel) 9 (2019). https://www.ncbi.nlm.nih.gov/pubmed/31117275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Li H, Xu Y, Li X, Chen Y, Jiang Y, Zhang C, Lu B, Wang J, Ma Y, Chen Y, Huang Y, Ding M, Su H, Song G, Luo Y, Feng X, Epidermal Inorganic Optoelectronics for Blood Oxygen Measurement. Advanced Healthcare Materials 6 (2017) 1601013. 10.1002/adhm.201601013. [DOI] [PubMed] [Google Scholar]
- [125].Chen H, Yang W, Zhang C, Wu M, Li W, Zou Y, Lv L, Yu H, Ke H, Liu R, Xu Y, Wang J, Li Z, Performance-enhanced and cost-effective triboelectric nanogenerator based on stretchable electrode for wearable SpO2 monitoring. Nano Research 15 (2021) 2465–2471. 10.1016/j.seta.2022.102982. [DOI] [Google Scholar]
- [126].Xu Q, Gao X, Zhao S, Liu Y-N, Zhang D, Zhou K, Khanbareh H, Chen W, Zhang Y, Bowen C, Construction of Bio-Piezoelectric Platforms: From Structures and Synthesis to Applications. Adv. Mater. 33 (2021) 2008452. 10.1002/adma.202008452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Jeong CK, Toward bioimplantable and biocompatible flexible energy harvesters using piezoelectric ceramic materials. MRS Communications 10 (2020) 365–378. 10.1557/mrc.2020.48. [DOI] [Google Scholar]
- [128].AlTowireb SM, Goumri-Said S, Core-Shell structures for the enhancement of energy harvesting in piezoelectric Nanogenerators: A review. Sustainable Energy Technologies and Assessments 55 (2023) 102982. 10.1016/j.seta.2022.102982. [DOI] [Google Scholar]
- [129].Yan J, Liu M, Jeong YG, Kang W, Li L, Zhao Y, Deng N, Cheng B, Yang G, Performance enhancements in poly (vinylidene fluoride)-based piezoelectric nanogenerators for efficient energy harvesting. Nano Energy 56 (2019) 662–692. 10.1016/j.nanoen.2018.12.010. [DOI] [Google Scholar]
- [130].Le AT, Ahmadipour M, Pung S-Y, A review on ZnO-based piezoelectric nanogenerators: Synthesis, characterization techniques, performance enhancement and applications. J. Alloys Compd. 844 (2020) 156172. 10.1016/j.jallcom.2020.156172. [DOI] [Google Scholar]
- [131].Yu Y, Wang X, Chemical modification of polymer surfaces for advanced triboelectric nanogenerator development. Extreme Mechanics Letters 9 (2016) 514–530. 10.1016/j.eml.2016.02.019. [DOI] [Google Scholar]
- [132].Ibrahim M, Jiang J, Wen Z, Sun X, Surface engineering for enhanced triboelectric nanogenerator. Nanoenergy Advances 1 (2021) 58–80. 10.3390/nanoenergyadv1010004. [DOI] [Google Scholar]
- [133].Khalid S, Raouf I, Khan A, Kim N, Kim HS, A review of human-powered energy harvesting for smart electronics: Recent progress and challenges. International Journal of Precision Engineering and Manufacturing-Green Technology 6 (2019) 821–851. 10.1007/s40684-019-00144-y. [DOI] [Google Scholar]
- [134].Mukhopadhyay SC, Wearable sensors for human activity monitoring: A review. IEEE Sens. J. 15 (2014) 1321–1330. https://ieeexplore.ieee.org/abstract/document/6974987. [Google Scholar]
- [135].Dong K, Peng X, Wang ZL, Fiber/fabric-based piezoelectric and triboelectric nanogenerators for flexible/stretchable and wearable electronics and artificial intelligence. Adv. Mater. 32 (2020) 1902549. 10.1002/adma.201902549. [DOI] [PubMed] [Google Scholar]
- [136].Xiong J, Lee PS, Progress on wearable triboelectric nanogenerators in shapes of fiber, yarn, and textile. Science and technology of advanced materials 20 (2019) 837–857. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6720508/pdf/TSTA_20_1650396.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Singh G, Chanda A, Mechanical properties of whole-body soft human tissues: A review. Biomedical Materials 16 (2021) 062004. 10.1088/1748-605X/ac2b7a/meta. [DOI] [PubMed] [Google Scholar]
- [138].Wang H, Wu T, Zeng Q, Lee C, A review and perspective for the development of triboelectric nanogenerator (TENG)-Based self-powered neuroprosthetics. Micromachines 11 (2020) 865. https://mdpi-res.com/d_attachment/micromachines/micromachines-11-00865/article_deploy/micromachines-1100865.pdf?version=1600417184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Delgado-Alvarado E, Martínez-Castillo J, Zamora-Peredo L, Gonzalez-Calderon JA, López-Esparza R, Ashraf MW, Tayyaba S, Herrera-May AL, Triboelectric and Piezoelectric Nanogenerators for Self-Powered Healthcare Monitoring Devices: Operating Principles, Challenges, and Perspectives. Nanomaterials 12 (2022) 4403. https://mdpi-res.com/d_attachment/nanomaterials/nanomaterials-12-04403/article_deploy/nanomaterials-12-04403.pdf?version=1670597836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Shao Y, Yan S, Li J, Silva-Pedraza Z, Zhou T, Hsieh M, Liu B, Li T, Gu L, Zhao Y, Dong Y, Yin B, Wang X, Stretchable Encapsulation Materials with High Dynamic Water Resistivity and Tissue-Matching Elasticity. ACS Appl. Mater. Interfaces 14 (2022) 18935–18943. 10.1021/acsami.2c03110. [DOI] [PMC free article] [PubMed] [Google Scholar]


