Abstract
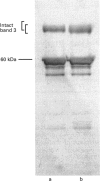
1. We have studied band 3 HT, a human red-cell band 3 variant with increased M(r), which is associated with abnormal red-cell shape (acanthocytosis) and increased anion-transport activity. 2. We have shown that the increased M(r) does not result from the presence of the band 3 Memphis mutation, and that the variant band 3 is covalently labelled by 4,4'-di-isothiocyanato-1,2-diphenylethane-2,2'-disulphonic acid (H2DIDS) less readily than normal. 3. cDNA cloning studies show that band 3 HT results from the mutation Pro-868-->Leu, and the possible significance of the mutation in the altered anion-transport activity and cytoskeleton binding properties of band 3 HT is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper S. L. The band 3-related anion exchanger (AE) gene family. Annu Rev Physiol. 1991;53:549–564. doi: 10.1146/annurev.ph.53.030191.003001. [DOI] [PubMed] [Google Scholar]
- Bartel D., Hans H., Passow H. Identification by site-directed mutagenesis of Lys-558 as the covalent attachment site of H2DIDS in the mouse erythroid band 3 protein. Biochim Biophys Acta. 1989 Nov 3;985(3):355–358. doi: 10.1016/0005-2736(89)90427-6. [DOI] [PubMed] [Google Scholar]
- Batenjany M. M., Mizukami H., Salhany J. M. Near-UV circular dichroism of band 3. Evidence for intradomain conformational changes and interdomain interactions. Biochemistry. 1993 Jan 19;32(2):663–668. doi: 10.1021/bi00053a035. [DOI] [PubMed] [Google Scholar]
- Bosman G. J., Kay M. M. Alterations of band 3 transport protein by cellular aging and disease: erythrocyte band 3 and glucose transporter share a functional relationship. Biochem Cell Biol. 1990 Dec;68(12):1419–1427. doi: 10.1139/o90-205. [DOI] [PubMed] [Google Scholar]
- Falke J. J., Kanes K. J., Chan S. I. The kinetic equation for the chloride transport cycle of band 3. A 35Cl and 37Cl NMR study. J Biol Chem. 1985 Aug 15;260(17):9545–9551. [PubMed] [Google Scholar]
- Garcia A. M., Lodish H. F. Lysine 539 of human band 3 is not essential for ion transport or inhibition by stilbene disulfonates. J Biol Chem. 1989 Nov 25;264(33):19607–19613. [PubMed] [Google Scholar]
- Hsu L., Morrison M. A new variant of the anion transport protein in human erythrocytes. Biochemistry. 1985 Jun 18;24(13):3086–3090. doi: 10.1021/bi00334a003. [DOI] [PubMed] [Google Scholar]
- Jarolim P., Palek J., Amato D., Hassan K., Sapak P., Nurse G. T., Rubin H. L., Zhai S., Sahr K. E., Liu S. C. Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11022–11026. doi: 10.1073/pnas.88.24.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul R. K., Murthy S. N., Reddy A. G., Steck T. L., Kohler H. Amino acid sequence of the N alpha-terminal 201 residues of human erythrocyte membrane band 3. J Biol Chem. 1983 Jul 10;258(13):7981–7990. [PubMed] [Google Scholar]
- Kay M. M., Bosman G. J., Lawrence C. Functional topography of band 3: specific structural alteration linked to functional aberrations in human erythrocytes. Proc Natl Acad Sci U S A. 1988 Jan;85(2):492–496. doi: 10.1073/pnas.85.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupka R. M. Role of substrate binding forces in exchange-only transport systems: II. Implications for the mechanism of the anion exchanger of red cells. J Membr Biol. 1989 Jul;109(2):159–171. doi: 10.1007/BF01870855. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Kopito R. R., Lodish H. F. Cloning and characterization of band 3, the human erythrocyte anion-exchange protein (AE1). Proc Natl Acad Sci U S A. 1989 Dec;86(23):9089–9093. doi: 10.1073/pnas.86.23.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Winardi R., Knowles D., Leung A., Parra M., George E., Conboy J., Chasis J. Molecular basis for membrane rigidity of hereditary ovalocytosis. A novel mechanism involving the cytoplasmic domain of band 3. J Clin Invest. 1992 Feb;89(2):686–692. doi: 10.1172/JCI115636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T. J., Morrison M. Detection of a variant of protein 3, the major transmembrane protein of the human erythrocyte. J Biol Chem. 1977 Oct 10;252(19):6573–6576. [PubMed] [Google Scholar]
- Salhany J. M., Sloan R. L., Cordes K. A. In situ cross-linking of human erythrocyte band 3 by bis(sulfosuccinimidyl)suberate. Evidence for ligand modulation of two alternate quaternary forms: covalent band 3 dimers and noncovalent tetramers formed by the association of two covalent dimers. J Biol Chem. 1990 Oct 15;265(29):17688–17693. [PubMed] [Google Scholar]
- Schofield A. E., Reardon D. M., Tanner M. J. Defective anion transport activity of the abnormal band 3 in hereditary ovalocytic red blood cells. Nature. 1992 Feb 27;355(6363):836–838. doi: 10.1038/355836a0. [DOI] [PubMed] [Google Scholar]
- Schofield A. E., Tanner M. J., Pinder J. C., Clough B., Bayley P. M., Nash G. B., Dluzewski A. R., Reardon D. M., Cox T. M., Wilson R. J. Basis of unique red cell membrane properties in hereditary ovalocytosis. J Mol Biol. 1992 Feb 20;223(4):949–958. doi: 10.1016/0022-2836(92)90254-h. [DOI] [PubMed] [Google Scholar]
- Spring F. A., Bruce L. J., Anstee D. J., Tanner M. J. A red cell band 3 variant with altered stilbene disulphonate binding is associated with the Diego (Dia) blood group antigen. Biochem J. 1992 Dec 15;288(Pt 3):713–716. doi: 10.1042/bj2880713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J., Bruce L., Martin P. G., Rearden D. M., Jones G. L. Melanesian hereditary ovalocytes have a deletion in red cell band 3. Blood. 1991 Nov 15;78(10):2785–2786. [PubMed] [Google Scholar]
- Tanner M. J., Martin P. G., High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J. 1988 Dec 15;256(3):703–712. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J. Molecular and cellular biology of the erythrocyte anion exchanger (AE1). Semin Hematol. 1993 Jan;30(1):34–57. [PubMed] [Google Scholar]
- Thevenin B. J., Low P. S. Kinetics and regulation of the ankyrin-band 3 interaction of the human red blood cell membrane. J Biol Chem. 1990 Sep 25;265(27):16166–16172. [PubMed] [Google Scholar]
- Wainwright S. D., Tanner M. J., Martin G. E., Yendle J. E., Holmes C. Monoclonal antibodies to the membrane domain of the human erythrocyte anion transport protein. Localization of the C-terminus of the protein to the cytoplasmic side of the red cell membrane and distribution of the protein in some human tissues. Biochem J. 1989 Feb 15;258(1):211–220. doi: 10.1042/bj2580211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannoukakos D., Vasseur C., Driancourt C., Blouquit Y., Delaunay J., Wajcman H., Bursaux E. Human erythrocyte band 3 polymorphism (band 3 Memphis): characterization of the structural modification (Lys 56----Glu) by protein chemistry methods. Blood. 1991 Aug 15;78(4):1117–1120. [PubMed] [Google Scholar]