Abstract
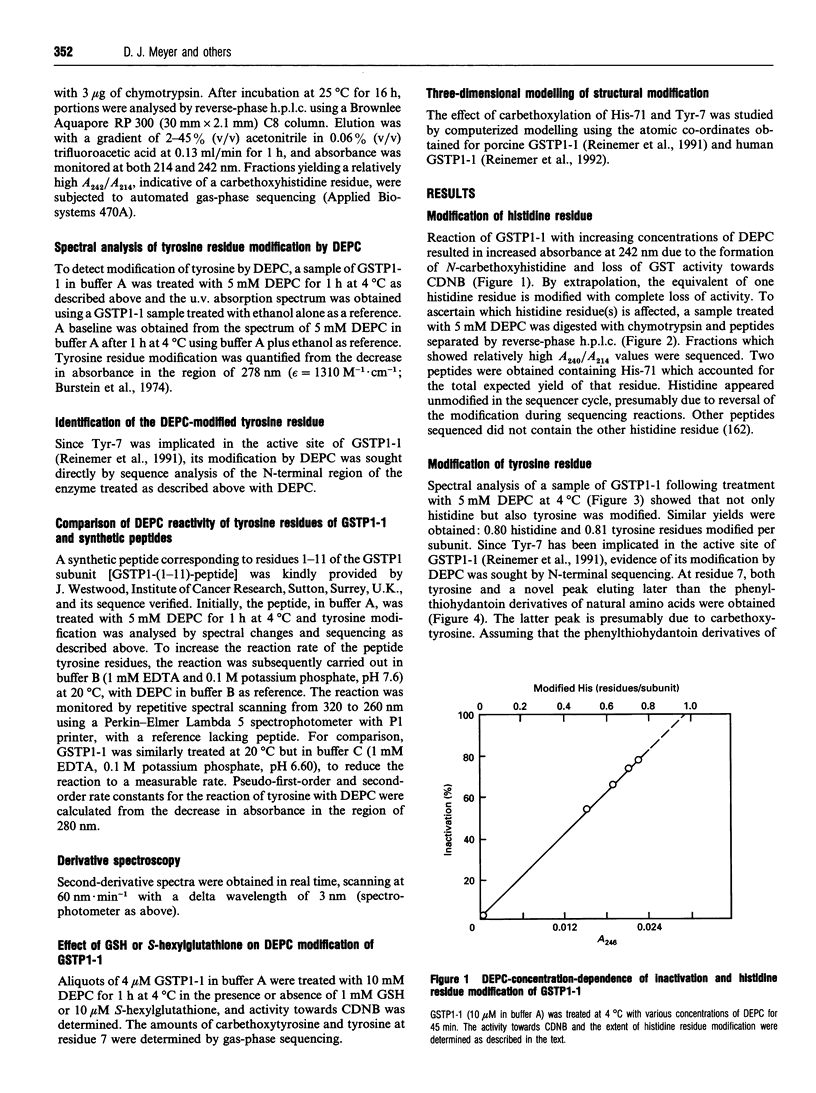
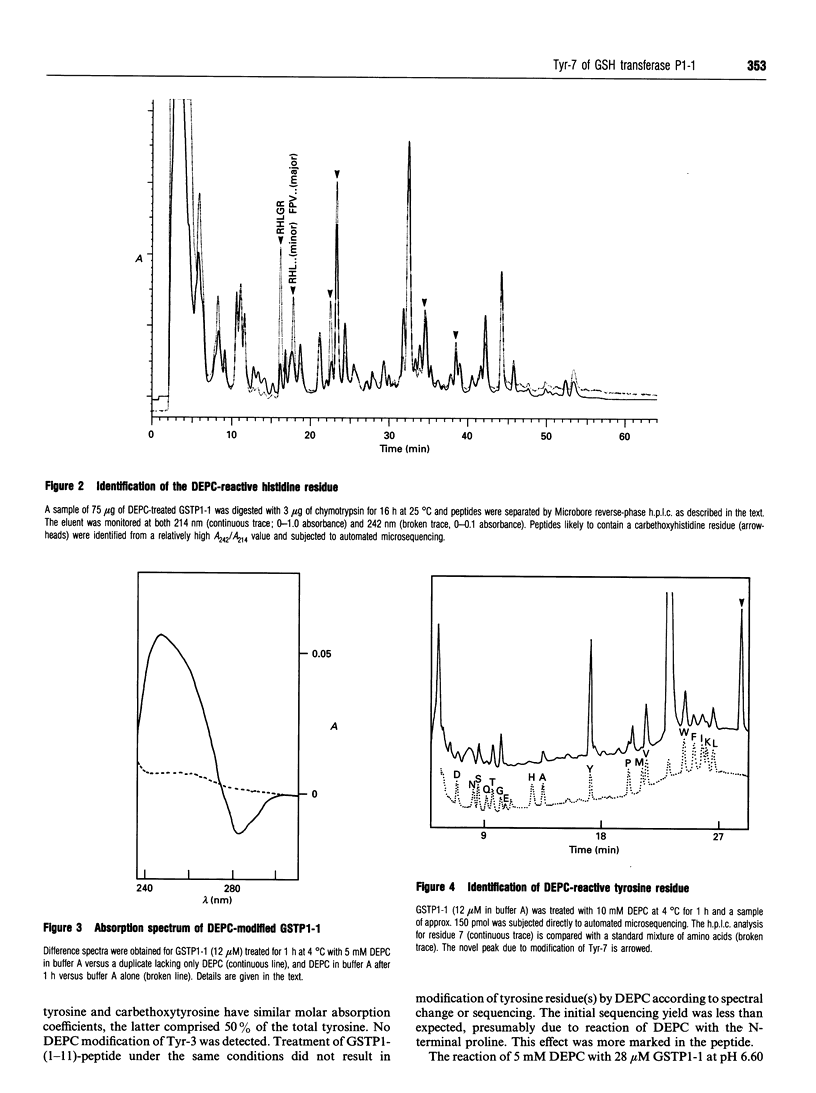
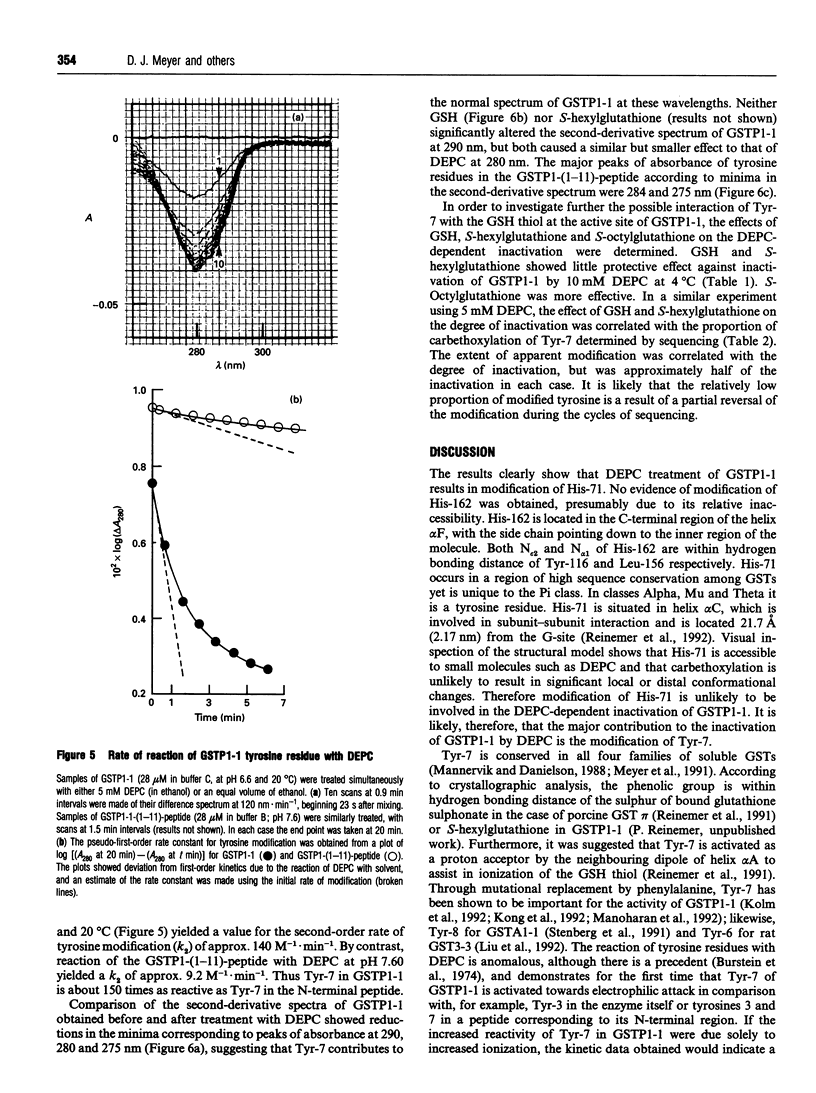
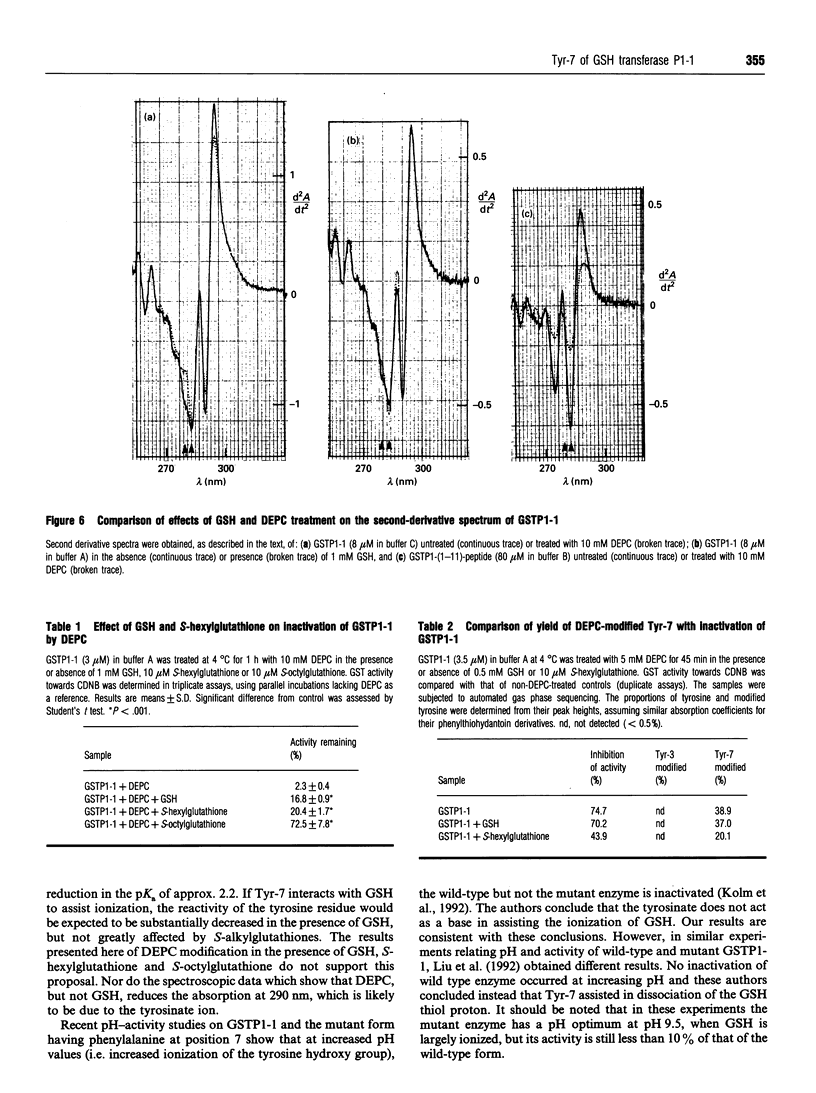
Reaction of human GSH transferase P1-1 (GSTP1-1) with diethylpyrocarbonate (DEPC) at pH 7.0 and 4 degrees C resulted in covalent modification of an equivalent of one histidine and one tyrosine residue per subunit, with loss of activity. Sequence analysis showed that His-71 and Tyr-7 were modified. Reference to the three-dimensional structure of GSTP1-1 [Reinemer, Dirr, Ladenstein, Huber, Lo Bello, Frederici and Parker (1992) J. Mol. Biol. 227, 214-226] shows that the modification of Tyr-7 is most likely to affect enzyme activity. Kinetic analysis of the DEPC modification of Tyr-7 in GSTP1-1 gave a k2 approx. 150 times that of a peptide comprising residues 1-11 of GSTP1-1. The reaction of Tyr-7 of GSTP1-1 with DEPC was poorly inhibited by 1 mM GSH (14%) or 10 microM S-hexylglutathione (18%). DEPC treatment of the enzyme altered the absorbance at 290 nm in second-derivative spectra, suggesting that a significant amount of tyrosinate ion occurs in the enzyme. GSH, however, did not significantly alter the A290. The data provide the first evidence of unusual chemical reactivity of Tyr-7 and are consistent with its proposed role as a proton acceptor during catalysis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awasthi Y. C., Bhatnagar A., Singh S. V. Evidence for the involvement of histidine at the active site of glutathione S-transferase psi from human liver. Biochem Biophys Res Commun. 1987 Mar 30;143(3):965–970. doi: 10.1016/0006-291x(87)90345-7. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Walsh K. A., Neurath H. Evidence of an essential histidine residue in thermolysin. Biochemistry. 1974 Jan 1;13(1):205–210. doi: 10.1021/bi00698a030. [DOI] [PubMed] [Google Scholar]
- Carne T., Tipping E., Ketterer B. The binding and catalytic activities of forms of ligandin after modification of its thiol groups. Biochem J. 1979 Feb 1;177(2):433–439. doi: 10.1042/bj1770433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Ji X., Zhang P., Armstrong R. N., Gilliland G. L. The three-dimensional structure of a glutathione S-transferase from the mu gene class. Structural analysis of the binary complex of isoenzyme 3-3 and glutathione at 2.2-A resolution. Biochemistry. 1992 Oct 27;31(42):10169–10184. doi: 10.1021/bi00157a004. [DOI] [PubMed] [Google Scholar]
- Kolm R. H., Sroga G. E., Mannervik B. Participation of the phenolic hydroxyl group of Tyr-8 in the catalytic mechanism of human glutathione transferase P1-1. Biochem J. 1992 Jul 15;285(Pt 2):537–540. doi: 10.1042/bj2850537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong K. H., Inoue H., Takahashi K. Non-essentiality of cysteine and histidine residues for the activity of human class PI glutathione S-transferase. Biochem Biophys Res Commun. 1991 Dec 16;181(2):748–755. doi: 10.1016/0006-291x(91)91254-a. [DOI] [PubMed] [Google Scholar]
- Kong K. H., Nishida M., Inoue H., Takahashi K. Tyrosine-7 is an essential residue for the catalytic activity of human class PI glutathione S-transferase: chemical modification and site-directed mutagenesis studies. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1122–1129. doi: 10.1016/0006-291x(92)91848-k. [DOI] [PubMed] [Google Scholar]
- Liu S., Zhang P., Ji X., Johnson W. W., Gilliland G. L., Armstrong R. N. Contribution of tyrosine 6 to the catalytic mechanism of isoenzyme 3-3 of glutathione S-transferase. J Biol Chem. 1992 Mar 5;267(7):4296–4299. [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Manoharan T. H., Gulick A. M., Reinemer P., Dirr H. W., Huber R., Fahl W. E. Mutational substitution of residues implicated by crystal structure in binding the substrate glutathione to human glutathione S-transferase pi. J Mol Biol. 1992 Jul 20;226(2):319–322. doi: 10.1016/0022-2836(92)90949-k. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Coles B., Pemble S. E., Gilmore K. S., Fraser G. M., Ketterer B. Theta, a new class of glutathione transferases purified from rat and man. Biochem J. 1991 Mar 1;274(Pt 2):409–414. doi: 10.1042/bj2740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. J., Lalor E., Coles B., Kispert A., Alin P., Mannervik B., Ketterer B. Single-step purification and h.p.l.c. analysis of glutathione transferase 8-8 in rat tissues. Biochem J. 1989 Jun 15;260(3):785–788. doi: 10.1042/bj2600785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penington C. J., Rule G. S. Mapping the substrate-binding site of a human class mu glutathione transferase using nuclear magnetic resonance spectroscopy. Biochemistry. 1992 Mar 24;31(11):2912–2920. doi: 10.1021/bi00126a010. [DOI] [PubMed] [Google Scholar]
- Reinemer P., Dirr H. W., Ladenstein R., Huber R., Lo Bello M., Federici G., Parker M. W. Three-dimensional structure of class pi glutathione S-transferase from human placenta in complex with S-hexylglutathione at 2.8 A resolution. J Mol Biol. 1992 Sep 5;227(1):214–226. doi: 10.1016/0022-2836(92)90692-d. [DOI] [PubMed] [Google Scholar]
- Reinemer P., Dirr H. W., Ladenstein R., Schäffer J., Gallay O., Huber R. The three-dimensional structure of class pi glutathione S-transferase in complex with glutathione sulfonate at 2.3 A resolution. EMBO J. 1991 Aug;10(8):1997–2005. doi: 10.1002/j.1460-2075.1991.tb07729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg G., Board P. G., Mannervik B. Mutation of an evolutionarily conserved tyrosine residue in the active site of a human class Alpha glutathione transferase. FEBS Lett. 1991 Nov 18;293(1-2):153–155. doi: 10.1016/0014-5793(91)81174-7. [DOI] [PubMed] [Google Scholar]
- Vander Jagt D. L., Hunsaker L. A., Garcia K. B., Royer R. E. Isolation and characterization of the multiple glutathione S-transferases from human liver. Evidence for unique heme-binding sites. J Biol Chem. 1985 Sep 25;260(21):11603–11610. [PubMed] [Google Scholar]
- Wang R. W., Newton D. J., Pickett C. B., Lu A. Y. Site-directed mutagenesis of glutathione S-transferase YaYa: functional studies of histidine, cysteine, and tryptophan mutants. Arch Biochem Biophys. 1992 Aug 15;297(1):86–91. doi: 10.1016/0003-9861(92)90644-c. [DOI] [PubMed] [Google Scholar]
- Wang R. W., Newton D. J., Pickett C. B., Lu A. Y. Site-directed mutagenesis of glutathione S-transferase YaYa: nonessential role of histidine in catalysis. Arch Biochem Biophys. 1991 May 1;286(2):574–578. doi: 10.1016/0003-9861(91)90082-t. [DOI] [PubMed] [Google Scholar]
- Widersten M., Mannervik B. A structural role of histidine 15 in human glutathione transferase M1-1, an amino acid residue conserved in class Mu enzymes. Protein Eng. 1992 Sep;5(6):551–557. doi: 10.1093/protein/5.6.551. [DOI] [PubMed] [Google Scholar]
- Zhang P. H., Graminski G. F., Armstrong R. N. Are the histidine residues of glutathione S-transferase important in catalysis? An assessment by 13C NMR spectroscopy and site-specific mutagenesis. J Biol Chem. 1991 Oct 15;266(29):19475–19479. [PubMed] [Google Scholar]


