Abstract
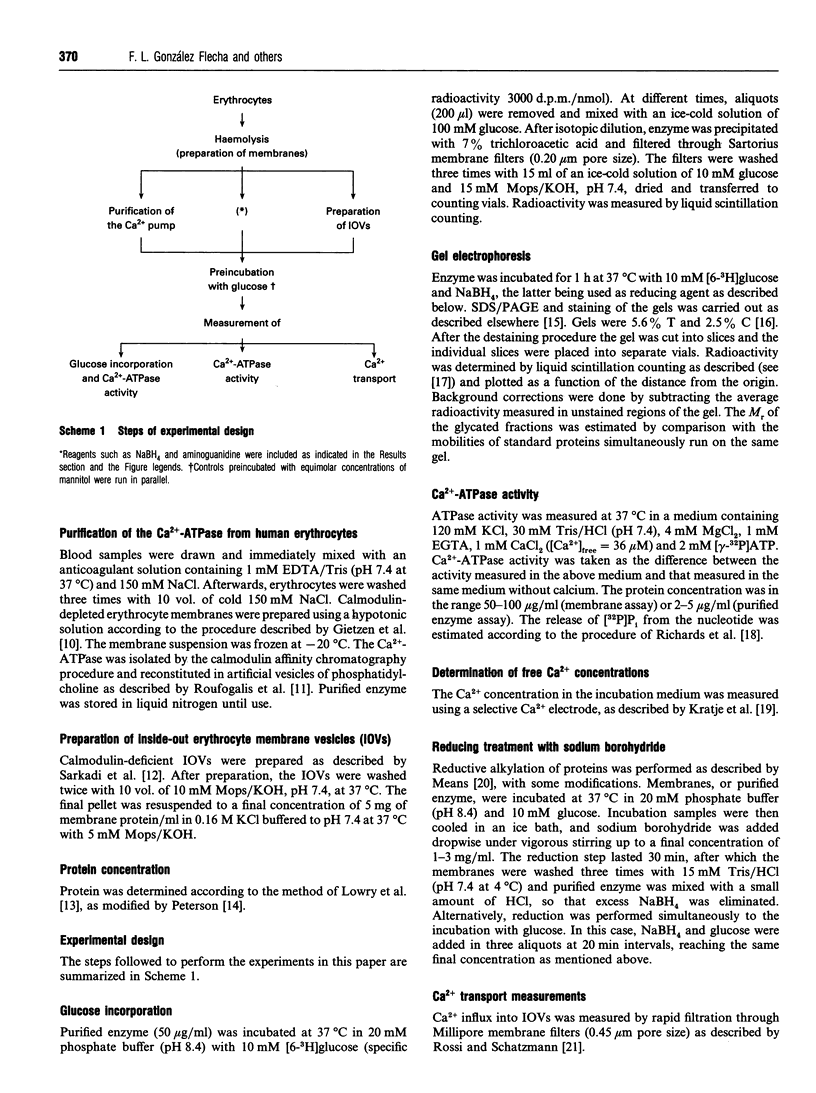
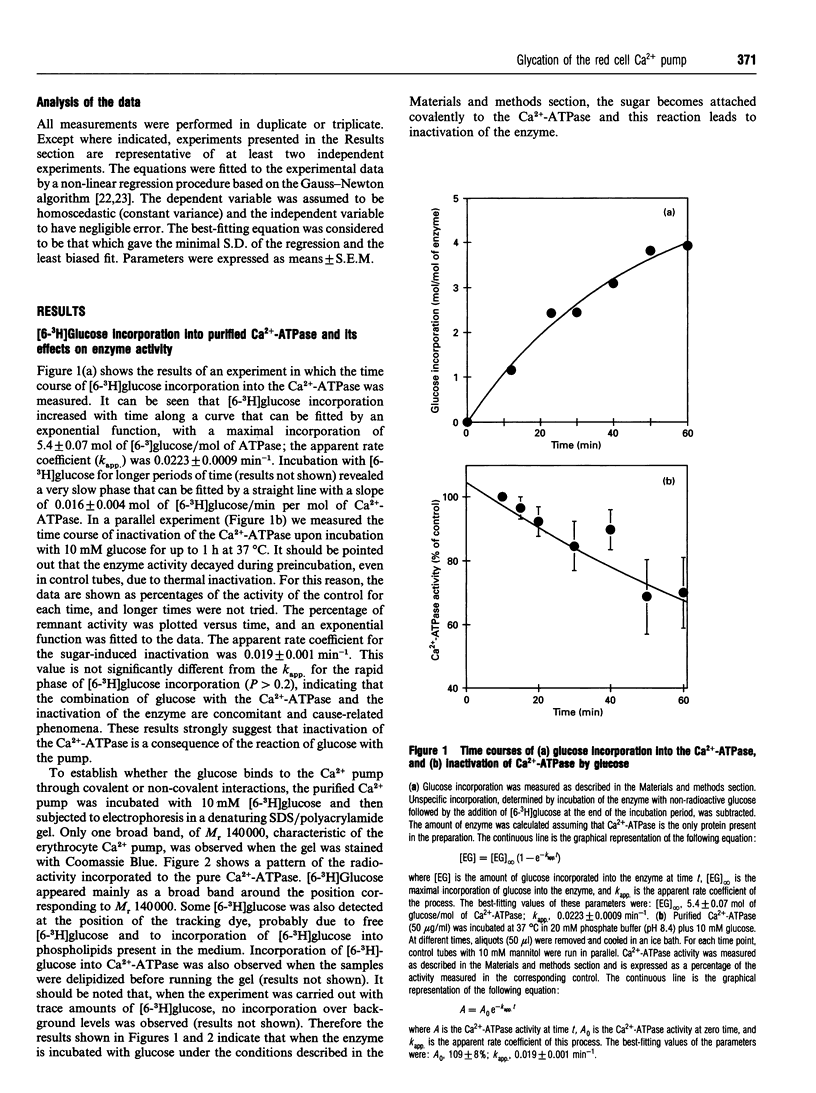
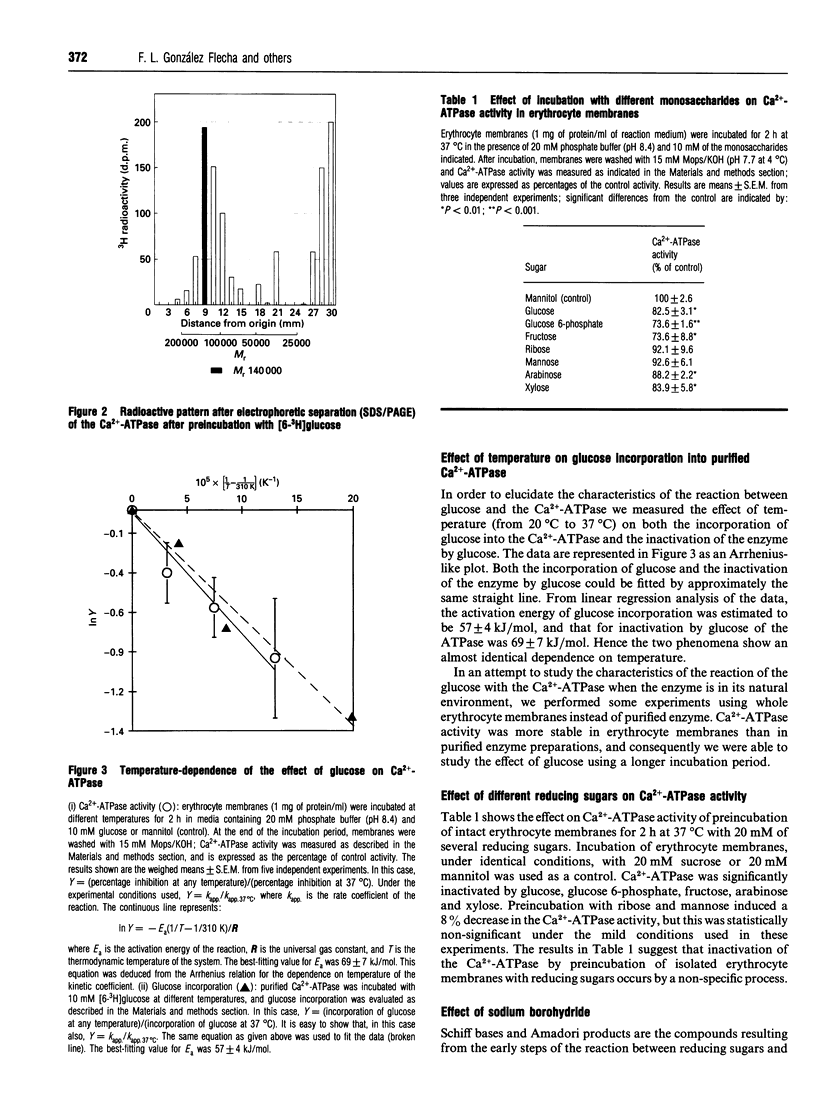
In a previous paper we demonstrated that incubation of either intact erythrocytes or erythrocytes membranes with glucose decreases the activity of the membrane Ca(2+)-ATPase [González Flecha, Bermúdez, Cédola, Gagliardino and Rossi (1990) Diabetes 39, 707-711]. The aim of the present work was to obtain information about the mechanism of this inhibition. For this purpose, experiments were carried out with purified Ca(2+)-ATPase, inside-out vesicles and membranes from human erythrocytes. Incubation of the purified Ca(2+)-ATPase with glucose led to a decay in the enzyme activity of up to 50% of the control activity under the conditions used. The decrease in ATPase activity was concomitant with labelling by [6-3H]glucose of the purified Ca2+ pump; the kinetic properties of both processes were almost identical, suggesting that inhibition is a consequence of the incorporation of glucose into the Ca(2+)-ATPase molecule. In inside-out vesicles, glucose also promoted inhibition of Ca(2+)-ATPase activity as well as of active Ca2+ transport. Arabinose, xylose, mannose, ribose, fructose and glucose 6-phosphate (but not mannitol) were also able to inactive the ATPase. The activation energy for both the decrease in ATPase activity by glucose and the labelling of the pump with [6-3H]glucose was about 65 kJ/mol. Furthermore, inorganic phosphate enhanced the inactivation of the Ca(2+)-ATPase by glucose. This evidence strongly suggests that inhibition is a non-enzymically catalysed process. Inactivation of the Ca(2+)-ATPase by glucose was enhanced by reductive alkylation with sodium borohydride. Aminoguanidine, an inhibitor of the formation of the advanced end products of glycosylation, did not prevent the deleterious effect of glucose on the enzyme activity. Therefore it is concluded that inactivation of the Ca2+ pump is a consequence of the glycation of this protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee M., Vlassara H., Kooney A., Ulrich P., Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986 Jun 27;232(4758):1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Shapiro R., McManus M., Garrick L., McDonald M. J., Gallop P. M., Gabbay K. H. Structural heterogeneity of human hemoglobin A due to nonenzymatic glycosylation. J Biol Chem. 1979 May 25;254(10):3892–3898. [PubMed] [Google Scholar]
- Davis F. B., Davis P. J., Nat G., Blas S. D., MacGillivray M., Gutman S., Feldman M. J. The effect of in vivo glucose administration on human erythrocyte Ca2+-ATPase activity and on enzyme responsiveness in vitro to thyroid hormone and calmodulin. Diabetes. 1985 Jul;34(7):639–646. doi: 10.2337/diab.34.7.639. [DOI] [PubMed] [Google Scholar]
- Deziel M. R., Safeer R. S., Blas S. D., Davis F. B., Davis P. J. Hexose-specific inhibition in vitro of human red cell Ca(2+)-ATPase activity. Biochim Biophys Acta. 1992 Sep 21;1110(1):119–122. doi: 10.1016/0005-2736(92)90302-3. [DOI] [PubMed] [Google Scholar]
- Donnet C., Caride A. J., Fernández H. N., Rossi J. P. Differential reactivity of lysine residues of the red blood cell Ca2+ pump involved in the E1-E2 conformational equilibrium. Biochem J. 1991 Oct 1;279(Pt 1):121–127. doi: 10.1042/bj2790121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS G. P. The Maillard reaction. Adv Carbohydr Chem. 1959;14:63–134. doi: 10.1016/s0096-5332(08)60223-4. [DOI] [PubMed] [Google Scholar]
- Filoteo A. G., Gorski J. P., Penniston J. T. The ATP-binding site of the erythrocyte membrane Ca2+ pump. Amino acid sequence of the fluorescein isothiocyanate-reactive region. J Biol Chem. 1987 May 15;262(14):6526–6530. [PubMed] [Google Scholar]
- Garner M. H., Bahador A., Sachs G. Nonenzymatic glycation of Na,K-ATPase. Effects on ATP hydrolysis and K+ occlusion. J Biol Chem. 1990 Sep 5;265(25):15058–15066. [PubMed] [Google Scholar]
- Gietzen K., Wüthrich A., Bader H. R 24571: a new powerful inhibitor of red blood cell Ca++-transport ATPase and of calmodulin-regulated functions. Biochem Biophys Res Commun. 1981 Jul 30;101(2):418–425. doi: 10.1016/0006-291x(81)91276-6. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Flecha F. L., Bermúdez M. C., Cédola N. V., Gagliardino J. J., Rossi J. P. Decreased Ca2(+)-ATPase activity after glycosylation of erythrocyte membranes in vivo and in vitro. Diabetes. 1990 Jun;39(6):707–711. doi: 10.2337/diabetes.39.6.707. [DOI] [PubMed] [Google Scholar]
- HJERTEN S., MOSBACH R. "Molecular-sieve" chromatography of proteins on colums of cross-linked polyacrylamide. Anal Biochem. 1962 Feb;3:109–118. doi: 10.1016/0003-2697(62)90100-8. [DOI] [PubMed] [Google Scholar]
- KATCHALSKY A., SHARON N. Kinetics of aldose-amino acid interaction. Biochim Biophys Acta. 1953 Feb;10(2):290–301. doi: 10.1016/0006-3002(53)90252-2. [DOI] [PubMed] [Google Scholar]
- Kratje R. B., Garrahan P. J., Rega A. F. The effects of alkali metal ions on active Ca2+ transport in reconstituted ghosts from human red cells. Biochim Biophys Acta. 1983 May 26;731(1):40–46. doi: 10.1016/0005-2736(83)90395-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Means G. E. Reductive alkylation of amino groups. Methods Enzymol. 1977;47:469–478. doi: 10.1016/0076-6879(77)47047-2. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Richards D. E., Rega A. F., Garrahan P. J. Two classes of site for ATP in the Ca2+-ATPase from human red cell membranes. Biochim Biophys Acta. 1978 Aug 4;511(2):194–201. doi: 10.1016/0005-2736(78)90313-9. [DOI] [PubMed] [Google Scholar]
- Rossi J. P., Gronda C. M., Fernandez H. N., Gagliardino J. J. Characteristics of a Ca2+-ATPase activity measured in islet homogenates. Biochim Biophys Acta. 1988 Aug 18;943(2):175–182. doi: 10.1016/0005-2736(88)90549-4. [DOI] [PubMed] [Google Scholar]
- Rossi J. P., Schatzmann H. J. Is the red cell calcium pump electrogenic? J Physiol. 1982 Jun;327:1–15. doi: 10.1113/jphysiol.1982.sp014215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Szász I., Gárdos G. Characteristics and regulation of active calcium transport in inside-out red cell membrane vesicles. Biochim Biophys Acta. 1980 May 23;598(2):326–338. doi: 10.1016/0005-2736(80)90010-3. [DOI] [PubMed] [Google Scholar]
- Watkins N. G., Neglia-Fisher C. I., Dyer D. G., Thorpe S. R., Baynes J. W. Effect of phosphate on the kinetics and specificity of glycation of protein. J Biol Chem. 1987 May 25;262(15):7207–7212. [PubMed] [Google Scholar]
- Watkins N. G., Thorpe S. R., Baynes J. W. Glycation of amino groups in protein. Studies on the specificity of modification of RNase by glucose. J Biol Chem. 1985 Sep 5;260(19):10629–10636. [PubMed] [Google Scholar]
- Zvaritch E., James P., Vorherr T., Falchetto R., Modyanov N., Carafoli E. Mapping of functional domains in the plasma membrane Ca2+ pump using trypsin proteolysis. Biochemistry. 1990 Sep 4;29(35):8070–8076. doi: 10.1021/bi00487a012. [DOI] [PubMed] [Google Scholar]


