Abstract
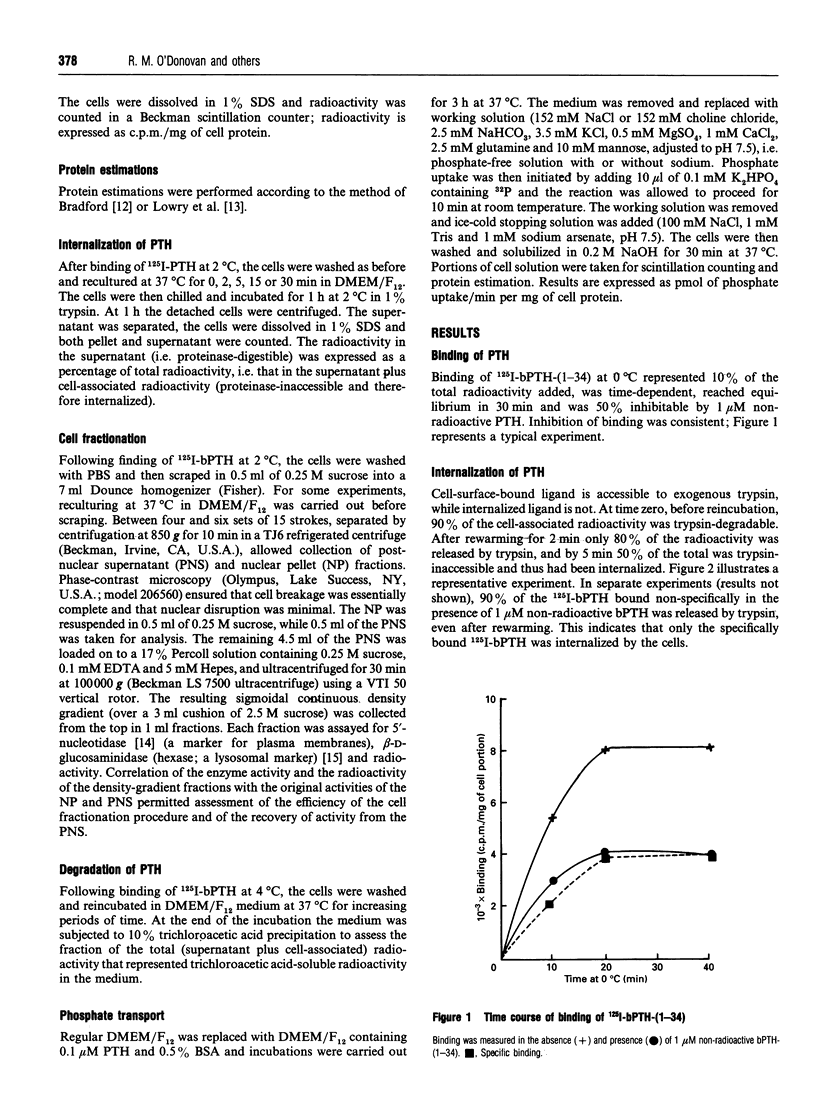
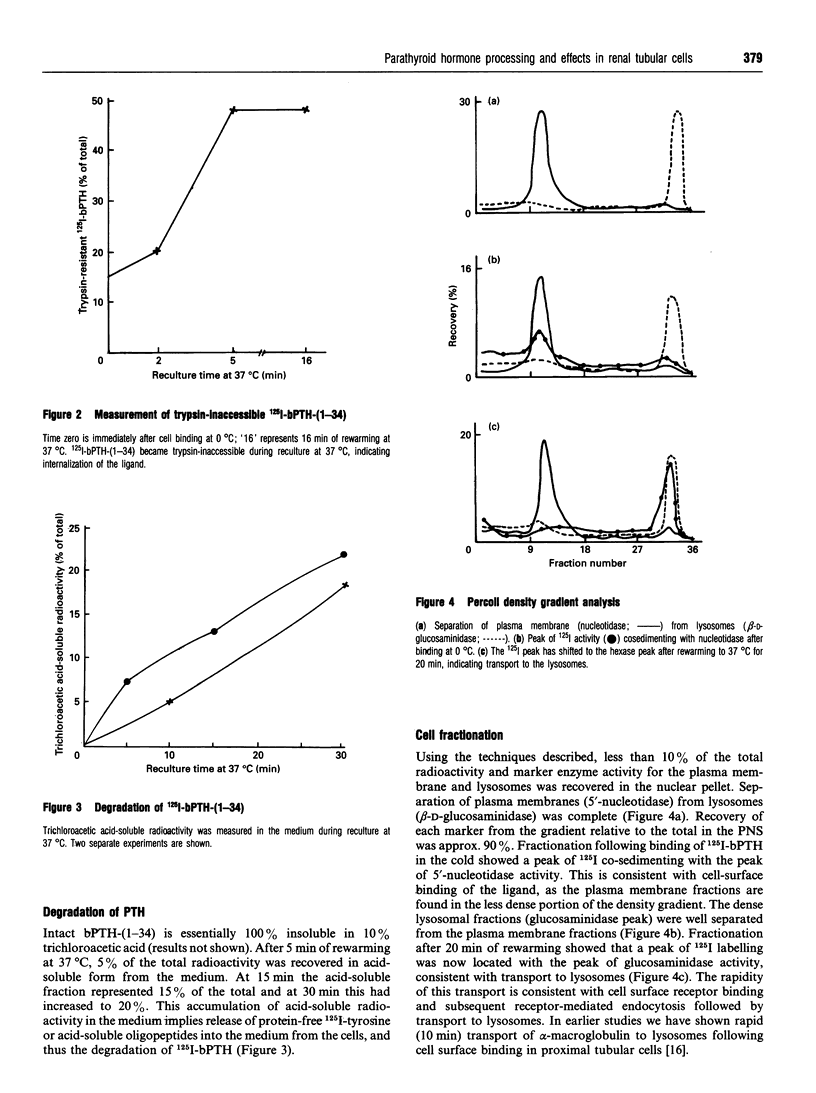
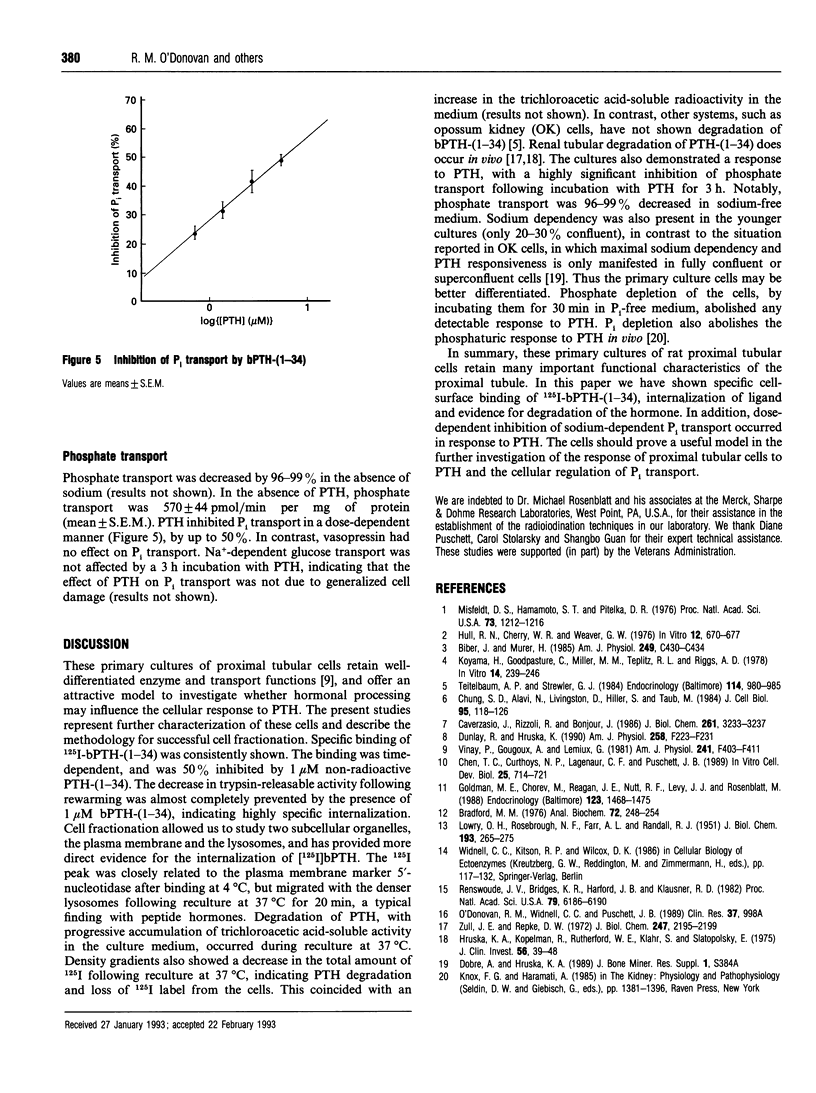
The development of satisfactory cell culture models for the study of parathyroid hormone (PTH)-induced inhibition of Pi transport has proven difficult. Using subcellular fractionation techniques we investigated the response of primary cultures of rat proximal tubular cells to PTH-(1-34). Specific binding of 125I-bPTH-(1-34) occurred at 2 degrees C. After 5 min of rewarming, trypsin-releasable radioactivity decreased from 90 to 50%, indicating internalization of the ligand. Cell disruption, followed by density centrifugation with 17% Percoll either directly after binding at 2 degrees C or post-rewarming for 20 min, showed a shift of 125I label from the plasma membrane (5'-nucleotidase) to lysosomal fractions (beta-D-glucosaminidase), confirming the sequential occurrence of cell surface binding, internalization and transport to lysosomes of 125I-bPTH-(1-34). Reculture at 37 degrees C revealed steady accumulation of trichloroacetic acid-soluble radioactivity in the medium, indicating degradation of 125I-bPTH-(1-34). Phosphate transport in the absence of sodium was minimal. Incubation of the cells with bPTH-(1-34) resulted in up to 50% inhibition of sodium-dependent phosphate transport. Prior phosphate depletion abrogated the response to PTH.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biber J., Murer H. Na-Pi cotransport in LLC-PK1 cells: fast adaptive response to Pi deprivation. Am J Physiol. 1985 Nov;249(5 Pt 1):C430–C434. doi: 10.1152/ajpcell.1985.249.5.C430. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caverzasio J., Rizzoli R., Bonjour J. P. Sodium-dependent phosphate transport inhibited by parathyroid hormone and cyclic AMP stimulation in an opossum kidney cell line. J Biol Chem. 1986 Mar 5;261(7):3233–3237. [PubMed] [Google Scholar]
- Chen T. C., Curthoys N. P., Lagenaur C. F., Puschett J. B. Characterization of primary cell cultures derived from rat renal proximal tubules. In Vitro Cell Dev Biol. 1989 Aug;25(8):714–722. doi: 10.1007/BF02623724. [DOI] [PubMed] [Google Scholar]
- Chung S. D., Alavi N., Livingston D., Hiller S., Taub M. Characterization of primary rabbit kidney cultures that express proximal tubule functions in a hormonally defined medium. J Cell Biol. 1982 Oct;95(1):118–126. doi: 10.1083/jcb.95.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlay R., Hruska K. PTH receptor coupling to phospholipase C is an alternate pathway of signal transduction in bone and kidney. Am J Physiol. 1990 Feb;258(2 Pt 2):F223–F231. doi: 10.1152/ajprenal.1990.258.2.F223. [DOI] [PubMed] [Google Scholar]
- Goldman M. E., Chorev M., Reagan J. E., Nutt R. F., Levy J. J., Rosenblatt M. Evaluation of novel parathyroid hormone analogs using a bovine renal membrane receptor binding assay. Endocrinology. 1988 Sep;123(3):1468–1475. doi: 10.1210/endo-123-3-1468. [DOI] [PubMed] [Google Scholar]
- Hruska K. A., Kopelman R., Rutherford W. E., Klahr S., Slatopolsky E., Greenwalt A., Bascom T., Markham J. Metabolism in immunoreactive parathyroid hormone in the dog. The role of the kidney and the effects of chronic renal disease. J Clin Invest. 1975 Jul;56(1):39–48. doi: 10.1172/JCI108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. N., Cherry W. R., Weaver G. W. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro. 1976 Oct;12(10):670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- Koyama H., Goodpasture C., Miller M. M., Teplitz R. L., Riggs A. D. Establishment and characterization of a cell line from the American opossum (Didelphys virginiana). In Vitro. 1978 Mar;14(3):239–246. doi: 10.1007/BF02616032. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum A. P., Strewler G. J. Parathyroid hormone receptors coupled to cyclic adenosine monophosphate formation in an established renal cell line. Endocrinology. 1984 Mar;114(3):980–985. doi: 10.1210/endo-114-3-980. [DOI] [PubMed] [Google Scholar]
- Vinay P., Gougoux A., Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol. 1981 Oct;241(4):F403–F411. doi: 10.1152/ajprenal.1981.241.4.F403. [DOI] [PubMed] [Google Scholar]
- Zull J. E., Repke D. W. The tissue localization of tritiated parathyroid hormone in thyroparathyroidectomized rats. J Biol Chem. 1972 Apr 10;247(7):2195–2199. [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]


