Abstract
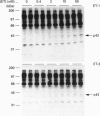
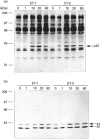
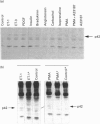
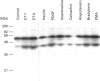
Endothelins (ET-1, -2, -3) display pleiotropic activities, by signalling through G-protein-coupled membrane receptors. We show here that ET-1 and ET-3 stimulate within minutes the tyrosine phosphorylation of a 42 kDa protein (p42) in primary cultures of mouse embryo astrocytes, but not in any of two subclones of rat astrocytoma C6 cells. This effect, measured by anti-phosphotyrosine immunoblotting of cell extracts, was also observed in response to bradykinin, platelet-derived growth factor, the phorbol ester phorbol 12-myristate 13-acetate and the G-protein activator fluoroaluminate. Pretreatment of cells with pertussis toxin, which inactivates Gi/G(o) proteins, did not affect these responses. However, down-regulation of protein kinase C completely blocked the response to phorbol ester and fluoroaluminate and at least partially impaired the ET-1-stimulated phosphorylation of p42. We have identified p42 as p42mapk, a mitogen-activated protein (MAP) kinase, on the basis of the following data: by sequential immunoblotting with antiphosphotyrosine and anti-MAP kinase antibodies, (i) similar kinetics are observed for p42 phosphorylation and the decrease in p42mapk electrophoretic mobility, likely corresponding to its tyrosine/threonine phosphorylation [de Vries-Smits, Boudewijn, Burgering, Leevers, Marshall and Bos (1992) Nature (London) 357, 602-604]; (ii) p42 and the shifted form of p42mapk co-migrate on SDS/PAGE; (iii) the myelin-basic-protein kinase activity of p42mapk is stimulated by ET-1, in parallel with the tyrosine phosphorylation of p42. In conclusion, these findings strongly suggest that endothelins can stimulate the tyrosine phosphorylation and activation of p42mapk in astrocytes, via pertussis-toxin-insensitive G protein and protein kinase C-dependent and -independent pathways.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. D., Parker P. J. Activation of mitogen-activated protein (MAP) kinase by a MAP kinase-kinase. J Biol Chem. 1992 Jul 5;267(19):13135–13137. [PubMed] [Google Scholar]
- Anderson N. G., Kilgour E., Sturgill T. W. Activation of mitogen-activated protein kinase in BC3H1 myocytes by fluoroaluminate. J Biol Chem. 1991 Jun 5;266(16):10131–10135. [PubMed] [Google Scholar]
- Boulton T. G., Cobb M. H. Identification of multiple extracellular signal-regulated kinases (ERKs) with antipeptide antibodies. Cell Regul. 1991 May;2(5):357–371. doi: 10.1091/mbc.2.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M. Cross-talk between second messengers. Ann N Y Acad Sci. 1990;594:120–129. doi: 10.1111/j.1749-6632.1990.tb40473.x. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Cotton P. C., Queral A. E., Barrett J. N., Nonner D., Keane R. W. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985 Aug 8;316(6028):554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Bowen-Pope D. F., Raines E., Ross R., Hunter T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell. 1982 Nov;31(1):263–273. doi: 10.1016/0092-8674(82)90426-3. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Major substrate for growth factor-activated protein-tyrosine kinases is a low-abundance protein. Mol Cell Biol. 1985 Nov;5(11):3304–3309. doi: 10.1128/mcb.5.11.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countaway J. L., McQuilkin P., Gironès N., Davis R. J. Multisite phosphorylation of the epidermal growth factor receptor. Use of site-directed mutagenesis to examine the role of serine/threonine phosphorylation. J Biol Chem. 1990 Feb 25;265(6):3407–3416. [PubMed] [Google Scholar]
- Couraud P. O., Durieu-Trautmann O., Nguyen D. L., Marin P., Glibert F., Strosberg A. D. Functional endothelin-1 receptors in rat astrocytoma C6. Eur J Pharmacol. 1991 Mar 25;206(3):191–198. doi: 10.1016/s0922-4106(05)80018-5. [DOI] [PubMed] [Google Scholar]
- Durieu-Trautmann O., Foignant-Chaverot N., Perdomo J., Gounon P., Strosberg A. D., Couraud P. O. Immortalization of brain capillary endothelial cells with maintenance of structural characteristics of the blood-brain barrier endothelium. In Vitro Cell Dev Biol. 1991 Oct;27A(10):771–778. doi: 10.1007/BF02631242. [DOI] [PubMed] [Google Scholar]
- Durieu-Trautmann O., Fédérici C., Créminon C., Foignant-Chaverot N., Roux F., Claire M., Strosberg A. D., Couraud P. O. Nitric oxide and endothelin secretion by brain microvessel endothelial cells: regulation by cyclic nucleotides. J Cell Physiol. 1993 Apr;155(1):104–111. doi: 10.1002/jcp.1041550114. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Platelet tyrosine-specific protein phosphorylation is regulated by thrombin. Mol Cell Biol. 1988 Sep;8(9):3603–3610. doi: 10.1128/mcb.8.9.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force T., Kyriakis J. M., Avruch J., Bonventre J. V. Endothelin, vasopressin, and angiotensin II enhance tyrosine phosphorylation by protein kinase C-dependent and -independent pathways in glomerular mesangial cells. J Biol Chem. 1991 Apr 5;266(10):6650–6656. [PubMed] [Google Scholar]
- Giaid A., Gibson S. J., Ibrahim B. N., Legon S., Bloom S. R., Yanagisawa M., Masaki T., Varndell I. M., Polak J. M. Endothelin 1, an endothelium-derived peptide, is expressed in neurons of the human spinal cord and dorsal root ganglia. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7634–7638. doi: 10.1073/pnas.86.19.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Brugge J. S. Thrombin treatment induces rapid changes in tyrosine phosphorylation in platelets. Proc Natl Acad Sci U S A. 1989 Feb;86(3):901–905. doi: 10.1073/pnas.86.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameshita I., Fujisawa H. A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1989 Nov 15;183(1):139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Kohno M., Pouysségur J. Alpha-thrombin-induced tyrosine phosphorylation of 43,000- and 41,000-Mr proteins is independent of cytoplasmic alkalinization in quiescent fibroblasts. Biochem J. 1986 Sep 1;238(2):451–457. doi: 10.1042/bj2380451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Allemain G., Pouyssegur J., Weber M. J. p42/mitogen-activated protein kinase as a converging target for different growth factor signaling pathways: use of pertussis toxin as a discrimination factor. Cell Regul. 1991 Aug;2(8):675–684. doi: 10.1091/mbc.2.8.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenheim R. G., Lacroix I., Foignant-Chaverot N., Strosberg A. D., Couraud P. O. Endothelins stimulate c-fos and nerve growth factor expression in astrocytes and astrocytoma. J Neurochem. 1993 Jan;60(1):260–266. doi: 10.1111/j.1471-4159.1993.tb05846.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Song X. H. Bradykinin and bombesin rapidly stimulate tyrosine phosphorylation of a 120-kDa group of proteins in Swiss 3T3 cells. J Biol Chem. 1991 Apr 25;266(12):7746–7749. [PubMed] [Google Scholar]
- MacCumber M. W., Ross C. A., Snyder S. H. Endothelin in brain: receptors, mitogenesis, and biosynthesis in glial cells. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2359–2363. doi: 10.1073/pnas.87.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin P., Delumeau J. C., Durieu-Trautmann O., Le Nguyen D., Prémont J., Strosberg A. D., Couraud P. O. Are several G proteins involved in the different effects of endothelin-1 in mouse striatal astrocytes? J Neurochem. 1991 Apr;56(4):1270–1275. doi: 10.1111/j.1471-4159.1991.tb11421.x. [DOI] [PubMed] [Google Scholar]
- Nakamura K. D., Martinez R., Weber M. J. Tyrosine phosphorylation of specific proteins after mitogen stimulation of chicken embryo fibroblasts. Mol Cell Biol. 1983 Mar;3(3):380–390. doi: 10.1128/mcb.3.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakielny S., Campbell D. G., Cohen P. MAP kinase kinase from rabbit skeletal muscle. A novel dual specificity enzyme showing homology to yeast protein kinases involved in pheromone-dependent signal transduction. FEBS Lett. 1992 Aug 17;308(2):183–189. doi: 10.1016/0014-5793(92)81271-m. [DOI] [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Hernández-Sotomayor S. M., Tonks N. K., Rhee S. G., Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990 Nov 30;250(4985):1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S. MAP kinases: charting the regulatory pathways. Science. 1992 Sep 4;257(5075):1355–1356. doi: 10.1126/science.1382311. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S. Mitogen-activated protein kinases: versatile transducers for cell signaling. Trends Biochem Sci. 1992 Jun;17(6):233–238. doi: 10.1016/s0968-0004(00)80005-5. [DOI] [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossomando A., Wu J., Weber M. J., Sturgill T. W. The phorbol ester-dependent activator of the mitogen-activated protein kinase p42mapk is a kinase with specificity for the threonine and tyrosine regulatory sites. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5221–5225. doi: 10.1073/pnas.89.12.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strosberg A. D. Structure/function relationship of proteins belonging to the family of receptors coupled to GTP-binding proteins. Eur J Biochem. 1991 Feb 26;196(1):1–10. doi: 10.1111/j.1432-1033.1991.tb15778.x. [DOI] [PubMed] [Google Scholar]
- Tobe K., Kadowaki T., Tamemoto H., Ueki K., Hara K., Koshio O., Momomura K., Gotoh Y., Nishida E., Akanuma Y. Insulin and 12-O-tetradecanoylphorbol-13-acetate activation of two immunologically distinct myelin basic protein/microtubule-associated protein 2 (MBP/MAP2) kinases via de novo phosphorylation of threonine and tyrosine residues. J Biol Chem. 1991 Dec 25;266(36):24793–24803. [PubMed] [Google Scholar]
- Tuy F. P., Henry J., Rosenfeld C., Kahn A. High tyrosine kinase activity in normal nonproliferating cells. 1983 Sep 29-Oct 5Nature. 305(5933):435–438. doi: 10.1038/305435a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wang Y., Simonson M. S., Pouysségur J., Dunn M. J. Endothelin rapidly stimulates mitogen-activated protein kinase activity in rat mesangial cells. Biochem J. 1992 Oct 15;287(Pt 2):589–594. doi: 10.1042/bj2870589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Gil J., Lehmann W., Sinnett-Smith J., Rozengurt E. Bombesin, vasopressin, and endothelin rapidly stimulate tyrosine phosphorylation in intact Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4577–4581. doi: 10.1073/pnas.88.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J., Rozengurt E. Stimulation of tyrosine kinase activity in anti-phosphotyrosine immune complexes of Swiss 3T3 cell lysates occurs rapidly after addition of bombesin, vasopressin, and endothelin to intact cells. J Biol Chem. 1991 Dec 15;266(35):24126–24133. [PubMed] [Google Scholar]
- de Vries-Smits A. M., Burgering B. M., Leevers S. J., Marshall C. J., Bos J. L. Involvement of p21ras in activation of extracellular signal-regulated kinase 2. Nature. 1992 Jun 18;357(6379):602–604. doi: 10.1038/357602a0. [DOI] [PubMed] [Google Scholar]
- el-Etr M., Cordier J., Torrens Y., Glowinski J., Prémont J. Pharmacological and functional heterogeneity of astrocytes: regional differences in phospholipase C stimulation by neuromediators. J Neurochem. 1989 Mar;52(3):981–984. doi: 10.1111/j.1471-4159.1989.tb02551.x. [DOI] [PubMed] [Google Scholar]