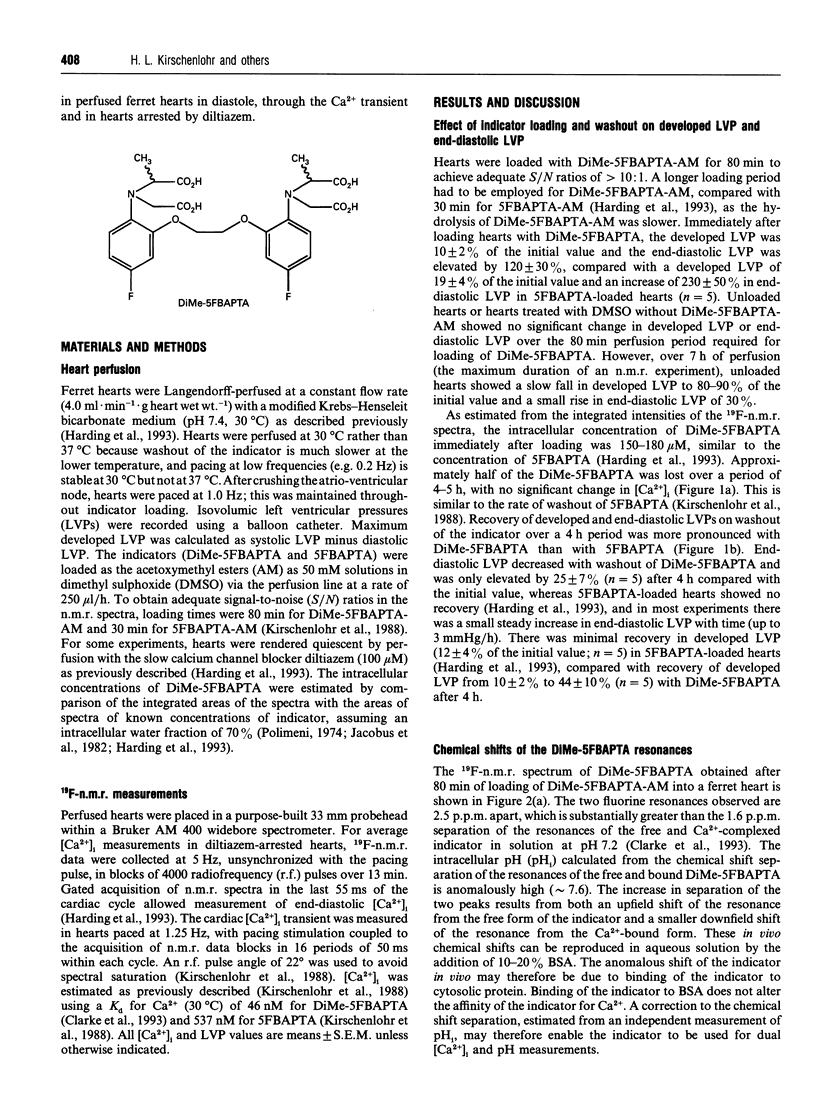
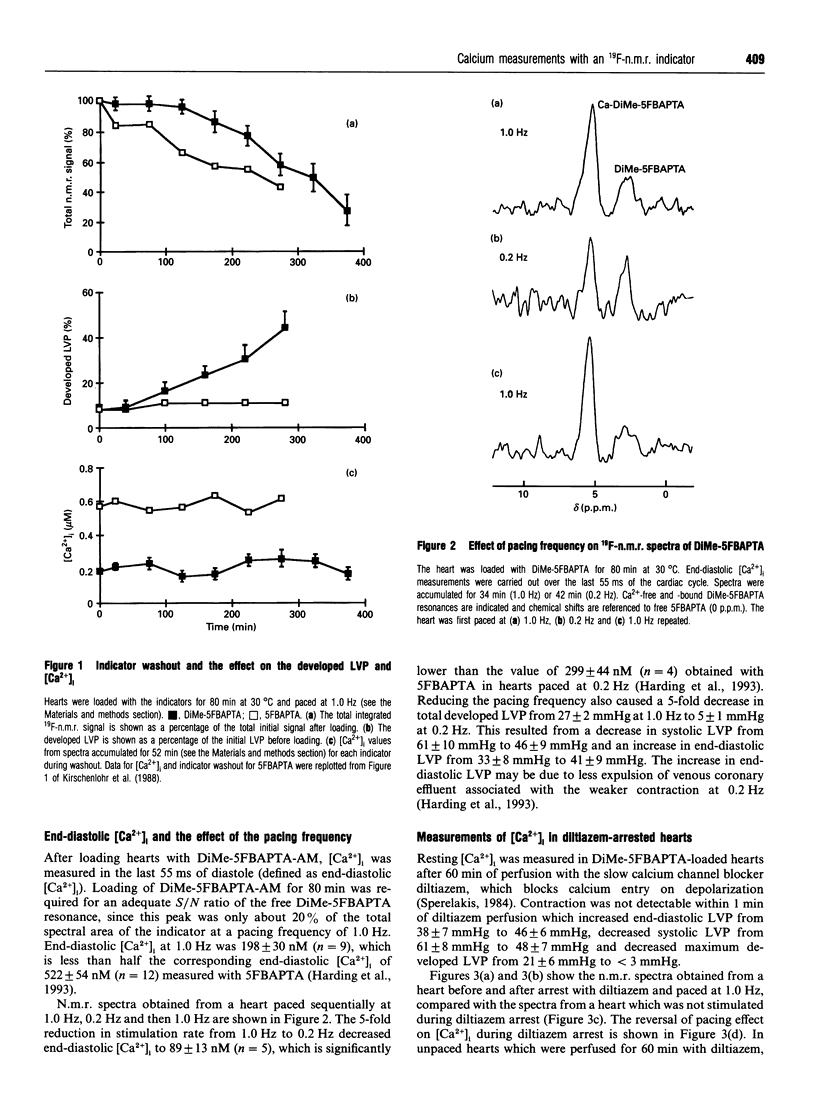
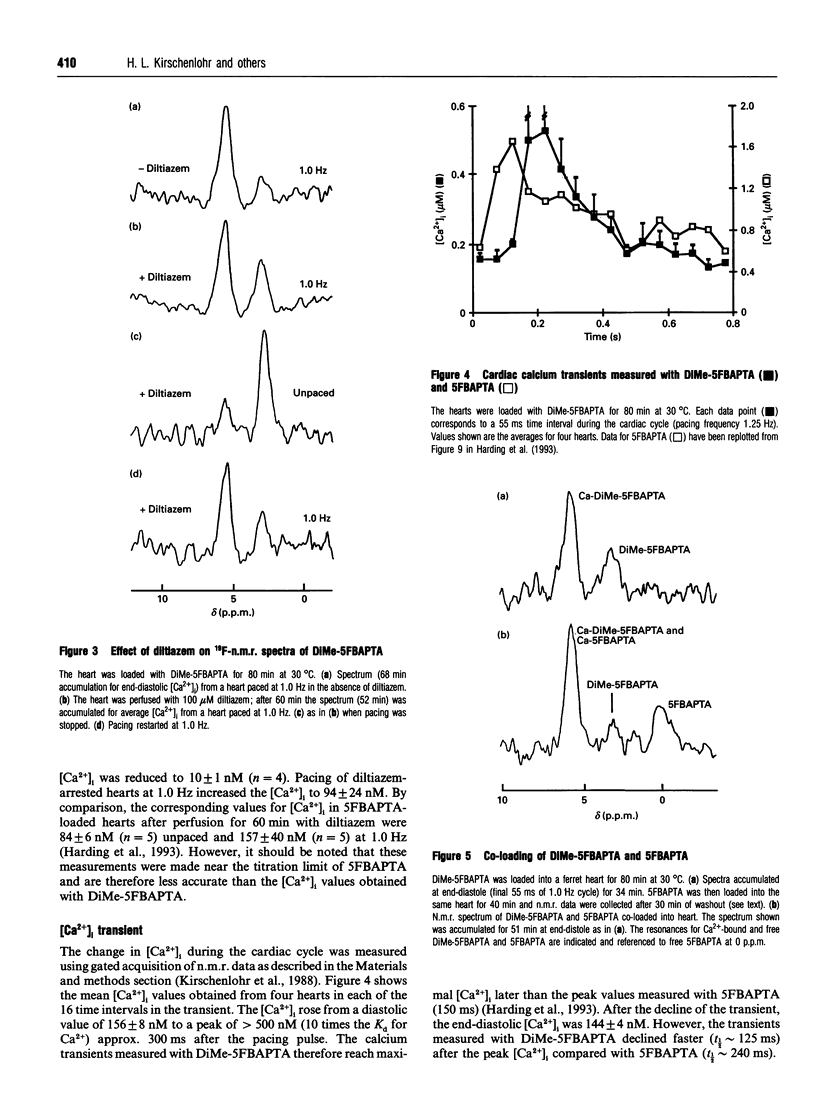
Abstract
A new n.m.r. indicator, 1,2-bis-(2-[1-(hydroxycarbony)ethyl- (hydroxycarbonylmethyl)]amino-5-fluorophenoxy)ethane (DiMe-5FBAPTA), with a higher affinity for calcium (apparent Kd 46 nM, pH 7.2, 30 degrees C) than the parent 5FBAPTA chelator (Kd 537 nM, pH 7.1, 30 degrees C) has been used to measure the cardiac intracellular free Ca2+ ([Ca2+]i). DiMe-5FBAPTA was loaded into Langendorff-perfused ferret hearts maintained at 30 degrees C using the acetoxymethyl ester (AM) derivative. The intracellular concentration required to achieve an adequate signal-to-noise (S/N) ratio (> 10:1) for the n.m.r. spectra caused a similar reduction in developed pressure to that obtained using 5FBAPTA-AM. The DiMe-5FBAPTA was used to estimate [Ca2+]i in diastole, through the calcium transient and at rest in the presence of the slow calcium channel blocker diltiazem. At a pacing frequency of 1.0 Hz, end-diastolic [Ca2+]i was 198 +/- 30 nM (n = 9), and reducing the pacing frequency to 0.2 Hz lowered [Ca2+]i to 89 +/- 13 nM (n = 5). Perfusion with diltiazem (100 microM) for 60 min lowered [Ca2+]i to 10 +/- 1 nM (n = 4) in unpaced hearts and to 94 +/- 24 nM (n = 4) in hearts paced at 1.0 Hz. The [Ca2+]i transient measured with DiMe-5FBAPTA was sharper and delayed compared with the transient measured previously with 5FBAPTA. Co-loading the two indicators provided evidence that the indicator with the higher Kd had a dominant effect on the end-diastolic [Ca2+]i. The lower values for end-diastolic [Ca2+]i obtained with DiMe-5FBAPTA are consistent with fluorescent indicator measurements. These observations suggest that perturbations of [Ca2+]i caused by the new indicator are less than those induced by 5FBAPTA. DiMe-5FBAPTA therefore represents a useful step in the development of 19F-n.m.r. calcium indicators.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borzak S., Kelly R. A., Krämer B. K., Matoba Y., Marsh J. D., Reers M. In situ calibration of fura-2 and BCECF fluorescence in adult rat ventricular myocytes. Am J Physiol. 1990 Sep;259(3 Pt 2):H973–H981. doi: 10.1152/ajpheart.1990.259.3.H973. [DOI] [PubMed] [Google Scholar]
- Frampton J. E., Orchard C. H., Boyett M. R. Diastolic, systolic and sarcoplasmic reticulum [Ca2+] during inotropic interventions in isolated rat myocytes. J Physiol. 1991 Jun;437:351–375. doi: 10.1113/jphysiol.1991.sp018600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenlohr H. L., Metcalfe J. C., Morris P. G., Rodrigo G. C., Smith G. A. Ca2+ transient, Mg2+, and pH measurements in the cardiac cycle by 19F NMR. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9017–9021. doi: 10.1073/pnas.85.23.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban E., Kitakaze M., Chacko V. P., Pike M. M. Ca2+ transients in perfused hearts revealed by gated 19F NMR spectroscopy. Circ Res. 1988 Sep;63(3):673–678. doi: 10.1161/01.res.63.3.673. [DOI] [PubMed] [Google Scholar]
- Marban E., Kitakaze M., Koretsune Y., Yue D. T., Chacko V. P., Pike M. M. Quantification of [Ca2+]i in perfused hearts. Critical evaluation of the 5F-BAPTA and nuclear magnetic resonance method as applied to the study of ischemia and reperfusion. Circ Res. 1990 May;66(5):1255–1267. doi: 10.1161/01.res.66.5.1255. [DOI] [PubMed] [Google Scholar]
- Marban E., Kitakaze M., Kusuoka H., Porterfield J. K., Yue D. T., Chacko V. P. Intracellular free calcium concentration measured with 19F NMR spectroscopy in intact ferret hearts. Proc Natl Acad Sci U S A. 1987 Aug;84(16):6005–6009. doi: 10.1073/pnas.84.16.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J. C., Hesketh T. R., Smith G. A. Free cytosolic Ca2+ measurements with fluorine labelled indicators using 19FNMR. Cell Calcium. 1985 Apr;6(1-2):183–195. doi: 10.1016/0143-4160(85)90043-0. [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Seguchi M., Tsuchiya T., Yasukouchi S., Takao A. Effect of acidosis on intracellular pH and calcium concentration in the newborn and adult rabbit myocardium. Circ Res. 1990 Jul;67(1):111–123. doi: 10.1161/01.res.67.1.111. [DOI] [PubMed] [Google Scholar]
- Polimeni P. I. Extracellular space and ionic distribution in rat ventricle. Am J Physiol. 1974 Sep;227(3):676–683. doi: 10.1152/ajplegacy.1974.227.3.676. [DOI] [PubMed] [Google Scholar]
- Smith G. A., Hesketh R. T., Metcalfe J. C., Feeney J., Morris P. G. Intracellular calcium measurements by 19F NMR of fluorine-labeled chelators. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7178–7182. doi: 10.1073/pnas.80.23.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N. Hormonal and neurotransmitter regulation of Ca++ influx through voltage-dependent slow channels in cardiac muscle membrane. Membr Biochem. 1984;5(2):131–166. doi: 10.3109/09687688409150275. [DOI] [PubMed] [Google Scholar]
- Steenbergen C., Murphy E., Levy L., London R. E. Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circ Res. 1987 May;60(5):700–707. doi: 10.1161/01.res.60.5.700. [DOI] [PubMed] [Google Scholar]


