Abstract
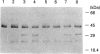
In order to elucidate the processing mechanism of the lysosomal cysteine proteinase, cathepsin B, in mammalian cells, recombinant rat and human cathepsin B precursors were expressed in Saccharomyces cerevisiae. The active-site cysteine residue was changed to serine to prevent autoprocessing. When the purified proenzymes were incubated with the soluble fraction of postnuclear organelles obtained from human hepatoma HepG2 cells, processing to a 33 kDa form corresponding to the mature endogenous single-chain enzyme was observed. Inhibitors of metallo-, serine and aspartic proteinases exerted no significant effect on procathepsin B processing in vitro. However, the processing activity was effectively blocked by cysteine proteinase inhibitors, in particular E-64 and its cathepsin-B-selective derivative CA-074. Processing positions were identified by using anti-peptide antibodies specific for epitopes in the N- and C-terminal cleavage regions. The single-chain form produced in vitro was thus shown to contain an N-terminal extension of at least four residues relative to the mature lysosomal enzyme, as well as a C-terminal extension present in the proenzyme but usually absent in fully processed cathepsin B. On expression of the wild-type proenzyme in yeast, procathepsin B undergoes autoprocessing, yielding a single-chain form of the active enzyme, which contains similar N- and C-terminal extensions. These results indicate that maturation of procathepsin B in vivo in mammalian tissues relies on the proteolytic activity of cathepsin B itself.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstetter T., Wolf D. H. Proteinases, proteolysis and biological control in the yeast Saccharomyces cerevisiae. Yeast. 1985 Dec;1(2):139–157. doi: 10.1002/yea.320010203. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Bernatowicz M. S., Matsueda G. R. Preparation of peptide-protein immunogens using N-succinimidyl bromoacetate as a heterobifunctional crosslinking reagent. Anal Biochem. 1986 May 15;155(1):95–102. doi: 10.1016/0003-2697(86)90231-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chan S. J., San Segundo B., McCormick M. B., Steiner D. F. Nucleotide and predicted amino acid sequences of cloned human and mouse preprocathepsin B cDNAs. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7721–7725. doi: 10.1073/pnas.83.20.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conyers S. M., Kidwell D. A. Chromogenic substrates for horseradish peroxidase. Anal Biochem. 1991 Jan;192(1):207–211. doi: 10.1016/0003-2697(91)90208-b. [DOI] [PubMed] [Google Scholar]
- Felleisen R., Klinkert M. Q. In vitro translation and processing of cathepsin B of Schistosoma mansoni. EMBO J. 1990 Feb;9(2):371–377. doi: 10.1002/j.1460-2075.1990.tb08120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieselmann V., Pohlmann R., Hasilik A., Von Figura K. Biosynthesis and transport of cathepsin D in cultured human fibroblasts. J Cell Biol. 1983 Jul;97(1):1–5. doi: 10.1083/jcb.97.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanewinkel H., Glössl J., Kresse H. Biosynthesis of cathepsin B in cultured normal and I-cell fibroblasts. J Biol Chem. 1987 Sep 5;262(25):12351–12355. [PubMed] [Google Scholar]
- Hara K., Kominami E., Katunuma N. Effect of proteinase inhibitors on intracellular processing of cathepsin B, H and L in rat macrophages. FEBS Lett. 1988 Apr 11;231(1):229–231. doi: 10.1016/0014-5793(88)80737-3. [DOI] [PubMed] [Google Scholar]
- Jones E. W. Three proteolytic systems in the yeast saccharomyces cerevisiae. J Biol Chem. 1991 May 5;266(13):7963–7966. [PubMed] [Google Scholar]
- Mach L., Stüwe K., Hagen A., Ballaun C., Glössl J. Proteolytic processing and glycosylation of cathepsin B. The role of the primary structure of the latent precursor and of the carbohydrate moiety for cell-type-specific molecular forms of the enzyme. Biochem J. 1992 Mar 1;282(Pt 2):577–582. doi: 10.1042/bj2820577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Green G. D., Barrett A. J. Human liver cathepsin L. Biochem J. 1985 Feb 15;226(1):233–241. doi: 10.1042/bj2260233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClary J. A., Witney F., Geisselsoder J. Efficient site-directed in vitro mutagenesis using phagemid vectors. Biotechniques. 1989 Mar;7(3):282–289. [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Nikawa T., Towatari T., Katunuma N. Purification and characterization of cathepsin J from rat liver. Eur J Biochem. 1992 Feb 15;204(1):381–393. doi: 10.1111/j.1432-1033.1992.tb16647.x. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Kawabata T., Kato K. Identification of latent procathepsins B and L in microsomal lumen: characterization of enzymatic activation and proteolytic processing in vitro. Arch Biochem Biophys. 1988 Feb 15;261(1):64–71. doi: 10.1016/0003-9861(88)90104-x. [DOI] [PubMed] [Google Scholar]
- Rich D. H., Brown M. A., Barrett A. J. Purification of cathepsin B by a new form of affinity chromatography. Biochem J. 1986 May 1;235(3):731–734. doi: 10.1042/bj2350731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnboutt S., Stoorvogel W., Geuze H. J., Strous G. J. Identification of subcellular compartments involved in biosynthetic processing of cathepsin D. J Biol Chem. 1992 Aug 5;267(22):15665–15672. [PubMed] [Google Scholar]
- Rowan A. D., Mason P., Mach L., Mort J. S. Rat procathepsin B. Proteolytic processing to the mature form in vitro. J Biol Chem. 1992 Aug 5;267(22):15993–15999. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz W. N., Barrett A. J. Human cathepsin H. Biochem J. 1980 Nov 1;191(2):487–497. doi: 10.1042/bj1910487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towatari T., Nikawa T., Murata M., Yokoo C., Tamai M., Hanada K., Katunuma N. Novel epoxysuccinyl peptides. A selective inhibitor of cathepsin B, in vivo. FEBS Lett. 1991 Mar 25;280(2):311–315. doi: 10.1016/0014-5793(91)80319-x. [DOI] [PubMed] [Google Scholar]
- Tsai P. K., Frevert J., Ballou C. E. Carbohydrate structure of Saccharomyces cerevisiae mnn9 mannoprotein. J Biol Chem. 1984 Mar 25;259(6):3805–3811. [PubMed] [Google Scholar]
- Vernet T., Khouri H. E., Laflamme P., Tessier D. C., Musil R., Gour-Salin B. J., Storer A. C., Thomas D. Y. Processing of the papain precursor. Purification of the zymogen and characterization of its mechanism of processing. J Biol Chem. 1991 Nov 15;266(32):21451–21457. [PubMed] [Google Scholar]
- Wilcox D., Mason R. W. Inhibition of cysteine proteinases in lysosomes and whole cells. Biochem J. 1992 Jul 15;285(Pt 2):495–502. doi: 10.1042/bj2850495. [DOI] [PMC free article] [PubMed] [Google Scholar]