Abstract
Hereditary leiomyomatosis and renal cell cancer (HLRCC) is a rare autosomal-dominant disorder caused by a heterozygous germline mutation in the fumarate hydratase (FH) gene. HLRCC is clinically characterized by the development of three tumors: uterine leiomyomata, cutaneous leiomyomata, and renal cell carcinoma (RCC). HLRCC-associated RCC is aggressive and diagnosed at a much earlier age than sporadic RCC. It is essential for carriers of HLRCC to undergo annual renal screening by magnetic resonance imaging to detect early stage RCCs. Metastatic HLRCC-associated RCC must be treated by systemic therapy; however, it is unclear which medicines are most effective in treating this cancer owing to its low incidence rate. Immune checkpoint inhibitor (ICI) combinations or ICIs plus tyrosine kinase inhibitors are administered as systemic therapy for clear cell RCC. Here, we report a patient with HLRCC-associated RCC treated with sequential therapy, including ipilimumab plus nivolumab combination and cabozantinib, after diagnosis of HLRCC-associated RCC using FoundationOne Liquid CDx and single-site analysis. We also investigated familial FH mutations and describe a new family pedigree for HLRCC.
Keywords: FoundationOne Liquid CDx, fumarate hydratase, hereditary leiomyomatosis and renal cell cancer, immune checkpoint inhibitor
HLRCC is an autosomal-dominant disorder. The optimal therapeutic approach for metastatic HLRCC-associated renal cell carcinoma remains uncertain. It is imperative to examine blood relatives for the early identification and intervention of the condition.
Introduction
Hereditary leiomyomatosis and renal cell cancer (HLRCC; OMIN #150800) is a rare autosomal-dominant disorder caused by a heterozygous germline mutation in the fumarate hydratase (FH) gene, which encodes an enzyme that is part of the tricarboxylic acid cycle [1]. A previous study estimated the prevalence of HLRCC to be 10 in 2 000 000 people [2]. HLRCC is clinically characterized by the development of three types of tumors: uterine leiomyomata (UL), cutaneous piloleiomyomata (CL) and renal cell carcinoma (RCC). The rates of UL and CL in at-risk individuals can be as high as 70%, whereas the rate of RCC in at-risk individuals is approximately 15%–20% [1, 2].
It is unclear why HLRCC-associated RCC is so aggressive. However, this characteristic of HLRCC-associated RCC has been recognized by clinicians. The mean age at diagnosis is in the early forties, indicating that this disease occurs much earlier than sporadic RCC; the youngest patient diagnosed with HLRCC-associated RCC was 7 years old [3]. Similar to sporadic RCC, the mean survival of individuals diagnosed with stage III/IV RCC is significantly shorter than that of individuals diagnosed with stage I/II RCC [2]. Therefore, it is essential for carriers of HLRCC to undergo renal screening annually with magnetic resonance imaging (MRI) to detect early stage RCC [1].
However, it is clinically difficult to detect nonmetastatic HLRCC-associated RCC owing to its aggressive nature. Although metastatic HLRCC-associated RCC should be treated with systemic therapy, the most effective medicines have not yet been identified due to the low incidence rate of this cancer. Currently, immune checkpoint inhibitor (ICI) combinations or ICIs plus tyrosine kinase inhibitors (TKIs) are administered as systemic therapies to treat clear cell RCC. Therefore, HLRCC-associated RCC has been treated with ICI combinations or ICIs plus TKIs [4–8].
Here, we report a patient with HLRCC-associated RCC treated with sequential therapy, including ipilimumab (IPI) plus nivolumab (NIVO) combination and cabozantinib (CABO), after diagnosis using FoundationOne Liquid CDx and single-site analysis. We also investigated familial FH mutations by single-site analysis, and we present a new family pedigree for HLRCC.
Case presentation
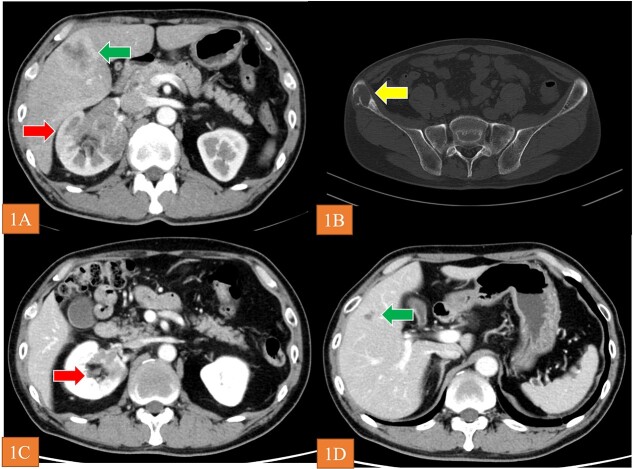
A 56-year-old man visited a previous hospital due to an 8-month history of back pain. Contrast-enhanced computed tomography revealed a hypovascular solid mass in the right kidney and right lobe of the liver as well as osteolysis in the right ilium and lumbar spine at L4 (Fig. 1A and B). He was referred to shimane university hospital (Shimane, Japan) for further evaluation and treatment. His medical history was unremarkable. Blood tests revealed low hemoglobin level (10.7 g/dl), high blood platelet level (55.8 × 104/μl), high C-related protein level (12.86 mg/dl), normal serum calcium level, and normal neutrophil counts. Urine test showed no microhematuria. The Karnofsky performance status was 90%. We then performed needle biopsy of the tumor in the right lobe of the liver. Histopathological findings for the specimen revealed unclassified RCC. There was no metastasis to the brain according to MRI. Accordingly, we diagnosed the patient with RCC at TNM stage cT3aN1M1. Based on the International Metastatic RCC Database Consortium risk classification, his risk was classified as poor. We selected IPI plus NIVO combination as the first-line systemic therapy. Because the patient was diagnosed with grade 1 myocarditis (CTCAE Version 5.0) after two courses of treatment, we halted administration of IPI plus NIVO. Instead, we started CABO 40 mg/day as second-line systematic therapy. The tumor volume decreased by 46% at 40 weeks (Fig. 1C and D), and the best overall response of CABO was a partial response within 73 weeks (RECIST Version 1.1). When disease progression was observed during CABO treatment, we prescribed axitinib 10 mg/day as third-line therapy. Utilizing FoundationOne Liquid on peripheral blood samples, we conducted an analysis of genetic mutations to aid in determining a more efficacious treatment approach. This analysis revealed a germline FH mutation (NM_000143.4) c.1189G>A (p.Gly397Arg) and we diagnosed as HLRCC-associated RCC. After AXI was no longer effective, we administered NIVO alone as fourth-line therapy. The patient is still alive as of March, 2023 (27 months after the start of treatment).
Figure 1.
(A and B) CT revealed the renal tumor (arrow), liver tumor (arrow), and bone metastasis (arrow). (C and D) arrows show that the tumor volume decreased by 46% at 40 weeks after initiation of CABO.
Because his older sister (60 years old) and younger brother (54 years old) wanted to know whether they had germline FH mutations, we performed single-site gene mutation analysis. Both siblings had FH mutations. Consequently, a family pedigree was established. Figure 2 shows a 5-generation family tree based on interviews with the patient and their family. III-2 is the index case. FH mutations were found in III-1 and III-3, who are his siblings. Furthermore, UL and kidney disease were both identified in the maternal aunts of the patients (II-5, 6), although the specific details are unknown. IV-1 and IV-2, who are the children of III-1, were diagnosed with UL at 30 and 35 years old, respectively, but have not yet undergone FH testing (Fig. 2). Hence, we will perform abdominal MRI for these FH mutation carriers annually to screen for renal cancer.
Figure 2.
The index patient is identified by an arrow and the letter P. *Patients with FH mutations. RCC: renal cell carcinoma UL: uterine leiomyomata.
Discussion
In this case report, we described a patient with HLRCC-associated RCC, which was detected by FoundationOne Liquid and single-site analysis, treated with IPI plus NIVO combination, CABO, AXI, and NIVO. Additionally, we assembled a pedigree delineating the tumor and clinical characteristics associated with HLRCC. In Japan, there have been 14 families with this cancer reported from 2007 to 2023 [3, 9, 10]. Given that the prevalence of HLRCC is approximately 10 in 2 000 000 people, it is estimated that there are approximately 600 carriers of HLRCC in Japan.
The optimal systemic therapy for metastatic HLRCC-associated RCC has not yet been identified owing to the low incidence rate of this cancer. In our case, we selected ICI combination therapy as the first-line treatment. A few case reports have demonstrated the efficacy of ICI and/or TKI treatment [5–7]. However, Xu et al. recently reported the ICI plus TKI combination therapy was associated with favorable overall survival in a multicenter retrospective study of 77 cases of FH-deficient RCC, including HLRCC [4]. Conventionally, although histopathological findings of HLRCC-associated RCC include papillary RCC, in practice, many histological types of RCC have been reported [2]. In the future, although further studies are needed to clarify the optimal therapy for HLRCC-associated RCC, systemic therapy focused on the histopathology or germline mutations of the disease, such as poly ADP (ribose) polymerase inhibitors, will be selected.
The FH mutation in this case was thought to have been inherited from the patient’s mother because there was a high incidence of UL on the maternal side and because the patient’s maternal aunts also had kidney disease. Therefore, screening for the early detection and treatment of renal cancer may be necessary for the children of the patient’s maternal aunts as well. In the future, we would like to proactively contact such families, provide information, and perform preventive screening if possible. When FH mutations are identified, it is important to reach a definitive diagnosis and to thoroughly investigate blood relatives from the perspective of preventive medicine as part of the treatment of hereditary tumors.
In conclusion, we report a patient with HLRCC-associated RCC treated with IPI plus NIVO combination, CABO, AXI, and rechallenge NIVO. We also created a new family tree for FH mutations.
Conflict of interest
The authors declare no conflicts of interest.
Approval of the research protocol by the Institutional Review Board (IRB)
This case report was approved by the IRB of Shimane University Hospital (approval no. 6892).
Consent
Written informed consent was obtained from patient.
Contributor Information
Ichiro Tsuboi, Department of Urology, Shimane University Faculty of Medicine, Izumo, Japan.
Momoko Araki, Department of Clinical Genetics Unit, Shimane University Faculty of Medicine, Izumo, Japan.
Shuhei Yokoyama, Department of Urology, Shimane University Faculty of Medicine, Izumo, Japan.
Gen Tanaka, Department of Urology, Shimane University Faculty of Medicine, Izumo, Japan.
Kazutaka Mitani, Department of Urology, Shimane University Faculty of Medicine, Izumo, Japan.
Saori Yosioka, Department of Urology, Shimane University Faculty of Medicine, Izumo, Japan.
Yusuke Kobayashi, Department of Urology, Shimane University Faculty of Medicine, Izumo, Japan.
Hirochika Nakajima, Department of Urology, Shimane University Faculty of Medicine, Izumo, Japan.
Taichi Nagami, Department of Urology, Shimane University Faculty of Medicine, Izumo, Japan.
Kohei Ogawa, Department of Urology, Shimane University Faculty of Medicine, Izumo, Japan.
Chiaki Koike, Department of Urology, Shimane University Faculty of Medicine, Izumo, Japan.
Koichiro Wada, Department of Urology, Shimane University Faculty of Medicine, Izumo, Japan.
References
- 1. Menko FH, Maher ER, Schmidt LS. et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer 2014;13:637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forde C, Lim DHK, Alwan Y. et al. Hereditary leiomyomatosis and renal cell cancer: clinical, molecular, and screening features in a cohort of 185 affected individuals. Eur Urol Oncol 2020;3:764–72. [DOI] [PubMed] [Google Scholar]
- 3. Taniguchi R, Muramatsu H, Okuno Y. et al. A patient with very early onset FH-deficient renal cell carcinoma diagnosed at age seven. Fam Cancer 2022;21:337–41. [DOI] [PubMed] [Google Scholar]
- 4. Xu Y, Kong W, Cao M. et al. Genomic profiling and response to immune checkpoint inhibition plus tyrosine kinase inhibition in FH-deficient renal cell carcinoma. Eur Urol 2023;83:163–72. [DOI] [PubMed] [Google Scholar]
- 5. Wang T, Huang Y, Huang X. et al. Complete response of hereditary leiomyomatosis and renal cell cancer (HLRCC)-associated renal cell carcinoma to pembrolizumab immunotherapy: a case report. Front Oncol 2021;11:735077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iribe Y, Furuya M, Shibata Y. et al. Complete response of hereditary leiomyomatosis and renal cell cancer (HLRCC)-associated renal cell carcinoma to nivolumab and ipilimumab combination immunotherapy by: a case report. Fam Cancer 2021;20:75–80. [DOI] [PubMed] [Google Scholar]
- 7. Gurruchaga Sotés I, Alves AN, Arregui SV. et al. Response to combination of pembrolizumab and axitinib in hereditary leyomiomatosis and renal cell cancer (HLRCC). Curr Oncol 2021;28:2346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park I, Shim YS, Go H. et al. Long-term response of metastatic hereditary leiomyomatosis and renal cell carcinoma syndrome associated renal cell carcinoma to bevacizumab plus erlotinib after temsirolimus and axitinib treatment failures. BMC Urol 2019;19:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furuya A, Hasumi H, Yao M. HLRCC (hereditary leiomyomatosis and renal cell cancer; HLRCC). J Heredit Tumors 2021;21:1–6(in Japansese). [Google Scholar]
- 10. Ueki A, Sugano K, Misu K. et al. Germline whole-gene deletion of FH diagnosed from tumor profiling. Int J Mol Sci 2021;22. 10.3390/ijms22157962. [DOI] [PMC free article] [PubMed] [Google Scholar]