Abstract
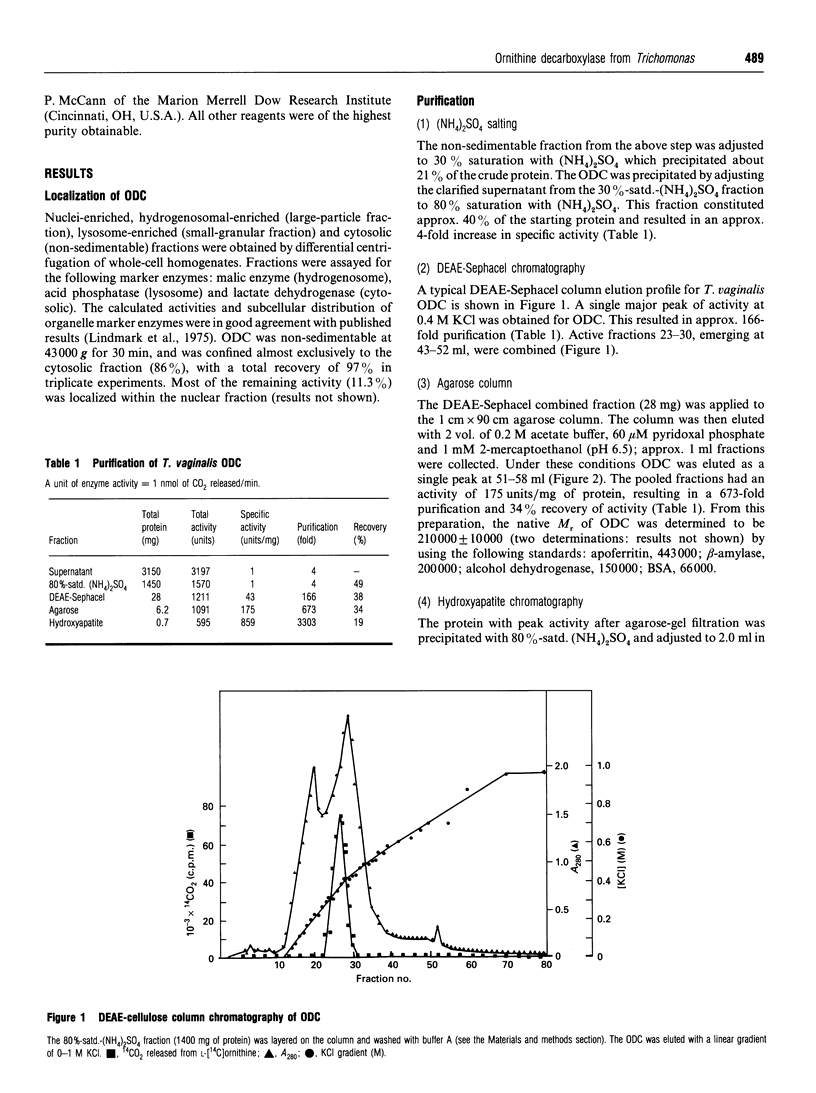
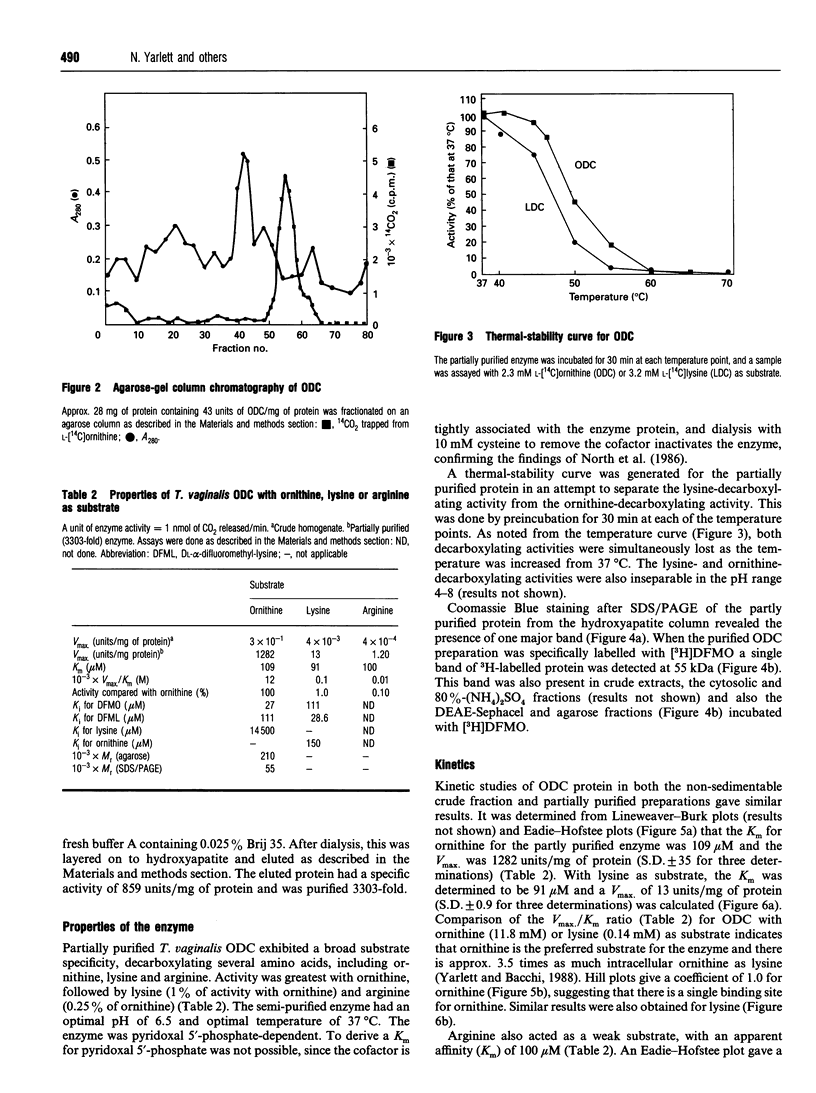
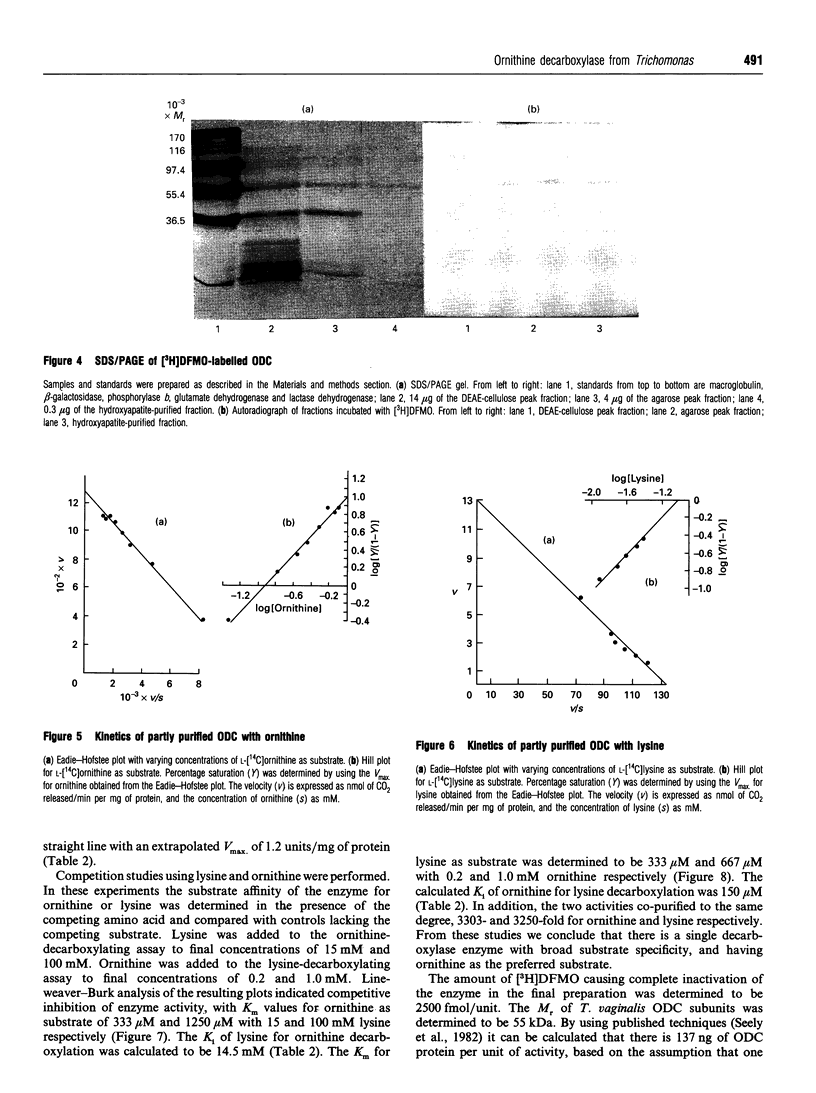
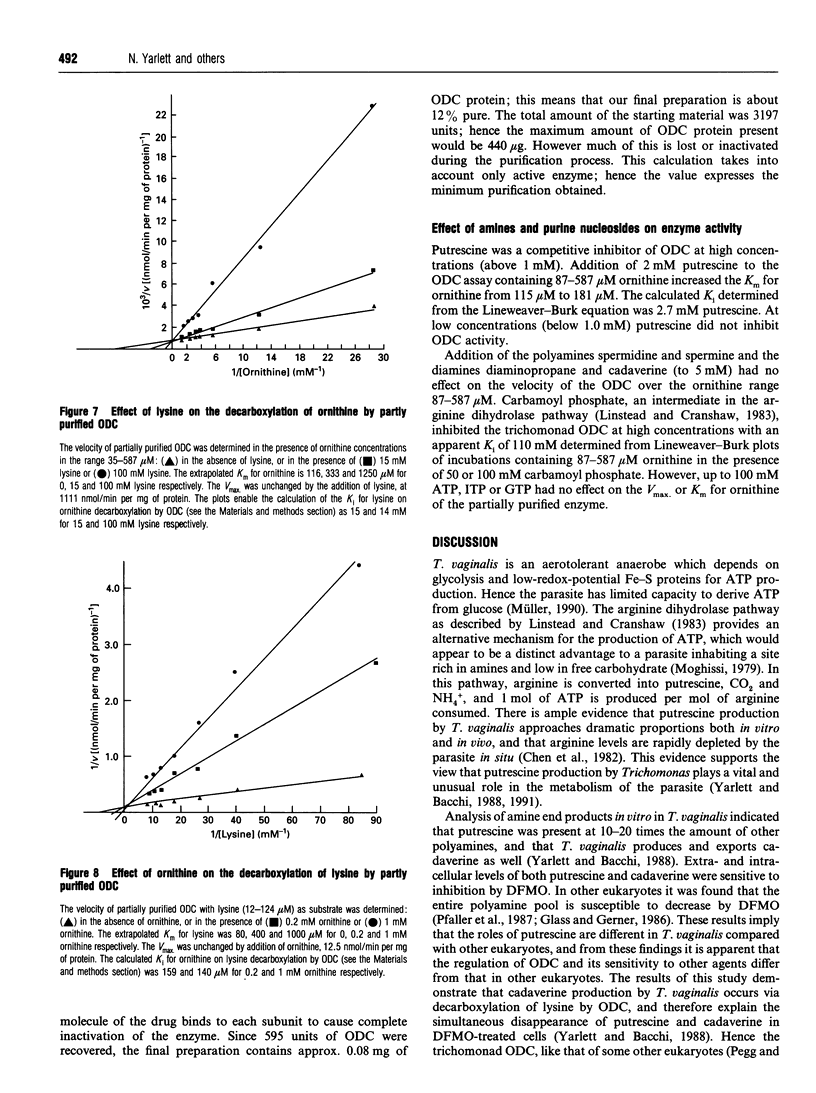
Ornithine decarboxylase (ODC), the lead enzyme in polyamine biosynthesis, was partially purified from Trichomonas vaginalis and its kinetic properties were studied. The enzyme appears to be of special significance in this anaerobic parasite, since the arginine dihydrolase pathway generates ATP as well as putrescine from arginine. ODC from T. vaginalis had a broad substrate specificity, decarboxylating ornithine (100%), lysine (1.0%) and arginine (0.1%). The enzyme had a pH optimum of 6.5, a temperature optimum of 37 degrees C and was pyridoxal 5'-phosphate-dependent. Attempts to separate ornithine- from lysine-decarboxylating activity by thermal-stability and pH-optima curves were not successful. Although Km values for ornithine and lysine were 109 and 91 microM respectively, and the Vmax values for these substrates were 1282 and 13 nmol/min per mg of protein respectively, the most important intracellular substrate is ornithine, since intracellular ornithine levels are 3.5 times those of lysine and extracellular putrescine levels are 7.5 times those of cadaverine. Ornithine was also an effective inhibitor of lysine-decarboxylating activity (Ki 150 microM), whereas lysine was relatively ineffective as inhibitor of ornithine-decarboxylating activity (Ki 14.5 mM). Crude ODC activity was localized (86%) in the 43,000 g supernatant and 3303-fold purification was obtained by (NH4)2SO4 salting and DEAE-Sephacel, agarose-gel and hydroxyapatite chromatography steps. The enzyme bound difluoro[3H]methylornithine ([3H]DFMO) with a ratio of drug bound to activity of 2500 fmol/unit, where 1 unit corresponds to 1 nmol of CO2 released from ornithine/min. The enzyme had a native M(r) of 210000 (gel filtration), with a subunit M(r) of 55,000 (by SDS/PAGE), suggesting that the trichomonad enzyme is a tetramer. From the subunit M(r) and binding ratio of DFMO, there is about 137 ng of ODC per mg of T. vaginalis protein (0.013%). The significant amount of ODC protein present supports the view that putrescine synthesis in T. vaginalis plays an important role in the metabolism of the parasite.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchi C. J., Garofalo J., Santana A., Hannan J. C., Bitonti A. J., McCann P. P. Trypanosoma brucei brucei: regulation of ornithine decarboxylase in procyclic forms and trypomastigotes. Exp Parasitol. 1989 May;68(4):392–402. doi: 10.1016/0014-4894(89)90124-0. [DOI] [PubMed] [Google Scholar]
- Barnett G. R., Kazarinoff M. N. Purification and properties of ornithine decarboxylase from Physarum polycephalum. J Biol Chem. 1984 Jan 10;259(1):179–183. [PubMed] [Google Scholar]
- Boeker E. A., Fischer E. H. Lysine decarboxylase (Escherichia coli B). Methods Enzymol. 1983;94:180–184. doi: 10.1016/s0076-6879(83)94030-2. [DOI] [PubMed] [Google Scholar]
- Chen K. C., Amsel R., Eschenbach D. A., Holmes K. K. Biochemical diagnosis of vaginitis: determination of diamines in vaginal fluid. J Infect Dis. 1982 Mar;145(3):337–345. doi: 10.1093/infdis/145.3.337. [DOI] [PubMed] [Google Scholar]
- Coons T., Hanson S., Bitonti A. J., McCann P. P., Ullman B. Alpha-difluoromethylornithine resistance in Leishmania donovani is associated with increased ornithine decarboxylase activity. Mol Biochem Parasitol. 1990 Feb;39(1):77–89. doi: 10.1016/0166-6851(90)90010-j. [DOI] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Glass J. R., Gerner E. W. Polyamine-mediated turnover of ornithine decarboxylase in Chinese-hamster ovary cells. Biochem J. 1986 Jun 1;236(2):351–357. doi: 10.1042/bj2360351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- Kitani T., Fujisawa H. Molecular properties of ornithine decarboxylase from mouse kidney: detailed comparison with those of the enzyme from rat liver. J Biochem. 1988 Mar;103(3):547–553. doi: 10.1093/oxfordjournals.jbchem.a122306. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linstead D., Cranshaw M. A. The pathway of arginine catabolism in the parasitic flagellate Trichomonas vaginalis. Mol Biochem Parasitol. 1983 Jul;8(3):241–252. doi: 10.1016/0166-6851(83)90046-4. [DOI] [PubMed] [Google Scholar]
- McNab W. L. The "other" venereal diseases: herpes simplex, trichomoniasis and candidiasis. J Sch Health. 1979 Feb;49(2):79–83. doi: 10.1111/j.1746-1561.1979.tb05292.x. [DOI] [PubMed] [Google Scholar]
- North M. J., Lockwood B. C., Bremner A. F., Coombs G. H. Polyamine biosynthesis in trichomonads. Mol Biochem Parasitol. 1986 Jun;19(3):241–249. doi: 10.1016/0166-6851(86)90006-x. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McGill S. Decarboxylation of ornithine and lysine in rat tissues. Biochim Biophys Acta. 1979 Jun 6;568(2):416–427. doi: 10.1016/0005-2744(79)90310-3. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988 Feb 15;48(4):759–774. [PubMed] [Google Scholar]
- Pfaller M. A., Gerarden T., Riley J. Growth inhibition of pathogenic yeast isolates by alpha-difluoromethylornithine: an inhibition of ornithine decarboxylase. Mycopathologia. 1987 Apr;98(1):3–8. doi: 10.1007/BF00431009. [DOI] [PubMed] [Google Scholar]
- Phillips M. A., Coffino P., Wang C. C. Trypanosoma brucei ornithine decarboxylase: enzyme purification, characterization, and expression in Escherichia coli. J Biol Chem. 1988 Dec 5;263(34):17933–17941. [PubMed] [Google Scholar]
- Pritchard M. L., Seely J. E., Pösö H., Jefferson L. S., Pegg A. E. Binding of radioactive alpha-difluoromethylornithine to rat liver ornithine decarboxylase. Biochem Biophys Res Commun. 1981 Jun;100(4):1597–1603. doi: 10.1016/0006-291x(81)90701-4. [DOI] [PubMed] [Google Scholar]
- Sanderson B. E., White E., Baldson M. J. Amine content of vaginal fluid from patients with trichomoniasis and gardnerella associated non-specific vaginitis. Br J Vener Dis. 1983 Oct;59(5):302–305. doi: 10.1136/sti.59.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer J. M., Donatelli M. R. Characterization of a high-affinity membrane-associated ornithine decarboxylase from the free-living nematode Caenorhabditis elegans. Biochem J. 1990 Sep 15;270(3):599–604. doi: 10.1042/bj2700599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Ornithine decarboxylase (mouse kidney). Methods Enzymol. 1983;94:158–161. doi: 10.1016/s0076-6879(83)94025-9. [DOI] [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Purification of ornithine decarboxylase from kidneys of androgen-treated mice. Biochemistry. 1982 Jul 6;21(14):3394–3399. doi: 10.1021/bi00257a023. [DOI] [PubMed] [Google Scholar]
- Sklaviadis T. K., Georgatsos J. G., Kyriakidis D. A. Purification and properties of ornithine decarboxylase from Tetrahymena pyriformis. Biochim Biophys Acta. 1985 Oct 18;831(3):288–296. doi: 10.1016/0167-4838(85)90109-8. [DOI] [PubMed] [Google Scholar]
- Tyagi A. K., Tabor C. W., Tabor H. Ornithine decarboxylase from Saccharomyces cerevisiae. Purification, properties, and regulation of activity. J Biol Chem. 1981 Dec 10;256(23):12156–12163. [PubMed] [Google Scholar]
- Yarlett N., Bacchi C. J. Effect of DL-alpha-difluoromethylornithine on methionine cycle intermediates in Trypanosoma brucei brucei. Mol Biochem Parasitol. 1988 Jan 1;27(1):1–10. doi: 10.1016/0166-6851(88)90019-9. [DOI] [PubMed] [Google Scholar]
- Yarlett N., Goldberg B., Moharrami M. A., Bacchi C. J. Inhibition of Trichomonas vaginalis ornithine decarboxylase by amino acid analogs. Biochem Pharmacol. 1992 Jul 22;44(2):243–250. doi: 10.1016/0006-2952(92)90006-5. [DOI] [PubMed] [Google Scholar]
- Yarlett N. Polyamine biosynthesis and inhibition in Trichomonas vaginalis. Parasitol Today. 1988 Dec;4(12):357–360. doi: 10.1016/0169-4758(88)90007-5. [DOI] [PubMed] [Google Scholar]



