Abstract
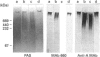
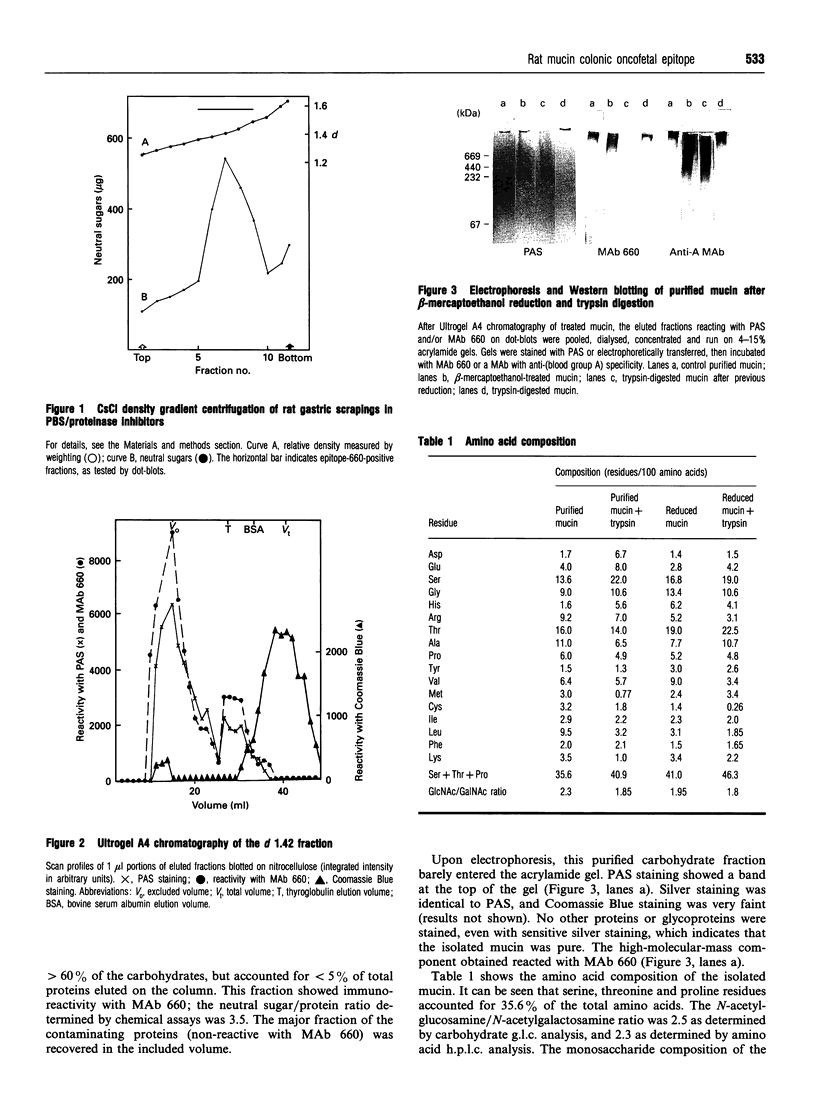
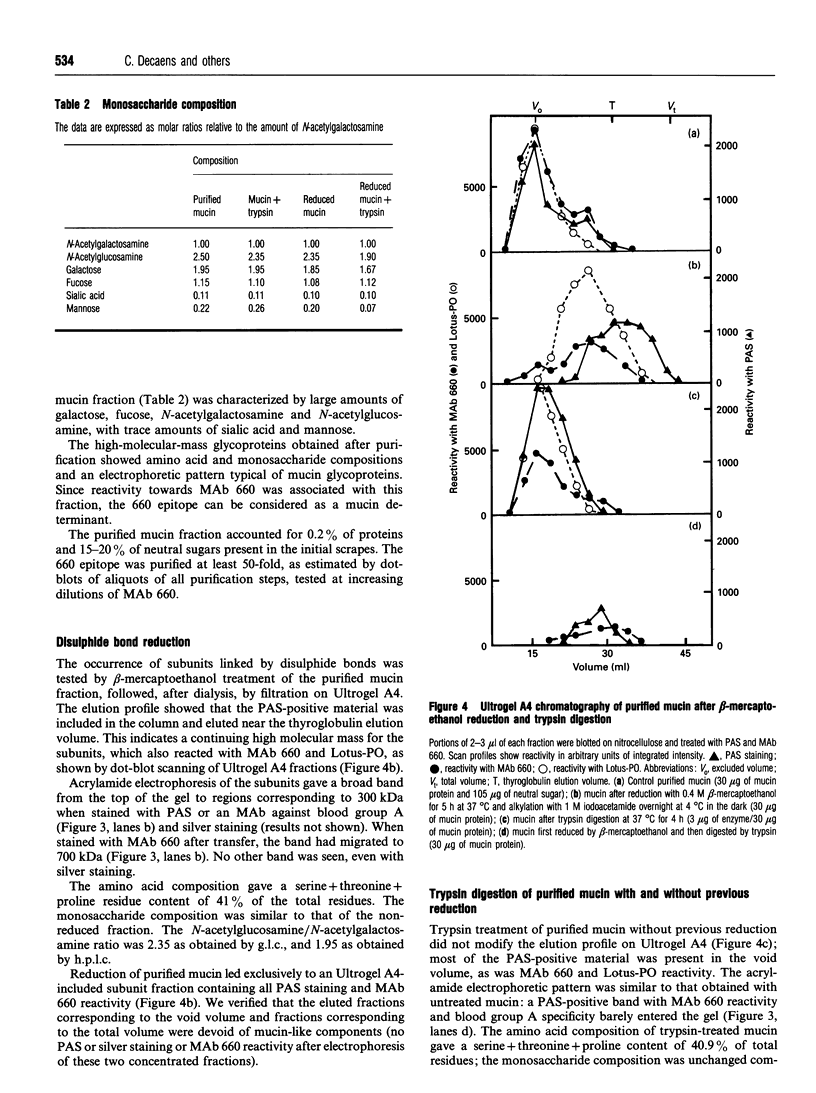
The 660 epitope was defined by a monoclonal antibody raised against rat gastric surface epithelium scrapings. This epitope, a marker of goblet cell differentiation, shows oncofetal behaviour in the colonic mucosa. We found that it co-purified with gastric mucin glycoproteins. We isolated rat gastric mucus glycoproteins using standard techniques: gastric scrapings in PBS were submitted to isopycnic density gradient centrifugation in CsCl in the presence of proteinase inhibitors. Fractions of relative density 1.4-1.45 with a high neutral sugar/protein ratio were chromatographed on an Ultrogel A4 column. According to the usual criteria, the high-molecular mass glycoproteins recovered in the excluded volume were purified mucins; when stained with periodic acid/Schiff reagent, they showed little migration on 4-15% gradient gel acrylamide electrophoresis. Serine+threonine+proline residues accounted for 35% of the total amino acids; the carbohydrate composition consisted of galactose, fucose, N-acetylgalactosamine and N-acetylglucosamine. These mucus glycoproteins carried the 660 epitope. After disulphide bond reduction, the remaining high-molecular-mass subunits were retained by the Ultrogel A4 column; amino acid and saccharide compositions were generally similar to those of the unreduced fraction. Trypsin digestion of the 660 epitope glycoprotein carrier did not modify its chromatographic and electrophoretic patterns, nor its chemical composition. The 660 epitope was still present after these treatments. However, trypsin digestion of subunits gave rise to smaller components that were retained by an Ultrogel A4 column. The saccharide composition of these fragments was unchanged, but the proportion of serine+threonine+proline residues rose to 46% of the total. These digested subunits had lost nearly all reactivity with monoclonal antibody 660. Our results fit well with the macromolecular model of Carlstedt, Lindgren and Sheehan [(1983) Biochem. J. 213, 427-435]: mucin glycoproteins are homopolymers of subunits assembled end-to-end via disulphide bonds into very large linear macromolecules. After disulphide bond reduction, proteolytic attack sites are uncovered and trypsin digestion results in glycopeptides bearing the typical oligosaccharidic units and with enhanced amounts of serine, threonine and proline, the characteristic amino acids of this hyperglycosylated region of the peptide core. These digested subunits have lost virtually all 660 epitope reactivity. We thus show that the 660 epitope, a determinant of a mucin molecule, is probably associated with the peptide core of the glycoprotein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Hutton D. A., Mantle D., Pain R. H. Structure and gel formation in pig gastric mucus. Biochem Soc Trans. 1984 Aug;12(4):612–615. doi: 10.1042/bst0120612. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Bara J., Gautier R., Daher N., Zaghouani H., Decaens C. Monoclonal antibodies against oncofetal mucin M1 antigens associated with precancerous colonic mucosae. Cancer Res. 1986 Aug;46(8):3983–3989. [PubMed] [Google Scholar]
- Bara J., Gautier R., Mouradian P., Decaens C., Daher N. Oncofetal mucin M1 epitope family: characterization and expression during colonic carcinogenesis. Int J Cancer. 1991 Jan 21;47(2):304–310. doi: 10.1002/ijc.2910470222. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Lindgren H., Sheehan J. K. The macromolecular structure of human cervical-mucus glycoproteins. Studies on fragments obtained after reduction of disulphide bridges and after subsequent trypsin digestion. Biochem J. 1983 Aug 1;213(2):427–435. doi: 10.1042/bj2130427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K., Corfield A. P., Gallagher J. T. Mucous glycoproteins: a gel of a problem. Essays Biochem. 1985;20:40–76. [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K. Macromolecular properties and polymeric structure of mucus glycoproteins. Ciba Found Symp. 1984;109:157–172. doi: 10.1002/9780470720905.ch11. [DOI] [PubMed] [Google Scholar]
- Chaplin M. F. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):336–341. doi: 10.1016/0003-2697(82)90455-9. [DOI] [PubMed] [Google Scholar]
- Decaens C., Gautier R., Bara J., Daher N., Le Pendu J., Burtin P. A new mucin-associated oncofetal antigen, a marker of early carcinogenesis in rat colon. Cancer Res. 1988 Mar 15;48(6):1571–1577. [PubMed] [Google Scholar]
- Dekker J., Aelmans P. H., Strous G. J. The oligomeric structure of rat and human gastric mucins. Biochem J. 1991 Jul 15;277(Pt 2):423–427. doi: 10.1042/bj2770423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J., Strous G. J. Covalent oligomerization of rat gastric mucin occurs in the rough endoplasmic reticulum, is N-glycosylation-dependent, and precedes initial O-glycosylation. J Biol Chem. 1990 Oct 25;265(30):18116–18122. [PubMed] [Google Scholar]
- Dekker J., Van Beurden-Lamers W. M., Oprins A., Strous G. J. Isolation and structural analysis of rat gastric mucus glycoprotein suggests a homogeneous protein backbone. Biochem J. 1989 Jun 15;260(3):717–723. doi: 10.1042/bj2600717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J., Van Beurden-Lamers W. M., Strous G. J. Biosynthesis of gastric mucus glycoprotein of the rat. J Biol Chem. 1989 Jun 25;264(18):10431–10437. [PubMed] [Google Scholar]
- Devine P. L., McKenzie I. F. Mucins: structure, function, and associations with malignancy. Bioessays. 1992 Sep;14(9):619–625. doi: 10.1002/bies.950140909. [DOI] [PubMed] [Google Scholar]
- Fahim R. E., Specian R. D., Forstner G. G., Forstner J. F. Characterization and localization of the putative 'link' component in rat small-intestinal mucin. Biochem J. 1987 May 1;243(3):631–640. doi: 10.1042/bj2430631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Feizi T., Gooi H. C., Childs R. A., Picard J. K., Uemura K., Loomes L. M., Thorpe S. J., Hounsell E. F. Tumour-associated and differentiation antigens on the carbohydrate moieties of mucin-type glycoproteins. Biochem Soc Trans. 1984 Aug;12(4):591–596. doi: 10.1042/bst0120591. [DOI] [PubMed] [Google Scholar]
- Gupta R., Jentoft N. Analysis of natural and modified amino acids and hexosamines by reversed-phase high-performance liquid chromatography. J Chromatogr. 1989 Jul 19;474(2):411–417. doi: 10.1016/s0021-9673(01)93936-6. [DOI] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Antigenic and structural features of goblet-cell mucin of human small intestine. Biochem J. 1984 Jan 1;217(1):159–167. doi: 10.1042/bj2170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Mantle D., Allen A. Polymeric structure of pig small-intestinal mucus glycoprotein. Dissociation by proteolysis or by reduction of disulphide bridges. Biochem J. 1981 Apr 1;195(1):277–285. doi: 10.1042/bj1950277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J., Allen A., Venables C. Gastric mucus: isolation and polymeric structure of the undegraded glycoprotein: its breakdown by pepsin. Gastroenterology. 1980 Apr;78(4):709–715. [PubMed] [Google Scholar]
- Sheahan D. G., Jervis H. R. Comparative histochemistry of gastrointestinal mucosubstances. Am J Anat. 1976 Jun;146(2):103–131. doi: 10.1002/aja.1001460202. [DOI] [PubMed] [Google Scholar]
- Shochat D., Pant K. D., Goldenberg D. M. Colon-specific antigen-p (CSAp). II. Further characterization in colorectal and pancreatic cancer. Cancer. 1982 Sep 1;50(5):927–931. doi: 10.1002/1097-0142(19820901)50:5<927::aid-cncr2820500521>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Slomiany A., Okazaki K., Tamura S., Slomiany B. L. Identity of mucin's "118-kDa link protein" with fibronectin fragment. Arch Biochem Biophys. 1991 May 1;286(2):383–388. doi: 10.1016/0003-9861(91)90055-n. [DOI] [PubMed] [Google Scholar]
- Snary D., Allen A., Pain R. H. Structural studies on gastric mucoproteins: lowering of molecular weight after reduction with 2-mercaptoethanol. Biochem Biophys Res Commun. 1970 Aug 24;40(4):844–851. doi: 10.1016/0006-291x(70)90980-0. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Katsuyama T., Sannes P. L. Ultrastructural carbohydrate cytochemistry of gastric epithelium. Histochem J. 1978 May;10(3):309–331. doi: 10.1007/BF01007562. [DOI] [PubMed] [Google Scholar]