Abstract
Introduction
This study sought to evaluate the cost-effectiveness of baloxavir marboxil compared with oseltamivir or no antiviral treatment from a US payer perspective using data from a real-world US administrative claims study. Given baloxavir’s ability to rapidly stop viral shedding, the potential health economic implications of a baloxavir-induced population-level reduction in viral transmission was also explored.
Methods
A decision tree cost-effectiveness model was developed for seasonal influenza (2018–2020) using a lifetime time horizon with 3.0% discounting for costs and quality-adjusted life-years (QALYs). Patients aged ≥ 12 years could receive baloxavir, oseltamivir or no antiviral treatment. Patient characteristics, complications, and costs were derived from the Merative™ MarketScan® Research Databases including US commercial claims and Medicare and Medicaid Supplemental databases. A scenario analysis explored the impact of reduced viral transmission with baloxavir.
Results
In the base case analysis, baloxavir was cost-effective within a willingness-to-pay threshold of US$100,000/QALY compared with oseltamivir [incremental cost-effectiveness ratio (ICER), $6813/QALY gained] or no antiviral treatment (ICER, $669/QALY gained). The net monetary benefit (NMB) of baloxavir was $1180 and $6208 compared with oseltamivir and no treatment, respectively. The NMB of baloxavir increased linearly with reductions in viral transmission, where a 5% transmission reduction yielded an NMB of $2592 versus oseltamivir and $7621 versus no treatment. Baloxavir became dominant (more effective and less costly, with ICERs < 0) starting with a 12.0% reduction in viral transmission versus oseltamivir and 6.0% versus no antiviral treatment.
Conclusion
Baloxavir was cost-effective compared with oseltamivir or no antiviral treatment. The potential of baloxavir to reduce viral transmission offers a substantial economic benefit from a US payer perspective.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-024-01027-9.
Keywords: Antiviral treatment, Baloxavir, Commercial payer, Cost-effectiveness, Influenza, Oseltamivir, Pandemic, Real-world data, United States, Viral transmission
Plain Language Summary
Baloxavir is a prescription medicine that reduces the duration of flu symptoms and reduces the likelihood of complications from the flu, including serious complications that may require hospitalization. Baloxavir may reduce the spread of the flu to healthy people by reducing the amount and duration of virus shedding from infected people. We designed a model to estimate the cost benefits of using baloxavir versus another flu treatment, known as oseltamivir, or no flu treatment at all. Using baloxavir led to more cost savings than oseltamivir or no treatment for people in the US who have commercial health insurance. Baloxavir was even more cost-effective in the scenario where it reduced the number of flu cases (transmission benefit). This could ultimately have a meaningful benefit across a large health insurance population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-024-01027-9.
Key Summary Points
| Antiviral treatment has been shown to reduce the severity, duration and complications of influenza, but the potential for viral resistance to neuraminidase inhibitors has highlighted the need for treatments with different mechanisms of action. |
| Baloxavir marboxil is an oral, single-dose cap-dependent endonuclease inhibitor that improves influenza symptoms and rapidly reduces the duration and amount of viral shedding, which may reduce infectiousness and viral transmission; however, the cost-effectiveness of baloxavir compared with oseltamivir has not been explored in a US population. |
| This study evaluated the cost-effectiveness of antiviral treatment with baloxavir versus oseltamivir or no antiviral treatment from a third-party US payer perspective using data from a real-world US administrative claims study, and explored how a reduction in viral transmission rate with baloxavir might impact its cost-effectiveness compared with oseltamivir or no treatment. |
| This modeling study showed that baloxavir is cost-effective compared with oseltamivir or no antiviral treatment from a US payer perspective. In the total population, baloxavir provided incremental cost-effectiveness ratios of $6813 per quality-adjusted life-year versus oseltamivir and $669 versus no treatment, well below a commonly accepted threshold of $100,000 per quality-adjusted life-year. |
| Based on a real-world US administrative claims study, this model estimated a meaningful health economic benefit of baloxavir in an overall insured US population, including among high-risk and otherwise healthy subsets of the population. Scenario analyses suggested that the ability of baloxavir to reduce viral transmission could offer a substantial additional economic benefit. |
Introduction
Influenza poses a substantial burden on individuals, employers, and population health managers in the United States (US), whether through seasonal outbreaks or periodic pandemics. An estimated 27–54 million individuals were infected with influenza in the US during the 2022–2023 season, resulting in 300,000–650,000 hospitalizations and 19,000–58,000 influenza-related deaths during that time period [1]. As many as 75% of working adults experience 2–3 days of absenteeism due to influenza whether they are infected or someone in their household has been infected, and most people report going to work while having symptoms [2].
Common influenza symptoms include fever, fatigue, headache, nasal congestion, and cough [3, 4]. Uncomplicated illness can resolve after 3–8 days, but some symptoms may persist for up to 2 weeks [3]. Individuals ≥ 65 years of age, those with certain chronic conditions, and pregnant women have an elevated risk of morbidity and mortality [5]. Approximately half (54%) of influenza-related hospitalizations and 77% of influenza-related deaths in the US were among those aged ≥ 65 years during the 2018–2019 season [5]. The inflammatory response to influenza can also worsen underlying comorbidities, such as cardiovascular disease, diabetes, liver and kidney disease, and other conditions associated with compromised immunity [6, 7].
Infectiousness, a key factor in influenza transmission, is driven by viral shedding in the upper respiratory tract prior to symptom onset and for up to 1 week thereafter [8, 9]. Household transmission typically occurs within 2–4 days after the initial infection [10]. Viral shedding can be longer for those with more severe infection, which is more likely for immunocompromised individuals and those older than 65 years of age [9, 11, 12]. Antiviral treatment has been shown to reduce the severity, duration, and complications of illness, especially if treated within 48 h of symptom onset [13–15]. Prophylactic use of antiviral treatment can also reduce the risk of infection by as much as 92% for individuals who have been exposed to the virus [16, 17]. Neuraminidase inhibitors such as oseltamivir have been the prominent antiviral treatment strategy [11], but the potential for viral resistance to emerge against this class of antivirals has highlighted the need for treatments with different mechanisms of action [18, 19].
Baloxavir marboxil is an oral, single-dose cap-dependent endonuclease inhibitor that improves symptoms and rapidly reduces the duration and amount of viral shedding, which may reduce infectiousness and viral transmission [13, 14]. In clinical trials, baloxavir demonstrated a similar median time to alleviation of symptoms (TTAS) among otherwise healthy (OwH) adults and adolescents infected with influenza compared with oseltamivir (53.7 vs. 53.8 h) and a significantly shorter TTAS compared with placebo (53.7 vs. 80.2 h; P < 0.001) [13]. Among high-risk individuals, baloxavir showed a median time to improvement of influenza symptoms (TTIS) of 73.2 h which was significantly shorter compared with placebo (102.3 h; difference, 29.1 h; P < 0.0001) and numerically shorter compared with oseltamivir (81.0 h; difference, 7.7 h; not statistically significant) [14]. Baloxavir is approved in the US for the treatment of acute uncomplicated influenza in those ≥ 5 years old who have been symptomatic ≤ 48 h, and in those ≥ 12 years old who are at high risk of developing complications [20]. Post-exposure prophylaxis to prevent infection with baloxavir is also indicated in the US, with an estimated protective efficacy of 86%, as shown in the BLOCKSTONE trial [17, 20]. Reducing onward viral transmission by treating infected cases has been shown to be more effective with baloxavir compared with oseltamivir in a prospective real-world US survey study, where infected individuals treated with baloxavir had numerically lower household transmission rates compared with those treated with oseltamivir (18% vs. 27%, respectively; P = 0.09) [21]. The ongoing phase 3 CENTERSTONE clinical trial (NCT03969212) is assessing the efficacy of baloxavir in reducing viral transmission within households.
Baloxavir has also been shown to reduce health care resource use and costs more than oseltamivir in a large real-world US administrative claims database study, showing fewer all-cause and respiratory-related emergency department visits and hospitalizations during the 2018–2019 influenza season [22]. However, the cost-effectiveness of baloxavir compared with oseltamivir has not been explored in a US population. This study evaluated the cost-effectiveness of antiviral treatment with baloxavir versus oseltamivir or no antiviral treatment from a third-party US payer perspective, using data from a real-world US administrative claims study. This study also explored how a reduction in viral transmission rate with baloxavir might impact its cost-effectiveness compared with oseltamivir or no treatment.
Methods
Overview
We developed a decision tree cost-effectiveness model (CEM) in Microsoft® Excel® from a US commercial payer perspective, using inputs from a real-world US administrative claims database study [23] and from the published literature. This cost-effectiveness model was conducted based on analyses of a secondary-use database and did not recruit any human participants or involve animals.
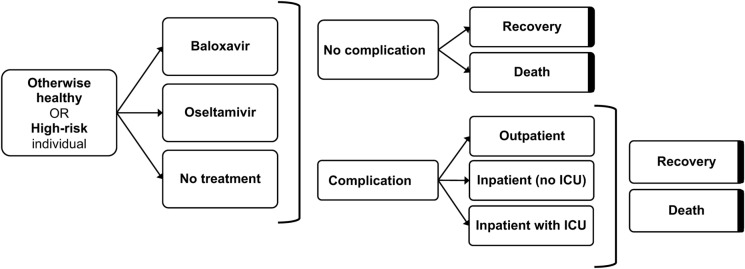
The base case CEM compared antiviral treatment with baloxavir versus oseltamivir or no antiviral treatment for symptomatic individuals with influenza in an insured US population. A lifetime time horizon with 3.0% discounting of costs and quality-adjusted life-years (QALYs) was used [24]. Results were reported for a hypothetical population of 100,000 individuals in terms of QALYs and incremental cost-effectiveness ratios (ICERs) over one influenza season. The CEM decision tree pathways were mutually exclusive at the individual level for both overall and high-risk populations, where an individual may or may not experience infection, receive treatment, develop a complication, and recover or die. Individuals with a complication could have outpatient care, or inpatient care with or without admission to the intensive care unit (ICU) (Fig. 1). A summary of key CEM inputs is provided in Table 1.
Fig. 1.
Decision tree structure of the cost-effectiveness model. Bolded sides indicate terminal nodes. ICU intensive care unit
Table 1.
Summary of key inputs from the base case CEM
| CEM inputs | Otherwise healthy | High-risk | Source |
|---|---|---|---|
| Currency, costing year | US dollars ($), 2022 | Assumption | |
| Time horizon | Lifetime | Assumption | |
| Discount rate, costs and QALYs | 3.0% | Assumption | |
| Population, % | 56% | 44% | Real-world US administrative claims study [23] |
| Overall rate of complications (95% CI) | Real-world US administrative claims study [23] | ||
| Baloxavir | 26.2% (25.3, 27.1) | 30.5% (29.3, 31.6) | |
| Oseltamivir | 28.1% (27.9, 28.2) | 31.3% (31.1, 31.5) | |
| No antiviral treatment | 31.8% (31.6, 32.0) | 37.8% (37.5, 38.1) | |
| Duration of symptoms, mean, days | NICE TA168 [4] | ||
| Baloxavir | 6.9 | 12.3 | |
| Oseltamivir | 6.9 | 12.3 | |
| No antiviral treatment | 8.9 | 13.8 | |
| Treatment-related adverse events, % |
Hayden 2018 [13] Ison 2020 [14] |
||
| Baloxavir | 4.4% | 5.6% | |
| Oseltamivir | 8.4% | 7.9% | |
| No antiviral treatment | – | – | |
| Treatment-related adverse event disutility | 0.21 | Smith 2002 [32] | |
| Treatment-related adverse event duration (cost) | 7 days ($10) | Assumption | |
| Drug costs (per episode of care) | AnalySource [34] | ||
| Baloxavir | $159.14 | ||
| Oseltamivir | $50.40 | ||
CEM cost-effectiveness model, CI confidence interval, QALY quality-adjusted life-year
Clinical trials have shown significantly faster declines in viral load by Day 2 with baloxavir treatment compared with oseltamivir or placebo [13, 14]. The ongoing CENTERSTONE study (NCT03969212) is exploring whether faster cessation of viral shedding with baloxavir treatment would lead to a reduction in viral transmission. Since the CENTERSTONE study was still ongoing when this CEM was developed, the base case CEM conservatively assumed there would be no additional transmission reduction benefit provided by treatment with baloxavir. A scenario analysis was then conducted to explore the potential impact of reduced viral transmission with baloxavir treatment in an insured US population.
CEM Population
Population Inputs from a Real-World Administrative Claims Study
A retrospective observational study was conducted using the Merative™ MarketScan® Research Databases from the 2018–2019 and 2019–2020 influenza seasons (October 1 through May 31, based on the Centers for Disease Control and Prevention [25]), which included the Commercial Claims and Encounters, MarketScan Medicare, and Medicaid Supplemental Research Databases [23]. In line with the US indication for baloxavir when the study was designed, patients in the real-world administrative claims study had to be ≥ 12 years of age at the time of influenza diagnosis, defined by International Classification of Diseases, 10th edition Clinical Modification (ICD-10-CM) codes (J09.xx, J10.xx, J11.xx), with continuous medical and pharmacy health plan enrollment for ≥ 6 months before and 30 days after the treatment date (or proxy treatment date for untreated patients). Treatment arms were defined by the presence of a prescription fill for baloxavir or oseltamivir within 2 days after the influenza diagnosis date (treated patients), or no record of any influenza treatment during the study period after influenza diagnosis (untreated patients). Patients were excluded if they received antiviral medication for prophylaxis, had a diagnosis code for coronavirus disease 2019 (COVID-19) during the 2019–2020 influenza season, received treatment with any other influenza antiviral medication (i.e., peramivir, zanamivir), or received baloxavir or oseltamivir > 2 days after influenza diagnosis.
The real-world US administrative claims study included a total of 753,721 patients (baloxavir, n = 14,868; oseltamivir, n = 466,420; no antiviral treatment, n = 272,433) [23]. Approximately 50,000 patients were excluded for having a COVID-19 diagnosis code and 28 patients were excluded for use of peramivir or zanamivir. The real-world study provided inputs for the CEM population including the mean age of 32 years and the proportions of individuals with influenza categorized as OwH (56%; n = 425,589/753,721) or high-risk (44%; n = 328,132/753,721) [23]. Definitions of OwH and high-risk were based on the Centers for Disease Control and Prevention [26] and the CAPSTONE-2 clinical trial [14]. CEM outcomes were modeled separately for the OwH and high-risk populations then combined to provide results for the total population (OwH + high-risk).
Complications
The rates of complications in the OwH and high-risk populations were based on patients who developed a complication within the 30 days after receiving baloxavir, oseltamivir, or no antiviral treatment in the real-world US administrative claims study [23]. Prespecified complications included pneumonia, bronchitis, upper respiratory tract infection, acute respiratory distress syndrome, sepsis, otitis media, gastrointestinal bleeding, sinusitis, exacerbation of asthma or chronic obstructive pulmonary disease, or influenza-related cardiovascular, renal, or central nervous system complications (Supplementary Table S1). The prevalence of complications was determined regardless of any history of the condition in the 6 months prior to influenza diagnosis. Complications where ≤ 1% of patients were managed in an inpatient setting (with or without an ICU stay) were redistributed to an outpatient care setting to avoid the influence of outliers (where very few observations would increase the uncertainty and possibly indicate exceptionally high or low costs for a particular care setting) [23]. Since very few observations were redistributed (acute respiratory distress syndrome, 0.07%; sepsis, 0.15%; renal, 0.79%; gastrointestinal bleeding, 0.60%), the impact of the redistributions on the model estimates was considered to be negligible.
In the OwH population, the proportions of individuals with a complication in the baloxavir, oseltamivir, and no treatment arms were 26.2% [95% confidence interval (CI) 25.3, 27.1], 28.1% (95% CI 27.9, 28.2), and 31.8% (95% CI 31.6, 32.0), respectively, based on the real-world US claims study [23]. In the high-risk population, the proportions of individuals with a complication were 30.5% (95% CI 29.3, 31.6), 31.3% (95% CI 31.1, 31.5), and 37.8% (95% CI 37.5, 38.1) in the baloxavir, oseltamivir and no treatment arms, respectively [23]. Proportions of patients with each complication (Supplementary Table S2), management of complications in the outpatient, inpatient (no ICU), or inpatient with ICU stay (Supplementary Table S3), and management costs for complications in each care setting (Supplementary Table S4) were based on the real-world US administrative claims study [23]. The proportions of cured patients in each care setting (Supplementary Table S5) were based on the published literature and on a published National Institute for Health and Care Excellence (NICE) technology appraisal (NICE TA168) of antiviral treatments that included oseltamivir [4, 27, 28]. The cost of an outpatient office visit ($68.10; code 99,213) was taken from the 2023 Centers for Medicare & Medicaid Services Physician Fee Schedule [29].
Duration of Influenza Symptoms
The duration of influenza symptoms was assumed to be the same for treated patients receiving either baloxavir or oseltamivir based on the generally similar reductions in median TTAS and TTIS observed in the CAPSTONE-1 [13] and CAPSTONE-2 [14] trials. Median TTAS was similar for baloxavir (53.7 h) compared with oseltamivir (53.8 h) among OwH individuals in CAPSTONE-1 (placebo, 80.2 h; P < 0.001 vs. baloxavir) [13], and median TTIS was numerically shorter than oseltamivir (73.2 vs. 81.0 h) among high-risk individuals in CAPSTONE-2 (placebo, 102.3 h; P < 0.0001 vs. baloxavir) [14]. The input values for total mean duration of symptoms were based on NICE TA168 for treated patients (reported for oseltamivir in NICE TA 168 and assumed to be the same for baloxavir) and untreated patients (reported for placebo in NICE TA168) [4]. Mean duration of symptoms for treated individuals was thus 6.9 days for the OwH population and 12.3 days for the high-risk population [4]. Mean duration of symptoms for untreated individuals was 8.9 days for the OwH population and 13.8 days for the high-risk population [4]. All inputs related to duration of symptoms are provided in Supplementary Table S6.
Health State Utilities
Mean baseline utility score (0.96), the utility associated with influenza (0.81, with or without complications), and the utility associated with recovery from an ICU stay (0.90) were the same as those used in an oseltamivir cost-effectiveness model by Kamal et al. [27, 30–32]. Utilities associated with specific complications and the durations of complications are provided in Supplementary Table S7 and Table S8, respectively, based on management in the outpatient, inpatient, or inpatient with ICU setting.
The disutility associated with adverse events was assumed to be 0.21 for both the OwH and high-risk populations [33]. The proportions of patients receiving baloxavir or oseltamivir who might experience a treatment-related adverse event were based on adverse events reported in the CAPSTONE-1 trial for the OwH population (baloxavir, 4.4%; oseltamivir, 8.4%) [13] and the CAPSTONE-2 trial for the high-risk population (baloxavir, 5.6%; oseltamivir, 7.9%) [14]. The most common treatment-related adverse events in both the baloxavir and oseltamivir groups were nausea and diarrhea in both CAPSTONE trials [13, 14]. A sensitivity analysis for this CEM was conducted to explore how identical occurrences of treatment-related adverse events between baloxavir and oseltamivir groups might impact the results. The durations of adverse events were 7 days (assumption) with a disutility of 0.21 [33]. The cost of treating the adverse events was assumed to be $10 for over-the-counter medication.
Drug Costs
Drug costs used in the model were calculated per episode of care, including one tablet for baloxavir [20] and 75 mg twice daily for a 5-day course of oseltamivir [34]. The model inputs used the wholesale acquisition costs (as of July 1, 2023) per episode of care of $159.14 for baloxavir and $50.40 for oseltamivir (calculated from the average of all generic oseltamivir drugs available in the US) [35].
Sensitivity Analyses
One-way deterministic and probabilistic sensitivity analyses were conducted to explore the robustness of the primary findings. Deterministic sensitivity analyses explored a range of complication rates among baloxavir-treated patients (10–50%), the cost of baloxavir treatment ($0 to $300) while the cost of oseltamivir remained constant, and an identical rate of adverse events with baloxavir and oseltamivir (8.4% each for the OwH subset; 7.9% each for the high-risk subset). The probabilistic sensitivity analysis (1000 simulations) generated a cost-effectiveness plane (incremental costs by incremental QALYs) with an expected ICER and a probability of baloxavir being cost-effective at a willingness-to-pay (WTP) of $100,000/QALY gained.
Scenario Analyses
Scenario analysis 1 evaluated the cost-effectiveness of baloxavir using the base case assumptions applied separately to the OwH and high-risk subpopulations. Since the base case analysis assumed no (zero) reduction in viral transmission with antiviral treatment, scenario analysis two explored the potential impact of a range of potential values for reduced viral transmission rates with baloxavir compared with oseltamivir or no antiviral treatment in terms of ICERs and net monetary benefit (NMB). NMB is a function of effectiveness (QALYs) and a WTP threshold of $100,000/QALY gained minus the cost of the intervention, such that an NMB > 0 indicates cost-effectiveness of the intervention [36].
Results
Base Case Cost-Effectiveness Results
From a total population perspective (OwH + high-risk), antiviral treatment with baloxavir provided 1266 incremental QALYs per 100,000 individuals at an incremental total cost of $8.62 million per 100,000 compared with oseltamivir, for an ICER of $6813 per QALY gained and an NMB of $1180 (Table 2). Baloxavir provided 6250 incremental QALYs per 100,000 at an incremental total cost of $4.18 million per 100,000 compared with no antiviral treatment, for an ICER of $669 per QALY gained and an NMB of $6208 (Table 2).
Table 2.
Base case cost-effectiveness results per 100,000 individuals (no reduction in viral transmission with antiviral treatment)
| Parameter (per 100,000) | Baloxavir | Oseltamivir | No treatment |
|---|---|---|---|
| General practitioner visit costs | $6.81 million | $6.81 million | $6.81 million |
| Antiviral drug cost | $15.91 million | $5.04 million | $0 |
| Direct costs of treatment-related adverse events | $49,000 | $82,000 | 0.00 |
| Direct costs of complications | $50.83 million | $53.05 million | $62.62 million |
| Total costs | $73.61 million | $64.98 million | $69.43 million |
| Incremental costs, baloxavir vs. comparator | – | $8.62 million | $4.18 million |
| Total QALYs lost due to influenza | 27,519 | 28,785 | 33,769 |
| Incremental QALYs, baloxavir vs. comparator | – | 1266 | 6250 |
| ICER, baloxavir vs. comparator | – | $6813 | $669 |
| NMB, baloxavir vs. comparator | – | $1180 | $6208 |
Total costs may not sum precisely from component rows in this table due to rounding
ICER incremental cost-effectiveness ratio, NMB net monetary benefit, QALY quality-adjusted life-year
Sensitivity Analyses
Deterministic Sensitivity Analysis
When the complication rate among baloxavir-treated patients in the total population (OwH + high-risk) was varied from 10% to 28%, baloxavir was still dominant (more effective and less costly, with ICERs < 0) or cost-effective (< $100,000/QALY) versus oseltamivir (Supplementary Table S9). At hypothetical complication rates of 32% or higher, baloxavir would be dominated by oseltamivir (more costly and less effective). While the cost of oseltamivir remained fixed at $50.40, baloxavir remained cost-effective (< $100,000/QALY) versus oseltamivir even through a hypothetical cost as high as $300 for baloxavir treatment (ICER, $17,939/QALY vs. oseltamivir) (Supplementary Table S9). When treatment-related adverse event rate inputs were set to be identical for baloxavir and oseltamivir (OwH, 8.4% each; high-risk, 7.9% each), baloxavir provided an ICER of $6847/QALY versus oseltamivir (Supplementary Table S9).
Probabilistic Sensitivity Analysis
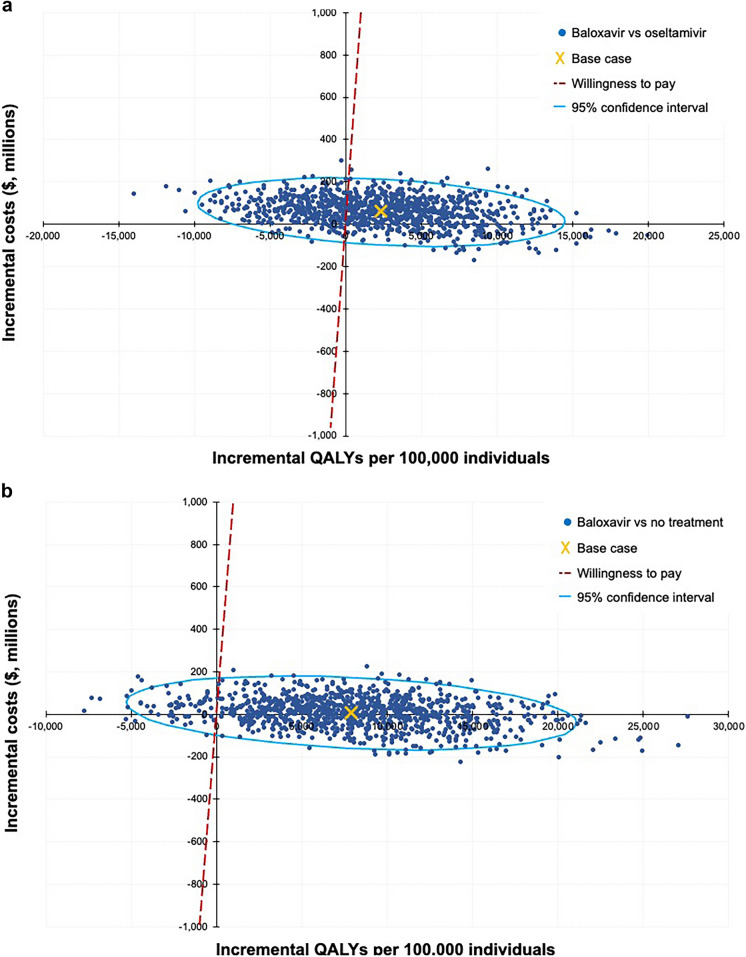
Probabilistic sensitivity analysis in the total population (OwH + high-risk) showed the probability of baloxavir being cost-effective at a willingness-to-pay threshold of $100,000/QALY to be 69.3% versus oseltamivir and 95.2% versus no antiviral treatment, with expected ICERs of $2569 and $87, respectively (Fig. 2).
Fig. 2.
Probabilistic sensitivity analysis. a Baloxavir versus oseltamivir. b Baloxavir versus no treatment. QALY quality-adjusted life-year, WTP willingness to pay
Scenario Analyses
Scenario Analysis 1: High-Risk and OwH Subpopulations
Scenario analysis 1 explored the cost-effectiveness of baloxavir versus oseltamivir or no antiviral treatment separately in the high-risk and OwH subpopulations (initially combined as the total population in the base case analysis). Baloxavir was cost-effective in the high-risk population compared with oseltamivir ($8597/QALY) and was dominant versus no antiviral treatment with cost savings of $2.3 million per 100,000 individuals (Table 3). In the OwH population, baloxavir provided an ICER of $5861/QALY versus oseltamivir and $2037/QALY versus no treatment (Table 4).
Table 3.
Cost-effectiveness of baloxavir in the high-risk population per 100,000 individuals (using the base case assumption of no reduction in viral transmission with antiviral treatment)
| Parameter (per 100,000) | Baloxavir | Oseltamivir | No treatment |
|---|---|---|---|
| Drug cost | $15.91 million | $5.04 million | $0 |
| Direct costs of treatment-related adverse events | $56,000 | $79,000 | 0.00 |
| Direct costs of complications | $75.49 million | $77.66 million | $93.75 million |
| Total costs | $98.27 million | $89.59 million | $100.56 million |
| Incremental costs, baloxavir vs. comparator | – | $8.68 million | –$2.29 million |
| Total QALYs lost due to influenza | 35,756 | 36,765 | 44,287 |
| Incremental QALYs, baloxavir vs. comparator | – | 1009 | 8531 |
| ICER, baloxavir vs. comparator | – | $8597 | Baloxavir dominanta |
ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year
aA dominant cost-effectiveness strategy is more effective and less costly than the comparator, producing an ICER < 0
Table 4.
Cost-effectiveness of baloxavir in the OwH population per 100,000 individuals (using the base case assumption of no reduction in viral transmission with antiviral treatment)
| Parameter (per 100,000) | Baloxavir | Oseltamivir | No treatment |
|---|---|---|---|
| Drug cost | $15.91 million | $5.04 million | $0 |
| Direct costs of treatment-related adverse events | $44,000 | $84,000 | 0.00 |
| Direct costs of complications | $31.82 million | $34.08 million | $38.62 million |
| Total costs | $54.59 million | $46.01 million | $45.43 million |
| Incremental costs, baloxavir vs. comparator | – | $8.58 million | $9.16 million |
| Total QALYs lost due to influenza | 21,199 | 22,663 | 25,698 |
| Incremental QALYs, baloxavir vs. comparator | – | 1464 | 4499 |
| ICER, baloxavir vs. comparator | – | $5861 | $2037 |
ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year
Scenario Analysis 2: Impact of Reducing Viral Transmission Rates with Baloxavir Treatment
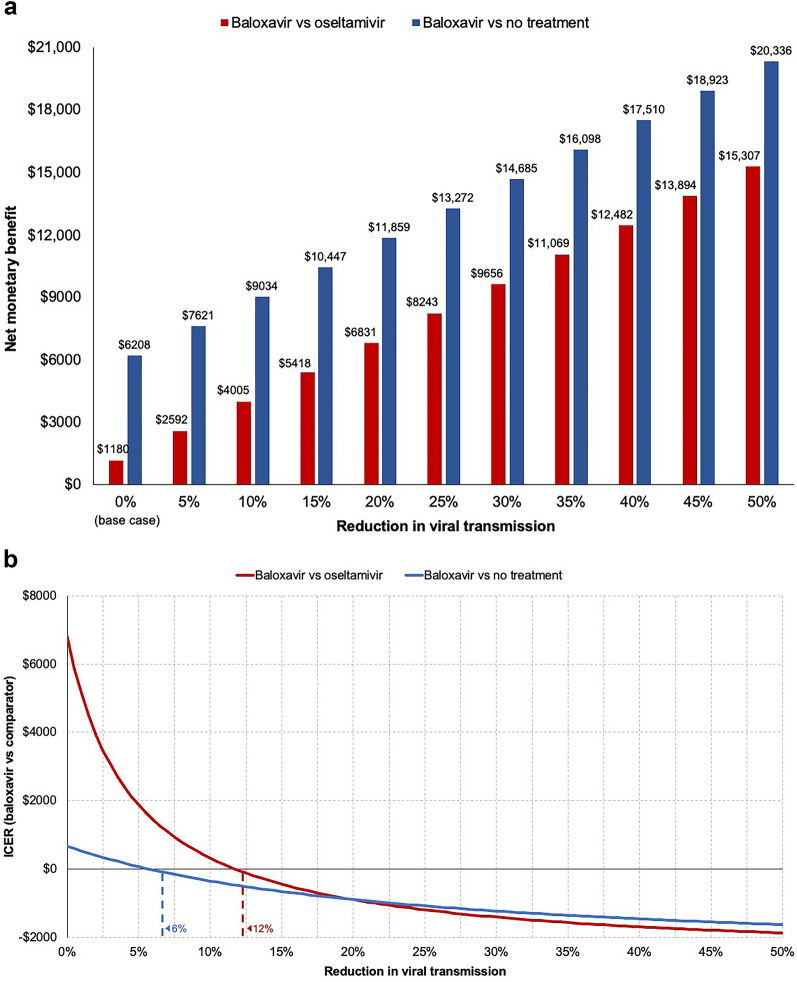
The base case analysis assumed no reduction (zero) in viral transmission rates with baloxavir treatment compared with oseltamivir or no treatment. Scenario analysis two explored the impact of reduced viral transmission with baloxavir using a range of values. This analysis showed that a reduction in viral transmission would have a considerable impact on the modeled cost-effectiveness of baloxavir. When baloxavir treatment was associated with a 5% reduction in viral transmission, the incremental NMB increased to $2592 versus oseltamivir (from $1180 in the base case analysis) and $7621 versus no treatment (from $6208 in the base case analysis) (Fig. 3a). The impact of reducing viral transmission on the NMB of baloxavir appeared to be generally linear, such that every 5% increase in reduced viral transmission improved the NMB of baloxavir by approximately $1413 compared with oseltamivir or no treatment (Fig. 3a). Reductions in viral transmission greater than 5% further increased the NMB of baloxavir, reducing the ICER until baloxavir would eventually be considered dominant (more effective and less costly, with ICERs < 0). Baloxavir was dominant starting with reduced viral transmission values of 12.0% (–$46/QALY gained) versus oseltamivir and 6.0% (–$30/QALY gained) versus no antiviral treatment (Fig. 3b).
Fig. 3.
Cost-effectiveness of baloxavir versus oseltamivir or no antiviral treatment by level of transmission reduction benefit. a NMB with baloxavir versus oseltamivir or no antiviral treatment; b ICER for baloxavir versus oseltamivir or no antiviral treatment. NMB was calculated using a willingness-to-pay threshold of $100,000 per QALY gained. Dashed lines indicate the reduction in viral transmission associated with the first ICER value < 0 for each comparison. ICER incremental cost-effectiveness ratio, NMB net monetary benefit, QALY quality-adjusted life-year
Discussion
This cost-effectiveness analysis used epidemiological and clinical assumptions from a real-world US administrative claims database analysis and from the published literature. The CEM showed that baloxavir is cost-effective compared with oseltamivir or no antiviral treatment from a US payer perspective. In the total population, baloxavir provided ICERs of $6813/QALY versus oseltamivir and $669/QALY versus no treatment, well below a commonly accepted threshold of $100,000/QALY. Baloxavir was dominant (more effective and less costly, with ICERs < 0) compared with oseltamivir when analyzed separately in the high-risk subset of the population (ICER vs. oseltamivir, $8597; dominant vs. no treatment), and cost-effective in the OwH subset (ICER vs. oseltamivir, $5861; ICER vs. no treatment, $2037). Overall, the high-risk population had more QALYs lost due to influenza than the OwH population, which may be expected given the higher rate of complications observed among high-risk individuals in the real-world US claims study [23], supporting the face validity of the model.
Sensitivity analyses explored how changes to model inputs would impact the base case results. The majority of the sensitivity analyses did not show substantive changes in the overall cost-effectiveness of baloxavir. However, baloxavir would be dominated by oseltamivir (less effective and more costly) if complication rates among patients treated with baloxavir were ≥ 32% while those among patients receiving oseltamivir remained at the base case value of 29.5%, demonstrating the importance of this particular input on the modeled results. Complication rates observed in the real-world US claims study were lower for patients receiving baloxavir (28.0%) compared with those receiving oseltamivir (29.5%) in the total population, as well as in the OwH (26.2% and 28.1%, respectively) and high-risk subsets (30.5% and 31.3%, respectively) [23]. It is worth noting that this is not a comparison of adverse events from clinical trials, but an observed occurrence of complications in a large population of beneficiaries where a 1.5% difference in complications may have a meaningful impact on direct costs to the health plan. The probabilistic sensitivity analysis provided consistent results with the deterministic base case findings and indicated that baloxavir had the highest probability of being cost-effective compared with oseltamivir or no treatment. The cost-effectiveness planes illustrated the 1000 simulations and the joint distribution on cost and effect. The majority of simulations were located in either the upper right quadrant (where baloxavir would be more effective and more costly) or lower right quadrant (where baloxavir would be more effective and less costly) for the comparisons with both oseltamivir and no treatment. However, the analysis against oseltamivir provided greater uncertainty in the results as seen from the cost-effectiveness plane, where several of the simulations were located in the upper left quadrant (where baloxavir would be less effective and more costly).
The scenario analysis exploring the impact of reducing viral transmission rates with baloxavir treatment showed a substantial economic benefit of baloxavir from a US commercial payer perspective. A 5% reduction in viral transmission increased the NMB of baloxavir versus oseltamivir to $2592 compared with $1180 in the base case analysis, and every subsequent 5% reduction in viral transmission improved the incremental NMB of baloxavir by approximately $1413 compared with oseltamivir or no treatment. Baloxavir was cost-dominant, starting with relatively low rates of reduced viral transmission of 12% compared with oseltamivir and 6% compared with no treatment.
The potential for antiviral treatment to reduce viral transmission is supported by published evidence from real-world research and modeling studies [21, 37–39], and the ongoing phase 3 CENTERSTONE clinical trial (NCT03969212) is assessing reduced viral transmission with baloxavir which may further inform projections of subsequent health economic outcomes. Reducing viral shedding and the duration of infectiousness for infected individuals in the community can reduce the number of secondary cases and subsequent hospitalizations, particularly in pandemic settings [37–39]. Real-world evidence has shown reduced viral transmission with antiviral treatment even among household members living in close proximity [21, 40]. Baloxavir showed numerically greater reductions in viral transmission compared with oseltamivir in a prospective real-world US study where infected individuals treated with baloxavir had 9% lower household transmission rates compared with oseltamivir (18% vs. 27%, respectively; P = 0.09) [21]. Baloxavir used for postexposure prophylaxis has also demonstrated efficacy in reducing influenza among household contacts of patients aged ≥ 5 years in Japan [17]. In this CEM, a 9% reduction in viral transmission with baloxavir was estimated to provide an NMB of $3723 compared with oseltamivir.
The results of this CEM should be interpreted in the context of certain strengths and limitations. This model only considered the basic direct costs of antiviral treatment, medical management of influenza complications, and adverse effects associated with antiviral treatment. It should be noted that treatment data from administrative claims is related to prescription fills, and this model assumed that patients were taking the medication correctly as indicated. Treatment compliance may have been more of a consideration for the multiple dosing regimen of oseltamivir than the single administration of baloxavir. Future work may also consider the potential influence of patients’ treatment preferences and compliance based on differences in antiviral treatment regimens; this model did not explore how a single-dose administration (baloxavir) may be generally preferred to a multiple-dose regimen (oseltamivir). Future work may also attempt to account for the impact of antiviral treatment on indirect costs such as those associated with the burden of influenza on work productivity and activities of daily living. As many as 75% of working adults have been reported to miss work for an average of 2–3 days when they have influenza, and influenza pandemics are associated with notable spikes in lost productivity across industries [41–43]. Further, approximately 50–75% of employed adults report missing work for an average of 1–2 days to care for a dependent with influenza [2, 44–46]. Thus, the cost-effectiveness of antiviral treatment estimated by this CEM is likely to be conservative, since the benefit of antiviral treatment and reduced viral transmission on indirect costs was not considered. It should be noted that more data are needed to better understand the real-world incidence influenza viruses with reduced susceptibility to baloxavir, which was not accounted for in this model. Current surveillance from the World Health Organization Global Influenza Surveillance and Response System (www.who.int/initiatives/global-influenza-surveillance-and-response-system) suggests a very low level or rare occurrence of resistance to baloxavir; however, ongoing surveillance and monitoring, especially in geographies with an increased use of baloxavir, remain essential.
This model showed that baloxavir was cost-effective compared with oseltamivir or no antiviral treatment in the total population, as well as in the high-risk and OwH subpopulations, and that the predicted reduction in viral transmission with baloxavir could result in further economic benefits. As a result, baloxavir is likely to have a meaningful health economic benefit in the commercially insured US population represented in the real-world administrative claims study that provided inputs for this model. The presence of influenza was determined by administrative claims that reflect the payer perspective but did not include test results for clinical confirmation, and did not include data related to use of over-the-counter medication. These findings may not be directly applicable to uninsured individuals or those with other types of coverage. Finally, these findings may be of further interest in the context of stockpiling antiviral treatment, which has been reported to be a cost-effective strategy particularly for pandemics [47].
Conclusion
This model estimated a meaningful health economic benefit of baloxavir treatment compared with oseltamivir or no antiviral treatment from a US payer perspective. Findings were robust in favor of baloxavir being cost-effective in the total insured US population as well as in the high-risk and OwH subpopulation analyses. Baloxavir provided a meaningful improvement in NMB compared with oseltamivir or no treatment when hypothetical rates of reduced viral transmission were explored in a scenario analysis. The ability of baloxavir to reduce viral transmission appeared to offer a substantial additional economic benefit.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank Devika Chawla for work supporting the real-world administrative claims study. Devika was an employee of Genentech Inc., a member of the Roche Group, when the work was performed.
Medical Writing and Editorial Assistance
Third-party editorial assistance was provided by The Nucleus Group Holdings, Inc, funded by F. Hoffmann-La Roche, Ltd.
Author Contributions
Conception and design: Svenn Alexander Kommandantvold, Shih-Chen Chang, Andy Surinach, Vince Yau, Marie-Helene Blanchet Zumofen. Analysis and interpretation of data: All authors. Drafting of the paper or revising it critically for intellectual content: All authors. Final approval of the version to be published: All authors. All authors agree to be accountable for all aspects of the work.
Funding
This study was supported by F. Hoffmann-La Roche, Ltd. The journal’s Rapid Service Fee was also funded by F. Hoffmann-La Roche, Ltd.
Data Availability
All data generated or analyzed in this study are included in this published article and supplementary materials.
Declarations
Conflict of Interest
Svenn Alexander Kommandantvold: Employee and shareholder of Roche Norge AS. Shih-Chen Chang: Employee and shareholder of Genentech Inc, a member of the Roche Group. Andy Surinach: Employee of Genesis Research, which received funding for this study from Genentech Inc, a member of the Roche Group. Vince Yau: Employee and shareholder of Genentech Inc, a member of the Roche Group. Jennie H. Best: Employee and shareholder of Genentech Inc, a member of the Roche Group. Hassan Zaraket: Employee and shareholder of Roche Products Ltd. Hao Zhou: Employee and shareholder of Genentech Inc, a member of the Roche Group. Marie-Helene Blanchet Zumofen: Employee and shareholder of F. Hoffmann-La Roche, Ltd.
Ethical Approval
This cost-effectiveness model was conducted based on analyses of a secondary-use database and did not recruit any human participants or involve animals.
Footnotes
Prior Presentation: This study was presented in part at IDWeek 2023; October 11–15, 2023; Boston, MA, USA.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention. 2022–2023 U.S. flu season: preliminary in-season burden estimates 2023 [August 11, 2023]. Available from: https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm
- 2.Blanchet Zumofen M-H, Frimpter J, Hansen SA. Impact of influenza and influenza-like illness on work productivity outcomes: a systematic literature review. Pharmacoeconomics. 2023;41(3):253–73. 10.1007/s40273-022-01224-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697–708. 10.1016/S0140-6736(17)30129-0 [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence. Amantadine, oseltamivir and zanamivir for the treatment of influenza. Technology appraisal guidance [TA168] 2009 [May 31, 2023]. Available from: https://www.nice.org.uk/guidance/ta168
- 5.Centers for Disease Control and Prevention. Estimated flu-related illnesses, medical visits, hospitalizations, and deaths in the United States — 2018–2019 flu season 2020 [June 27, 2023]. Available from: https://www.cdc.gov/flu/about/burden/2018-2019.html
- 6.Tavares LP, Teixeira MM, Garcia CC. The inflammatory response triggered by Influenza virus: a two edged sword. Inflamm Res. 2017;66(4):283–302. 10.1007/s00011-016-0996-0 [DOI] [PubMed] [Google Scholar]
- 7.Sellers SA, Hagan RS, Hayden FG, Fischer WA 2nd. The hidden burden of influenza: a review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017;11(5):372–93. 10.1111/irv.12470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167(7):775–85. 10.1093/aje/kwm375 [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization Writing Group. Non-pharmaceutical interventions for pandemic influenza, international measures. Emerg Infect Dis. 2006;12(1):81–7. 10.3201/eid1201.051370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallick C, Abbass IM, Sheinson D, Moawad D. Healthcare resource use and burden associated with influenza transmission among household members with a primary infection: commercial claims data analysis. Clinicoecon Outcomes Res. 2021;13:335–42. 10.2147/CEOR.S298992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(1):1–24. [PubMed] [Google Scholar]
- 12.Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492–500. 10.1086/600383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379(10):913–23. 10.1056/NEJMoa1716197 [DOI] [PubMed] [Google Scholar]
- 14.Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2020;20(10):1204–14. 10.1016/S1473-3099(20)30004-9 [DOI] [PubMed] [Google Scholar]
- 15.Ng S, Cowling BJ, Fang VJ, et al. Effects of oseltamivir treatment on duration of clinical illness and viral shedding and household transmission of influenza virus. Clin Infect Dis. 2010;50(5):707–14. 10.1086/650458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ison MG. Clinical use of approved influenza antivirals: therapy and prophylaxis. Influenza Other Respir Viruses. 2013;7(Suppl 1):7–13. 10.1111/irv.12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikematsu H, Hayden FG, Kawaguchi K, et al. Baloxavir marboxil for prophylaxis against influenza in household contacts. N Engl J Med. 2020;383(4):309–20. 10.1056/NEJMoa1915341 [DOI] [PubMed] [Google Scholar]
- 18.Hurt AC, Chotpitayasunondh T, Cox NJ, et al. Antiviral resistance during the 2009 influenza A H1N1 pandemic: public health, laboratory, and clinical perspectives. Lancet Infect Dis. 2012;12(3):240–8. 10.1016/S1473-3099(11)70318-8 [DOI] [PubMed] [Google Scholar]
- 19.Li TC, Chan MC, Lee N. Clinical implications of antiviral resistance in influenza. Viruses. 2015;7(9):4929–44. 10.3390/v7092850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.XOFLUZA® (baloxavir marboxil). US prescribing information. South San Francisco: Genentech Inc; 2024. [Google Scholar]
- 21.Best JH, Sadeghi MH, Sun X, et al. Household flu transmission and healthcare resource use among patients treated with baloxavir versus oseltamivir for influenza: an outpatient prospective survey in the United States. Open Forum Infect Dis. 2023;10(Suppl 2): ofad500.964. 10.1093/ofid/ofad500.964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuberger E, Wallick C, Chawla D, Castro RC. Baloxavir vs. oseltamivir: reduced utilization and costs in influenza. Am J Manag Care. 2022;28(3):e88–95. [DOI] [PubMed] [Google Scholar]
- 23.Data on File. F. Hoffmann-La Roche Ltd. 2023.
- 24.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–103. 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Flu season [July 22, 2024]. Available from: https://www.cdc.gov/flu/about/season/
- 26.Centers for Disease Control and Prevention. People at higher risk of flu complications 2022 [July 13, 2023]. Available from: https://www.cdc.gov/flu/highrisk/index.htm
- 27.Kamal MA, Smith PF, Chaiyakunapruk N, et al. Interdisciplinary pharmacometrics linking oseltamivir pharmacology, influenza epidemiology and health economics to inform antiviral use in pandemics. Br J Clin Pharmacol. 2017;83(7):1580–94. 10.1111/bcp.13229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marti J, Hall P, Hamilton P, et al. One-year resource utilisation, costs and quality of life in patients with acute respiratory distress syndrome (ARDS): secondary analysis of a randomised controlled trial. J Intensive Care. 2016;4:56. 10.1186/s40560-016-0178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Medicare & Medicaid Services. Physician fee schedule 2023 [July 13, 2023]. Available from: https://www.cms.gov/medicare/medicare-fee-for-service-payment/physicianfeesched
- 30.Khazeni N, Hutton DW, Garber AM, Owens DK. Effectiveness and cost-effectiveness of expanded antiviral prophylaxis and adjuvanted vaccination strategies for an influenza A (H5N1) pandemic. Ann Intern Med. 2009;151(12):840–53. 10.7326/0000605-200912150-00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fryback DG, Dasbach EJ, Klein R, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13(2):89–102. 10.1177/0272989X9301300202 [DOI] [PubMed] [Google Scholar]
- 32.Turner DA, Wailoo AJ, Cooper NJ, et al. The cost-effectiveness of influenza vaccination of healthy adults 50–64 years of age. Vaccine. 2006;24(7):1035–43. 10.1016/j.vaccine.2004.12.033 [DOI] [PubMed] [Google Scholar]
- 33.Smith KJ, Roberts MS. Cost-effectiveness of newer treatment strategies for influenza. Am J Med. 2002;113(4):300–7. 10.1016/S0002-9343(02)01222-6 [DOI] [PubMed] [Google Scholar]
- 34.TAMIFLU® (oseltamivir phosphate). US prescribing information. South San Francisco: Genentech Inc; 2019. [Google Scholar]
- 35.AnalySource. AnalySource: premier access to the First Databack drug pricing database 2023 [February 2, 2024]. Available from: www.analysource.com
- 36.Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Int J Epidemiol. 2006;36(2):476–7. [Google Scholar]
- 37.Yechezkel M, Ndeffo Mbah ML, Yamin D. Optimizing antiviral treatment for seasonal influenza in the USA: a mathematical modeling analysis. BMC Med. 2021;19(1):54. 10.1186/s12916-021-01926-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pepin KM, Riley S, Grenfell BT. Effects of influenza antivirals on individual and population immunity over many epidemic waves. Epidemiol Infect. 2013;141(2):366–76. 10.1017/S0950268812000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Hagan JJ, Wong KK, Campbell AP, et al. Estimating the United States demand for influenza antivirals and the effect on severe influenza disease during a potential pandemic. Clin Infect Dis. 2015;60(Suppl 1):S30-41. 10.1093/cid/civ084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayden FG, Asher J, Cowling BJ, et al. Reducing influenza virus transmission: the potential value of antiviral treatment. Clin Infect Dis. 2022;74(3):532–40. 10.1093/cid/ciab625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ablah E, Konda K, Tinius A, et al. Influenza vaccine coverage and presenteeism in Sedgwick County. Kansas Am J Infect Control. 2008;36(8):588–91. 10.1016/j.ajic.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 42.Karve S, Meier G, Davis KL, et al. Influenza-related health care utilization and productivity losses during seasons with and without a match between the seasonal and vaccine virus B lineage. Vaccine. 2013;31(33):3370–88. 10.1016/j.vaccine.2013.04.081 [DOI] [PubMed] [Google Scholar]
- 43.Sendi P, Drager S, Batzer B, et al. The financial burden of an influenza outbreak in a small rehabilitation centre. Influenza Other Respir Viruses. 2020;14(1):72–6. 10.1111/irv.12696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortega-Sanchez IR, Molinari NA, Fairbrother G, et al. Indirect, out-of-pocket and medical costs from influenza-related illness in young children. Vaccine. 2012;30(28):4175–81. 10.1016/j.vaccine.2012.04.057 [DOI] [PubMed] [Google Scholar]
- 45.Heikkinen T, Silvennoinen H, Heinonen S, Vuorinen T. Clinical and socioeconomic impact of moderate-to-severe versus mild influenza in children. Eur J Clin Microbiol Infect Dis. 2016;35(7):1107–13. 10.1007/s10096-016-2641-9 [DOI] [PubMed] [Google Scholar]
- 46.Tsuzuki S, Yoshihara K. The characteristics of influenza-like illness management in Japan. BMC Public Health. 2020;20(1):568. 10.1186/s12889-020-08603-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson SI, Chen YF, Nguyen-Van-Tam JS, et al. Evidence synthesis and decision modelling to support complex decisions: stockpiling neuraminidase inhibitors for pandemic influenza usage. F1000 Res. 2016;5:2293. 10.12688/f1000research.9414.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed in this study are included in this published article and supplementary materials.