Abstract
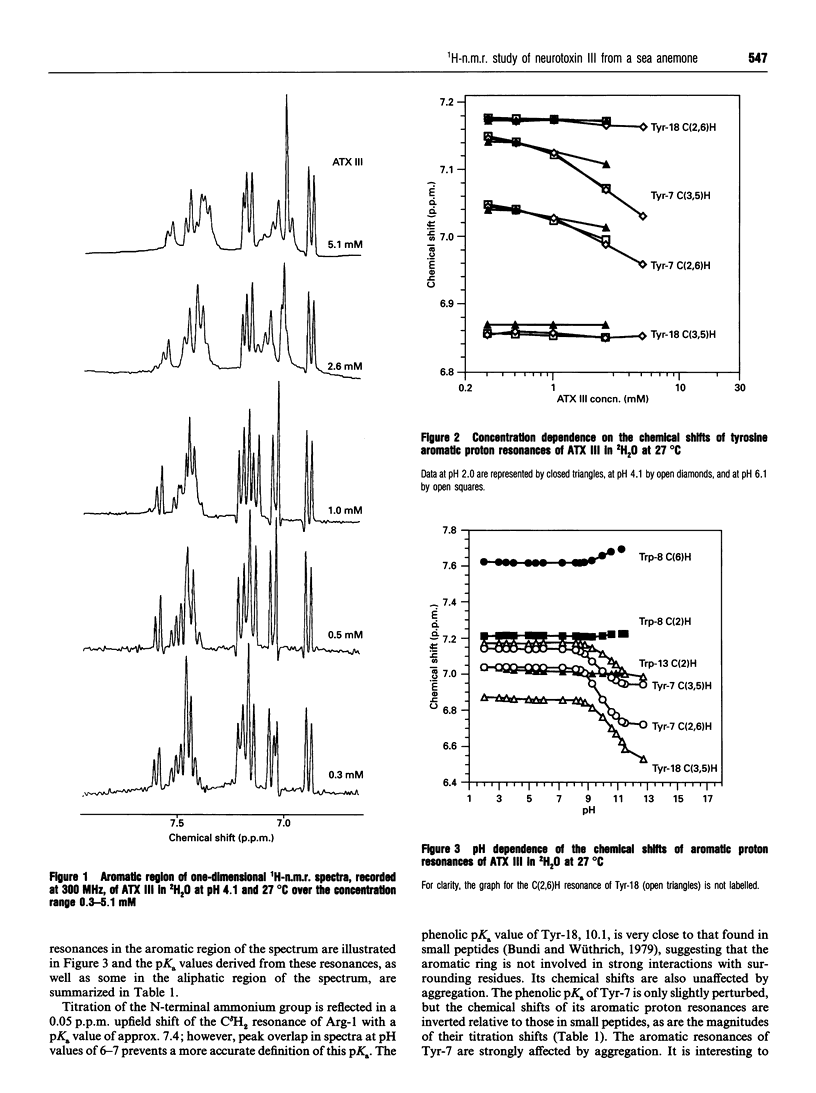
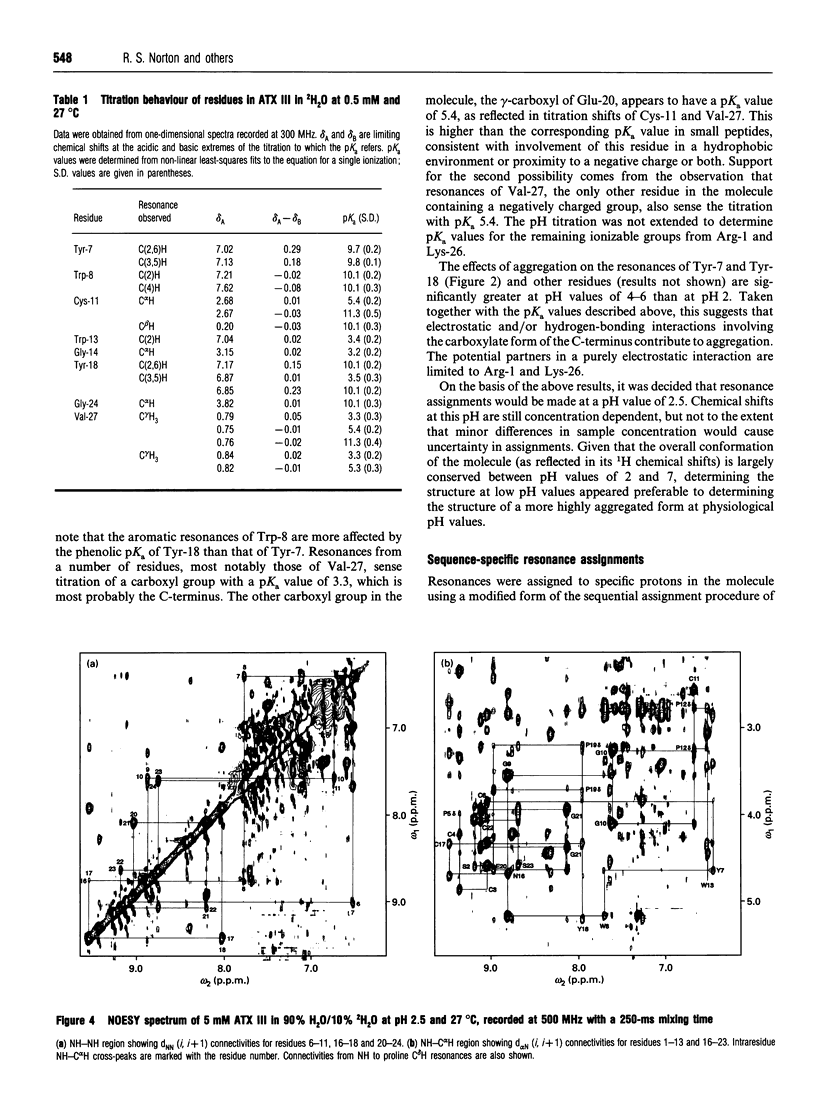
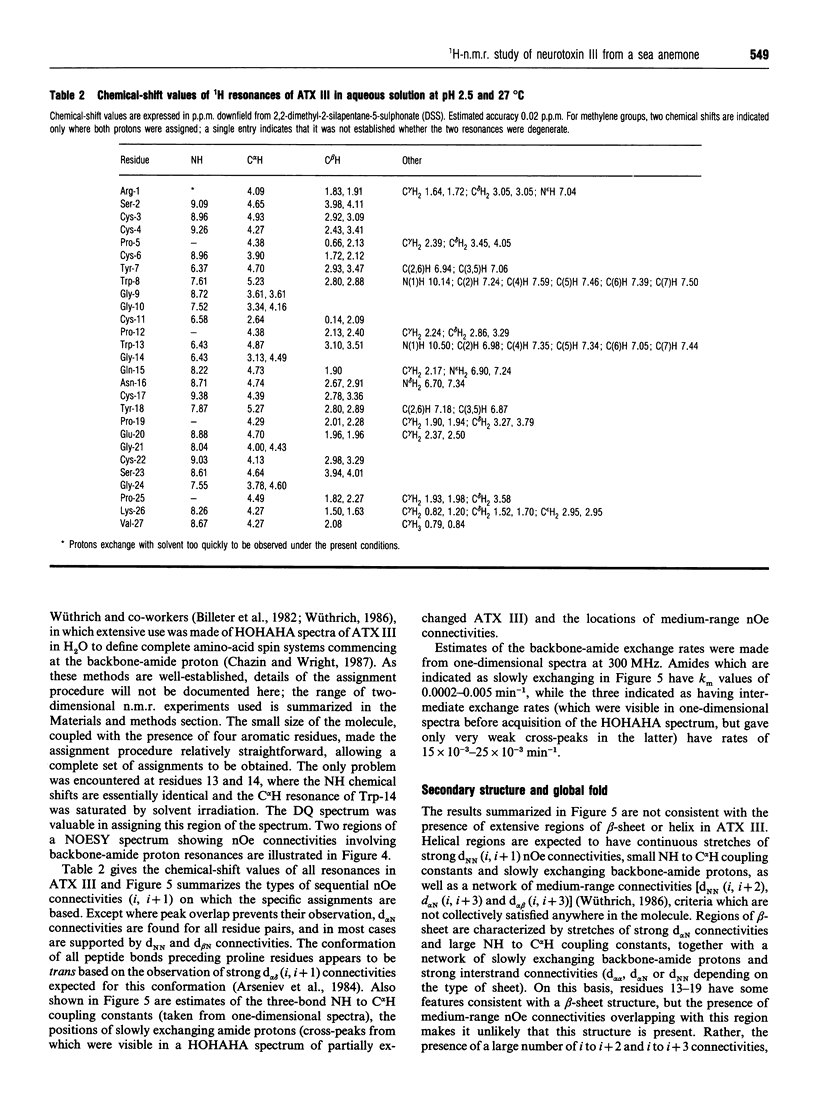
The solution properties, secondary structure and global fold of the 27-residue polypeptide neurotoxin III (ATX III), from the sea anemone Anemonia sulcata, have been investigated using high-resolution 1H-n.m.r. spectroscopy. Studies of the concentration dependence of the n.m.r. spectrum indicate that the molecule self-associates in the millimolar concentration range useable for n.m.r. analysis, the association being less pronounced at acidic pH values. The dependence on pH of association implies that electrostatic interactions play a role in this process, while the significant concentration-dependent shifts of the aromatic resonances of Tyr-7 and Trp-13 indicate that hydrophobic interactions also contribute. Individual pKa values have been determined for most ionizable groups in the molecule. Sequence-specific resonance assignments were obtained for all protons using a range of two-dimensional homonuclear-correlated and nuclear-Overhauser-effect (nOe) spectra. The secondary structure of the polypeptide was identified from sequential (i, i+1) and medium-range (i, i+2/3/4) nOe connectivities, NH to C alpha H coupling constants, C alpha H chemical shifts, and the location of slowly exchanging backbone-amide protons. ATX III contains no regular alpha-helix or beta-sheet, consisting instead of a network of reverse turns. nOe connectivities between half-cystine residues are consistent with the disulphide pairings 3-17, 4-11 and 6-22. ATX III has a well-defined structure and appears to lack the disordered loop which, in the longer sea anemone toxins (46-49 residues), may be part of the receptor-binding surface.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash P., Hider R. C., Ménez A., Wunderer G. Surface activity of polypeptide toxins isolated from Anemonia sulcata. Biochim Biophys Acta. 1981 Jul 28;669(2):231–235. doi: 10.1016/0005-2795(81)90245-2. [DOI] [PubMed] [Google Scholar]
- Bahraoui E. M., el Ayeb M., Granier C., Beress L., Rochat H. Specificity of antibodies to sea anemone toxin III and immunogenicity of the pharmacological site of anemone and scorpion toxins. Eur J Biochem. 1989 Mar 1;180(1):55–60. doi: 10.1111/j.1432-1033.1989.tb14614.x. [DOI] [PubMed] [Google Scholar]
- Barhanin J., Hugues M., Schweitz H., Vincent J. P., Lazdunski M. Structure-function relationships of sea anemone toxin II from Anemonia sulcata. J Biol Chem. 1981 Jun 10;256(11):5764–5769. [PubMed] [Google Scholar]
- Billeter M., Braun W., Wüthrich K. Sequential resonance assignments in protein 1H nuclear magnetic resonance spectra. Computation of sterically allowed proton-proton distances and statistical analysis of proton-proton distances in single crystal protein conformations. J Mol Biol. 1982 Mar 5;155(3):321–346. doi: 10.1016/0022-2836(82)90008-0. [DOI] [PubMed] [Google Scholar]
- Béress L., Béress R., Wunderer G. Isolation and characterisation of three polypeptides with neurotoxic activity from Anemonia sulcata. FEBS Lett. 1975 Feb 15;50(3):311–314. doi: 10.1016/0014-5793(75)80517-5. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Chazin W. J., Wright P. E. A modified strategy for identification of 1H spin systems in proteins. Biopolymers. 1987 Jun;26(6):973–977. doi: 10.1002/bip.360260615. [DOI] [PubMed] [Google Scholar]
- Driscoll P. C., Gronenborn A. M., Beress L., Clore G. M. Determination of the three-dimensional solution structure of the antihypertensive and antiviral protein BDS-I from the sea anemone Anemonia sulcata: a study using nuclear magnetic resonance and hybrid distance geometry-dynamical simulated annealing. Biochemistry. 1989 Mar 7;28(5):2188–2198. doi: 10.1021/bi00431a033. [DOI] [PubMed] [Google Scholar]
- Fogh R. H., Kem W. R., Norton R. S. Solution structure of neurotoxin I from the sea anemone Stichodactyla helianthus. A nuclear magnetic resonance, distance geometry, and restrained molecular dynamics study. J Biol Chem. 1990 Aug 5;265(22):13016–13028. doi: 10.2210/pdb2sh1/pdb. [DOI] [PubMed] [Google Scholar]
- Fontecilla-Camps J. C., Habersetzer-Rochat C., Rochat H. Orthorhombic crystals and three-dimensional structure of the potent toxin II from the scorpion Androctonus australis Hector. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7443–7447. doi: 10.1073/pnas.85.20.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould A. R., Mabbutt B. C., Norton R. S. Structure-function relationships in the polypeptide cardiac stimulant, anthopleurin-A. Effects of limited proteolysis by trypsin. Eur J Biochem. 1990 Apr 20;189(1):145–153. doi: 10.1111/j.1432-1033.1990.tb15471.x. [DOI] [PubMed] [Google Scholar]
- Llewellyn L. E., Norton R. S. Binding of the sea anemone polypeptide BDS II to the voltage-gated sodium channel. Biochem Int. 1991 Jul;24(5):937–946. [PubMed] [Google Scholar]
- Marion D., Wüthrich K. Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem Biophys Res Commun. 1983 Jun 29;113(3):967–974. doi: 10.1016/0006-291x(83)91093-8. [DOI] [PubMed] [Google Scholar]
- Nishida S., Fujita S., Warashina A., Satake M., Tamiya N. Amino acid sequence of a sea anemone toxin from Parasicyonis actinostoloides. Eur J Biochem. 1985 Jul 1;150(1):171–173. doi: 10.1111/j.1432-1033.1985.tb09003.x. [DOI] [PubMed] [Google Scholar]
- Norton R. S. Nuclear magnetic resonance (NMR) spectroscopy: applications to protein structure and engineering. Aust J Biotechnol. 1990 Apr;4(2):114–120. [PubMed] [Google Scholar]
- Norton R. S. Structure and structure-function relationships of sea anemone proteins that interact with the sodium channel. Toxicon. 1991;29(9):1051–1084. doi: 10.1016/0041-0101(91)90205-6. [DOI] [PubMed] [Google Scholar]
- Smythies J. R. A model of the molecular structure of toxin III from Anemonia sulcata. Med Hypotheses. 1981 Jun;7(6):707–710. doi: 10.1016/0306-9877(81)90081-5. [DOI] [PubMed] [Google Scholar]
- Torda A. E., Mabbutt B. C., van Gunsteren W. F., Norton R. S. Backbone folding of the polypeptide cardiac stimulant anthopleurin-A determined by nuclear magnetic resonance, distance geometry and molecular dynamics. FEBS Lett. 1988 Nov 7;239(2):266–270. doi: 10.1016/0014-5793(88)80931-1. [DOI] [PubMed] [Google Scholar]
- Wagner G., Zuiderweg E. R. Two-dimensional double quantum 1H NMR spectroscopy of proteins. Biochem Biophys Res Commun. 1983 Jun 29;113(3):854–860. doi: 10.1016/0006-291x(83)91077-x. [DOI] [PubMed] [Google Scholar]
- Widmer H., Billeter M., Wüthrich K. Three-dimensional structure of the neurotoxin ATX Ia from Anemonia sulcata in aqueous solution determined by nuclear magnetic resonance spectroscopy. Proteins. 1989;6(4):357–371. doi: 10.1002/prot.340060403. [DOI] [PubMed] [Google Scholar]
- Wishart D. S., Sykes B. D., Richards F. M. The chemical shift index: a fast and simple method for the assignment of protein secondary structure through NMR spectroscopy. Biochemistry. 1992 Feb 18;31(6):1647–1651. doi: 10.1021/bi00121a010. [DOI] [PubMed] [Google Scholar]


