Abstract
Background
A major drawback of traditional pulp testing procedures is that they depend on neural responses rather than vascular circulation. Hence, this study aimed to develop a custom-modified dental sensor using a finger pulse oximeter (PO) that is applicable to any type of tooth so as to test its ability and accuracy in evaluating the oxygen saturation (SaO2) values of teeth at different developmental stages as a measure of pulp vitality.
Materials and methods
A customized finger PO was employed to determine the systemic and pulp SaO2 levels in 300 children. A total of 600 teeth (primary and permanent) were divided into group I (100 primary molars), group II (200 permanent molars), group III (200 permanent incisors), and 100 endodontically treated (primary and permanent) teeth were included in group IV. The mean SaO2 values thus obtained in various groups were compared.
Results
Intragroup comparison of mean SaO2% of the patient's finger (systemic), test teeth, and control teeth showed significant differences (p ≤ 0.001). Intergroup comparisons also revealed significant differences in most of the groups.
Conclusions
The modified PO probe can be applied to any type of tooth. It was found to be accurate and sensitive enough to detect changes in SaO2 levels in various clinical situations irrespective of developmental stage. It proved to be a direct method of evaluation of pulp vitality by objective means.
How to cite this article
Patil A, Garg N, Pathivada L, et al. Evaluation of Oxygen Saturation Levels Using a Custom-modified Finger Pulse Oximeter for Assessment of Pulp Vitality in Various Clinical Situations in Pediatric Dental Practice: An In Vivo Study. Int J Clin Pediatr Dent 2024;17(S-1):S30–S36.
Keywords: Oxygen saturation, Pulse oximeter, Pulp vitality
Introduction
Pulp vitality testing is a prerequisite for establishing an accurate and rapid diagnosis in various clinical situations for pediatric dentists. Traditionally, pediatric dentists have depended upon various testing methods that reproduce symptoms linked with pulpal malady. All have the probability to produce an occasional pain sensation, which may lead to erroneous results.1 Melting ice drips can produce false positives onto adjacent teeth, gingiva,2 or periodontal ligament conducts electrical current that is applied onto the tooth surfaces, which stimulates the periodontal nerve fiber.3 Calcification in teeth with immature root formation or impact injury can give a false negative response.4,5
The conventional pulp vitality testing methods measure the neural response and not the vascular circulation, which is one of its major limitations.6 Determining the status of vascular supply is the most accurate method for assessing the pulp.7 They either rely on light absorption patterns as they pass through the tooth, for example, pulse oximetry, photoplethysmography, and dual wavelength spectrophotometry, or the change in frequency of light which is reflected from the tooth, for example, laser Doppler flowmetry.8
Schnettler and Wallace9 reported a connection between systemic and pulpal oxygen saturation (SaO2) using a customized ear pulse oximeter (PO) probe on a tooth. Wallace and Schnettler10 established a 100% correlation between histological and clinical diagnosis of the vitality of pulp using the PO probe and recommended it as a potential tester for pulp vitality. Over the past 2 decades, various authors1,7,9,11,12 have modified the PO that is designed to be used in a medical setup. Recently, Sharma et al.12 have tested a customized miniature version of the fingertip PO (Dr Morepen PO-04, Morepen Labs. Ltd, Delhi, India) for evaluation of the vitality of pulp in nonvital, vital, and root-filled primary and permanent incisors but not molars. Also, there is no single study available regarding the applicability of such miniature PO probes in primary, immature permanent, and mature permanent teeth, including molars, simultaneously. Hence, this research was undertaken to develop a custom-modified dental sensor using a finger PO and evaluate and compare the SaO2 levels for determination of pulp vitality in various clinical situations encountered by a pediatric dentist.
Materials and Methods
The research protocol was accepted by the Ethical Committee of the institute before beginning the study. A total number of 956 children was checked, who visited the Department of Pedodontics and Preventive Dentistry, Teerthanker Mahaveer Dental College and Research Centre, Moradabad, India, and 300 children who fulfilled the criteria were included in the research. A total of 600 teeth (primary and permanent) in these 300 children were allocated into group I (n = 100), group II (n = 200), group III (n = 200), and group IV (n = 100) based on randomization protocol using a split-mouth design.13 Following power analysis, the sample size was calculated which was >90%. The inclusion criteria for the research were an age-group of 4–12 years, children having clinically deep carious lesions involving primary molar along with contralateral sound primary molar, clinically deep carious lesion with respect to mature or immature permanent molar along with contralateral sound permanent molar, traumatized mature or immature permanent incisor along with contralateral sound permanent incisor, at least one endodontically treated tooth and contralateral sound tooth (primary and permanent) and the contralateral sound teeth should be having no fracture or caries or discoloration and the periodontal tissues should be healthy. Patients presenting with any systemic conditions, anterior crowding, rotated incisors, partially erupted teeth, badly broken-down teeth where the sensor cannot be engaged to the full extent, and teeth with swelling and mobility were excluded from the study. The teeth categorized under group I included 100 primary molars of 50 patients (50 test teeth and 50 control contralateral sound teeth). The teeth categorized under group II included 200 permanent molars of 100 patients. Group II was further subdivided into 100 mature permanent molars (50 test teeth and 50 control contralateral sound teeth) (group IIA) and 100 immature permanent molars (50 test teeth and 50 control contralateral sound teeth) designated based on the dental age of the patient (group IIB). The teeth categorized under group III included 200 permanent incisors of 100 patients. Group III was further subdivided into 100 mature permanent incisors (50 test teeth and 50 control contralateral sound teeth) (group IIIA) and 100 immature permanent incisors (50 test teeth and 50 control contralateral sound teeth) designated based on the dental age of the patient (group IIIB). The teeth categorized under group IV (negative control) included 100 teeth (previously endodontically treated primary and permanent) of 50 patients. Group IV was further subdivided into 50 primary teeth (25 endodontically treated test teeth and 25 control contralateral sound teeth) (group IVA) and 50 permanent teeth (25 endodontically treated test teeth and 25 control contralateral sound teeth) (group IVB). The PO used in the current study was a commercially available fingertip PO (Model-SLC 302, Sumo Life Care Pvt. Ltd, New Delhi, India) (Fig. 1). This PO had a color organic light-emitting diode (OLED) display screen which displayed SaO2 levels in percentage, pulse rate in beats/minute, and plethysmograph. The OLED display was featured with autorotation (auto four-direction sensor). This PO was small in size, effortless to operate, lightweight, easy to carry, and adopted a low utilization design with resistance to ambient light interference capability. The PO was stripped down without hampering the manufacturer's configuration of the internal electronic circuit. Then, the light emitting and receiving photodetector sensors were carefully separated from the main internal assembly was then attached to a local Velcro band, and taped for an attractive look and ease of use (Fig. 2). This custom-modified PO was extensively tested to evaluate the systemic SaO2 values before its actual intraoral utilization. During testing of this modified version, an original version served as reference equipment (Fig. 3).
Fig. 1:

Commercially available finger PO
Fig. 2:

Custom modified finger PO
Fig. 3:
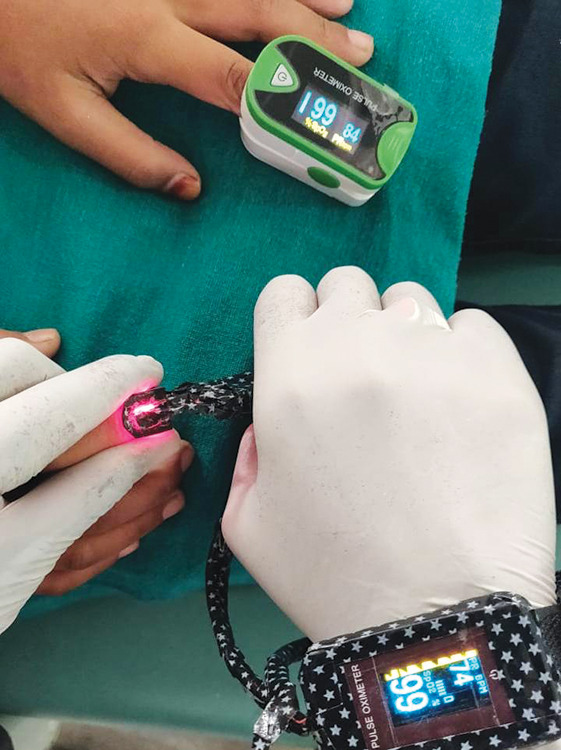
Accuracy of modified vs original PO in detecting systemic SaO2 levels
Procedure
The order of testing was firstly recording of systemic SaO2 levels followed by SaO2 levels of diseased teeth in each group and then SaO2 levels of control contralateral sound teeth in each group. The measurement of systemic SaO2 values on the index finger of the patient using customized dental probe (CDP) served as base readings, which would be compared with the SaO2 values of the teeth of the same patient. Surgical spirit was used for cold sterilization of CDP after every testing of the patient. The teeth to be tested were isolated with cotton rolls, and oil-free compressed air was used to dry the surface before the application of CDP. The sensor was placed on the labial or buccal surface, that is, the emitting diode, and the lingual or palatal surface, which is the receiving photodetector of the teeth to be tested (test tooth and control tooth). Maintaining a parallel position between both diodes of CDP was of utmost importance so that the photodetector receives as much light from the emitter through the tooth as possible. The interference of strong fluorescent ambient light and operating light was blocked during measurement to prevent interruption in capturing signals (Figs 4 to 6). All patients were instructed not to move their heads during vitality testing to avoid motion artifacts. After 30 seconds of monitoring the tooth, the values displayed by PO were recorded. Three consecutive readings on the OLED screen were noted at a gap of 30 seconds. For each tooth, the average of the three readings of SaO2% displayed by the PO was considered the final reading. If no values were displayed by PO at the end of the measurement period (three readings at an interval of 30 seconds), the SaO2 values of that particular tooth were recorded as negative.
Fig. 4:
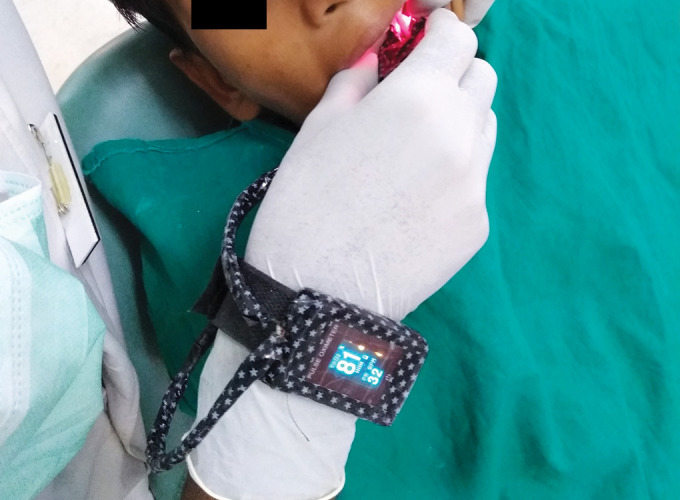
Custom-modified finger PO displaying 81% SaO2 in a primary molar
Fig. 6:
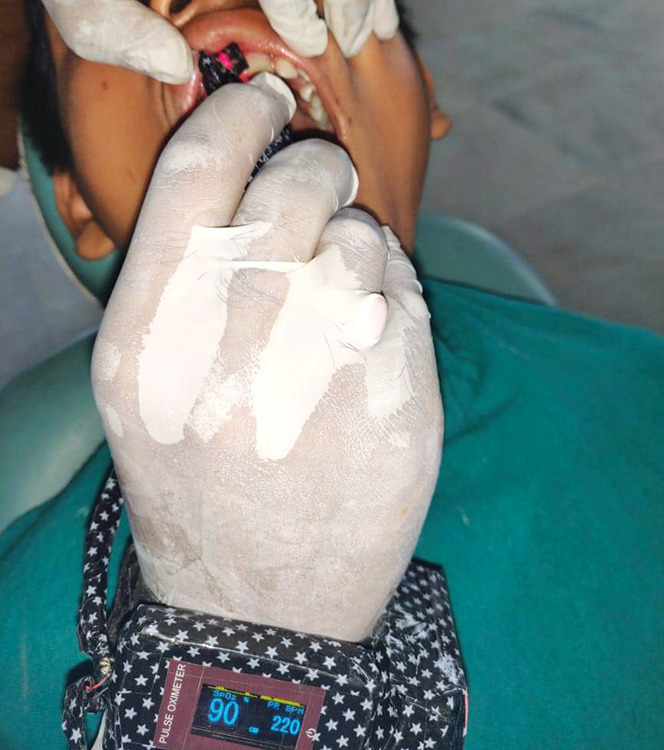
Custom-modified finger PO displaying 90% SaO2 in a permanent incisor
Fig. 5:
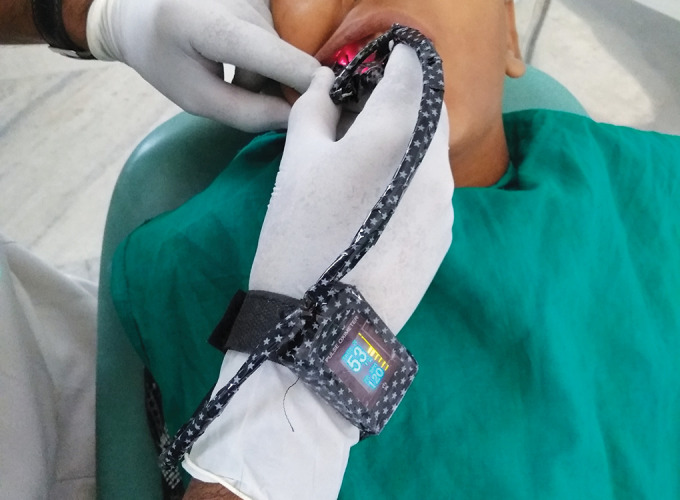
Custom-modified finger PO displaying 53% SaO2 in a permanent molar
The data thereby collected was statistically analyzed using Statistical Package for the Social Sciences (SPSS) software version “20” (IBM SPSS Statistics, IBM Corp., 2011) for Windows. Descriptive statistics, that is, mean and standard deviation, were calculated, and inferential statistics like the Wilcoxon signed rank test were utilized to evaluate the three variables (systemic SaO2, test, and control teeth SaO2) within the groups. Kruskal–Wallis test with post hoc Mann–Whitney U test was employed to evaluate systemic SaO2 values and control teeth SaO2 values in between the groups.
Results
Tables 1 to 3 represent the minimum, maximum, and mean SaO2 values of systemic, test tooth, and control tooth in all the groups, respectively. Comparison of the systemic-test tooth, test tooth-control tooth, and systemic-control tooth SaO2 values (Table 4) revealed a significant difference in the mean differences of SaO2 values of all the groups. Although there was no significant difference between the three variables, namely systemic (p = 0.83), test tooth (p = 0.79), and control tooth SaO2% (p = 0.54) as shown in Table 5. Table 6 shows a statistically significant difference between a few groups as a result of multiple comparisons. Based on the data of this research it was observed that the modified PO probe was capable enough to detect the varying SaO2 percentages of primary and permanent teeth at different developmental stages in various clinical situations.
Table 1:
Mean systemic SaO2 (%) values in all the groups
| N | Minimum | Maximum | Mean | Standard deviation | |
|---|---|---|---|---|---|
| Group I | 50 | 92.7 | 98.7 | 97.596 | 1.3060 |
| Group IIA | 50 | 92.0 | 99.0 | 97.760 | 1.2707 |
| Group IIB | 50 | 92.7 | 99.0 | 97.522 | 1.4629 |
| Group IIIA | 50 | 95.7 | 98.7 | 97.774 | .8386 |
| Group IIIB | 50 | 94.7 | 98.7 | 97.414 | 1.1450 |
| Group IVA | 25 | 93.7 | 98.7 | 97.228 | 1.2847 |
| Group IVB | 25 | 92.7 | 98.7 | 97.016 | 1.4801 |
Table 3:
Mean control tooth SaO2 (%) values in all the groups
| N | Minimum | Maximum | Mean | Standard deviation | |
|---|---|---|---|---|---|
| Group I | 50 | 70.7 | 97.0 | 89.326 | 4.6715 |
| Group IIA | 50 | 81.0 | 99.0 | 89.340 | 4.2552 |
| Group IIB | 50 | 78.3 | 91.7 | 85.382 | 4.0776 |
| Group IIIA | 50 | 71.3 | 98.3 | 89.520 | 4.4781 |
| Group IIIB | 50 | 80.7 | 94.0 | 87.990 | 3.2684 |
| Group IVA | 25 | 79.3 | 98.7 | 89.620 | 4.6791 |
| Group IVB | 25 | 81.3 | 94.3 | 87.128 | 3.1275 |
Table 4:
Comparison of mean differences of systemic, test, and control teeth SaO2 values within the groups (result of Wilcoxon signed rank test)
| Systemic: test tooth | Systemic: control tooth | Test tooth–control tooth | ||||
|---|---|---|---|---|---|---|
| Mean difference | p-value | Mean difference | p-value | Mean difference | p-value | |
| Group I | 38.77 | 0.00*** | 8.27 | 0.00*** | −30.50 | 0.00*** |
| Group IIA | 32.24 | 0.00*** | 8.42 | 0.00*** | −23.82 | 0.00*** |
| Group IIB | 35.22 | 0.00*** | 12.14 | 0.00*** | −23.08 | 0.00*** |
| Group IIIA | 32.53 | 0.00*** | 8.25 | 0.00*** | −24.27 | 0.00*** |
| Group IIIB | 39.65 | 0.00*** | 9.42 | 0.00*** | −30.22 | 0.00*** |
| Group IVA | 97.22 | 0.00*** | 7.60 | 0.00*** | −89.62 | 0.00*** |
| Group IVB | 97.01 | 0.00*** | 9.88 | 0.00*** | −87.12 | 0.00*** |
***Significant (p ≤ 0.001)
Table 5:
Comparison of systemic, test, and control teeth SaO2 values between the groups (result of Kruskal–Walli's test)
| Kruskal–Wallis | p-value | |
|---|---|---|
| Systemic | 0.046 | 0.83 (NS) |
| Test tooth | 0.06 | 0.79 (NS) |
| Control tooth | 0.36 | 0.54 (NS) |
NS, not significant (p > 0.05)
Table 6:
Result of post hoc Mann–Whitney U test
| Group | Groups | Systemic | Control | ||
|---|---|---|---|---|---|
| Mean difference | p-value | Mean difference | p-value | ||
| Group I | Group IIA | −0.16 | 0.83 | −0.01 | 0.54 |
| Group IIB | 0.07 | 0.94 | 3.94 | 0.00*** | |
| Group IIIA | −0.17 | 0.88 | −0.19 | 0.81 | |
| Group IIIB | 0.18 | 0.28 | 1.33 | 0.012* | |
| Group IVA | 0.36 | 0.14 | −0.29 | 0.90 | |
| Group IVB | 0.58 | 0.058 | 2.19 | 0.003** | |
| Group IIA | Group IIB | 0.23 | 0.22 | 3.95 | 0.00*** |
| Group IIIA | −0.01 | 0.48 | −0.18 | 0.44 | |
| Group IIIB | 0.34 | 0.11 | 1.35 | 0.08 | |
| Group IVA | 0.53 | 0.059 | −0.28 | 0.72 | |
| Group IVB | 0.74 | 0.005** | 2.21 | 0.018* | |
| Group IIB | Group IIIA | −0.25 | 0.84 | −4.13 | 0.00*** |
| Group IIIB | 0.10 | 0.28 | −2.60 | 0.005** | |
| Group IVA | 0.294 | 0.16 | −4.23 | 0.001*** | |
| Group IVB | 0.50 | 0.057 | −1.74 | 0.12 | |
| Group IIIA | Group IIIB | 0.36 | 0.16 | 1.53 | 0.007** |
| Group IVA | 0.54 | 0.081 | −0.10 | 0.94 | |
| Group IVB | 0.75 | 0.023* | 2.39 | 0.001*** | |
| Group IIIB | Group IVA | 0.1860 | 0.60 | −1.6 | 0.08 |
| Group IVB | 0.3980 | 0.34 | 0.86 | 0.43 | |
| Group IVA | Group IVB | 0.2120 | 0.74 | 2.49 | 0.037* |
Significant at *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001
Discussion
The skill of diagnosing the correct status of the dental pulp is one of the important pillars in uplifting the quality and success rate of endodontic treatment. The mechanism of traditional pulp vitality testing methods allows them to display/convey symptoms associated with pulpal condition. It is based on impulses generated by the pulp on thermal, electrical, and dentin stimuli. Traditional pulp vitality testing methods fall short of ideal pulp vitality testing methods. They are subjective in nature, and all conventional pulp vitality testing methods depend upon the neural stimulus of A δ-fibers. These methods have a common constraint in their mode of evaluation. Neural development varies at different developmental stages of teeth. In immature teeth, it is incomplete, while it greatly differs in newly matured teeth and complete in teeth that are older. The neural network also varies considerably for different regions of the pulp. The role of pain-sensory function is associated with certain myelinated nerve cell alterations during the development of the tooth.14
Pulpal circulation and not a neural network of pulp at any developmental stage is the major defining factor for the assessment of the pulpal status. There is a need for objective techniques for evaluating pulp vitality in pediatric dentistry (especially in children with behavioral problems) to avoid unpleasant sensations and improve patient cooperation. The purpose of this investigation was to assess the pulp vitality objectively using SaO2 values and also to evaluate the reliability of this device in different dentitions. The PO utilized in the present study was a portable fingertip PO, which is in contrast with previous studies,7–9 where hospital-setup POs with various types of probes were used. Pupim et al.15 confirmed that a portable fingertip PO can be used in dental treatments as it has similar accuracy to the traditional hospital PO. The data of the present research established the capability of PO to make a distinction between the nonvital and vital status of pulp in all different types of teeth. All permanent and primary teeth that were endodontically treated in group IV recorded SaO2 values of 0% with this device. Previous studies7,12 conducted by various authors also noted 0% SaO2 values on known teeth that were endodontically treated. In this research, a few test teeth gave negative responses, suggesting that their pulpal status might be altered or have become nonvital in groups I, IIA, IIB, IIIA, and IIIB. Studies conducted by Goho,16 Gopi Krishna et al.17 and Gopikrishna et al.18 Calil et al.,19 and Sharma et al.12 have revealed steady O2 saturation readings in all vital teeth using PO. Similarly, in the present study, all the test teeth in all groups (except in group IV) and control teeth in all groups showed SaO2 readings. Although all test teeth (diseased) except in group IV showed SaO2 readings, they might be on the verge of degeneration as there were varying amounts of percentage SaO2 values suggesting altered pulpal circulation.
In the present study, the SaO2 values obtained from primary molars, mature and immature permanent incisors, and molars were lower than those obtained from the patient's finger. Similar findings were reported by various authors in earlier studies.7,12,16,17,19 It was suggested by Fein et al.20 that the lesser O2 saturation readings for pulpal circulation may be credited to the light rays that get scattered through the gums. Gopi Krishna et al.17 evaluated pulpal vascular SaO2 in normal maxillary central incisors and found their mean SaO2 to be 79.30% (78–80). These readings are associated with the O2 saturation readings noted by Radhakrishnan et al.,7 where the mean value of O2 saturation for permanent central incisor (CI) was 81%. Pozzobon et al.21 evaluated permanent maxillary central incisors and recorded their average O2 saturation to be 85.27% (63–98). In this research, the average O2 saturation for control (sound) teeth in group IIIA (mature permanent incisors) was found to be 89.52% (71–98) (Table 3), which was close to the results established by Radhakrishnan et al.7 and Pozzobon et al.21
The findings of this study were approximately analogous to the results reported by Bargrizan et al.22 in the case of control (sound) teeth in group IIIB (immature permanent incisors), probably because of a similar age-group of sample patients. Goho16 assessed the pulp vitality in immature permanent incisors with a modified ear probe constructed by removal of the outer housing to help close the arrangement of sensors to the surface of the tooth. In results reported by Goho,16 the mean SaO2 found was 94%, which is higher than the mean percentage of SaO2 in the present study. Setzer et al.23 also conducted a study to investigate the correlation between pulp SaO2 rates and the diagnosis of pulpal status clinically. The authors found a mean SaO2 value of 74.6% for necrosed pulp. In the present study, the mean SaO2 values of test teeth (diseased teeth) are consistently lower in all different types of teeth at various levels of root developmental stages, suggesting an altered status of pulp vascularity.
According to manufacturer guidelines, the measurement range of the PO utilized in the present study is between 36 and 99%. However, Sharma et al.,12 in their study, suggested that all SaO2 readings <78% in permanent incisors and 80% in deciduous incisors should be considered nonspecific for diagnosis of nonvital teeth clinically. They compared PO with an electronic pulpal tester and thermal pulp testing. However, the present study was designed to evaluate only PO's efficiency to assess pulp vitality in various types of teeth at variable root development status. Wong24 elaborated on the reasons for false values with PO. Even though all the criteria for recording SaO2 of pulp, such as parallel alignment of sensors, the noise of the signals, and interruption with light transmission, were managed, dyshemoglobins that were there in the pulp that was nonvital may also be the reason for false values provided by PO. Furthermore, Wong24 affirmed that venous blood has an SaO2 level of 75%. In the present study, the results for test teeth (Table 2) in all the groups probably represent venous congestion (necrosis) and arterial ischemia in the pulp. Nevertheless, there was 100% accuracy of a PO with a customized dental probe for the tested range of SaO2 values for clinically sound teeth (control teeth) in all the groups (Table 3).
Table 2:
Mean test tooth SaO2 (%) values in all the groups
| N | Minimum | Maximum | Mean | Standard deviation | |
|---|---|---|---|---|---|
| Group I | 50 | 0.00 | 93.00 | 58.82 | 36.13 |
| Group IIA | 50 | 0.00 | 96.00 | 65.52 | 30.11 |
| Group IIB | 50 | 0.00 | 93.00 | 62.30 | 23.47 |
| Group IIIA | 50 | 0.00 | 97.30 | 65.24 | 33.12 |
| Group IIIB | 50 | 0.00 | 90.70 | 57.76 | 34.52 |
| Group IVA | 25 | 0.00 | 0.00 | 0.00 | 0E-7 |
| Group IVB | 25 | 0.00 | 0.00 | 0.00 | 0E-7 |
Karayilmaz et al.25 suggested that the pulpal blood flow of deciduous molars is amplified with age and physiological root resorption levels. They considered it to be linked with histological and morphological alterations in the pulp by age and physiologic root resorption. In this research as the mean O2 saturation value for primary molars recorded was 89.32% with a range of a minimum of 70.7% to a maximum of 97%, these findings concur with those of Karayilmaz et al.25 They also reported that higher SaO2 levels seen in primary molars might be due to hyperemia and dilated blood vessels of resorbing primary teeth. Similar changes in pulpal blood flow of permanent teeth during different stages of root development can occur. Bargrizan et al.22 suggested that a PO is also successful in the evaluation of O2 saturation in relation to root development status. They found that O2 saturation values in matured teeth with closed apex were lesser than those with immature teeth. This could be correlated to varied levels of vascular supply between the mature and immature permanent teeth. However, the results of groups IIA, IIB, IIIA, and IIIB (Table 3) in the present study are different, the reason being the design of the probe. The results also vary with the physiological parameters of the patient as they are never constant and are also based on the placement of sensors.
It is important to consider that readings may vary if this is put into practice, as readings depend on various factors, such as the placement of sensors and the probe used. Too much distance between the emitting diode and photodetector might affect the SaO2 level recordings. At the same time, the design of the probe utilized in the present study has both emitting diode and photodetector in close approximation, maintaining parallel alignment on the tooth to be tested. Hence, fewer variations in results might be obtained. Noting the lower values of SaO2 recorded for tooth, future studies must be aimed at understanding the light passage dynamics that pass through enamel and dentin in terms of reflection, transmission, absorption, diffraction, and other parameters. The information obtained should be functionalized to the monitor of PO so that it would be adjusted specifically for the measurement of SaO2. Although, according to Batchelder and Raley,26 as there is no certain reference to adjust POs, and verification of the correct calibration has no accepted procedure, other than human testing, every customized PO should be verified and tested for their calibration for recording the status of the vitality of pulp. Also, the nonadaptability of the customized probe sensor on a partially erupted tooth must be considered. Based on the observations of this research, it can be proposed that this device can be utilized even at a community level to screen patients who are in need of endodontic therapy. This device can also be used as a motivational factor in driving patients from rural areas to seek dental treatment, as it is noninvasive, painless, and patient-friendly.
Conclusion
The conclusions drawn from this study are:
The customized dental probe utilized in this study proved to be successfully applicable to any type of tooth (i.e., anterior/posterior, primary/permanent).
The portable fingertip PO is an accurate, very sensitive, and noninvasive method in diagnosing the pulpal status of teeth by detecting changes in percentage SaO2 levels in various clinical situations (i.e., teeth with deep carious lesions and/or traumatized teeth).
This modified PO probe proved to be helpful in assessing the status of the pulp of teeth at different developmental stages (i.e., primary, immature/ mature permanent teeth).
It proved to be a direct method of evaluation of pulp vitality by objective means and utmost reliable.
Pulse oximeter (PO) should be made popular by using a uniform design with verified ranges of SaO2 to diagnose pulpal status in various clinical conditions, which in-turn will help in improving the quality of endodontic diagnosis.
Orcid
Nishita Garg https://orcid.org/0000-0001-6594-9098
Ramakrishna Yeluri https://orcid.org/0000-0002-0320-2105
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Noblett WC, Wilcox LR, Scamman F, et al. Detection of pulpal circulation in vitro by pulse oximetry. J Endod. 1996;22(1):1–5. doi: 10.1016/S0099-2399(96)80226-3. [DOI] [PubMed] [Google Scholar]
- 2.Walton RE, Torabinejad M. Principles and Practice of Endodontics. Philadelphia: WB Saunders; 1989. pp. 61–62. [Google Scholar]
- 3.Rowe AH, Pitt Ford TR. The assessment of pulpal vitality. Int Endod J. 1990;23(2):77–83. doi: 10.1111/j.1365-2591.1990.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 4.Andreasen FM, Zhijie Y, Thomsen BL, et al. Occurrence of pulp canal obliteration after luxation injuries in the permanent dentition. Endod Dent Traumatol. 1987;3(3):103–115. doi: 10.1111/j.1600-9657.1987.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy DCM, Kiely MC, Keating PJ. Efficacy of electrical pulp testing. J Irish Dental Assoc. 1987;33(2-4):41–46. [PubMed] [Google Scholar]
- 6.Rapp R, Avery JK, Strachan DS. The distribution of nerves in human primary teeth. Anat Rec. 1967;159(1):89–103. doi: 10.1002/ar.1091590113. [DOI] [PubMed] [Google Scholar]
- 7.Radhakrishnan S, Munshi AK, Hegde AM. Pulse oximetry: a diagnostic instrument in pulpal vitality testing. J Clin Pediatr Dent. 2002;26(2):141–145. doi: 10.17796/jcpd.26.2.2j25008jg6u86236. [DOI] [PubMed] [Google Scholar]
- 8.Gazelius B, Olgart L, Edwall B, et al. Non-invasive recording of blood flow in human dental pulp. Endod Dent Traumatol. 1986;2(5):219–221. doi: 10.1111/j.1600-9657.1986.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 9.Schnettler JM, Wallace JA. Pulse oximetry as a diagnostic tool of pulpal vitality. J Endod. 1991;17(10):488–490. doi: 10.1016/S0099-2399(06)81795-4. [DOI] [PubMed] [Google Scholar]
- 10.Wallace JA, Schnettler JM. Pulse oximetry as a definitive diagnostic tool. J Endod. 1993;19:186. doi: 10.1016/S0099-2399(06)81795-4. [DOI] [PubMed] [Google Scholar]
- 11.Caldeira CL, Barletta FB, Ilha MC, et al. Pulse oximetry: a useful test for evaluating pulp vitality in traumatized teeth. Dent Traumatol. 2016;32(5):385–389. doi: 10.1111/edt.12279. [DOI] [PubMed] [Google Scholar]
- 12.Sharma DS, Mishra S, Banda NR, et al. In vivo evaluation of customized pulse oximeter and sensitivity pulp tests for assessment of pulp vitality. J Clin Pediatr Dent. 2019;43(1):11–15. doi: 10.17796/1053-4625-43.1.3. [DOI] [PubMed] [Google Scholar]
- 13.Hiremath SS. Biostatistics. Hiremath SS Textbook of Preventive and Community Dentistry. India: Elsevier; 2011. pp. 511–520. [Google Scholar]
- 14.Johnsen DC, Harshbarger J, Rymer HD. Quantitative assessment of neural development in human premolars. Anat Rec. 1983;205(4):421–429. doi: 10.1002/ar.1092050407. [DOI] [PubMed] [Google Scholar]
- 15.Pupim D, Iwaki Filho L, Takeshita WM, et al. Evaluation of accuracy of portable fingertip pulse oximeter, as compared to that of a hospital oximeter with digital sensor. Indian J Dent Res. 2013;24(5):542–546. doi: 10.4103/0970-9290.123362. [DOI] [PubMed] [Google Scholar]
- 16.Goho C. Pulse oximetry evaluation of vitality in primary and immature permanent teeth. Pediatr Dent. 1999;21(2):125–127. [PubMed] [Google Scholar]
- 17.Gopi Krishna V, Kandaswamy D, Gupta T. Assessment of the efficacy of an indigeniously developed pulse oximeter dental sensor holder for pulp vitality testing. Indian J Dent Res. 2006;17(3):111–113. doi: 10.4103/0970-9290.29880. [DOI] [PubMed] [Google Scholar]
- 18.Gopikrishna V, Tinagupta K, Kandaswamy D. Comparison of electrical, thermal, and pulse oximetry methods for assessing pulp vitality in recently traumatized teeth. J Endod. 2007;33(5):531–535. doi: 10.1016/j.joen.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Calil E, Caldeira CL, Gavini G, et al. Determination of pulp vitality in vivo with pulse oximetry. Int Endod J. 2008;41(9):741–746. doi: 10.1111/j.1365-2591.2008.01421.x. [DOI] [PubMed] [Google Scholar]
- 20.Fein ME, Gluskin AH, Goon WW, et al. Evaluation of optical methods of detecting dental pulp vitality. J Biomed Opt. 1997;2(1):58–73. doi: 10.1117/12.261679. [DOI] [PubMed] [Google Scholar]
- 21.Pozzobon MH, de Sousa Vieira R, Alves AM, et al. Assessment of pulp blood flow in primary and permanent teeth using pulse oximetry. Dent Traumatol. 2011;27(3):184–188. doi: 10.1111/j.1600-9657.2011.00976.x. [DOI] [PubMed] [Google Scholar]
- 22.Bargrizan M, Ashari MA, Ahmadi M, et al. The use of pulse oximetry in evaluation of pulp vitality in immature permanent teeth. Dent Traumatol. 2016;32(1):43–47. doi: 10.1111/edt.12215. [DOI] [PubMed] [Google Scholar]
- 23.Setzer FC, Kataoka SH, Natrielli F, et al. Clinical diagnosis of pulp inflammation based on pulp oxygenation rates measured by pulse oximetry. J Endod. 2012;38(7):880–883. doi: 10.1016/j.joen.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Wong JK. Pulse oximetry: technology and critical uses. Ont Dent. 2016;2:22–27. [Google Scholar]
- 25.Karayilmaz H, Kirzioğlu Z. Evaluation of pulpal blood flow changes in primary molars with physiological root resorption by laser Doppler flowmetry and pulse oximetry. J Clin Pediatr Dent. 2011;36(2):139–144. doi: 10.17796/jcpd.36.2.3l9lgu5292r08742. [DOI] [PubMed] [Google Scholar]
- 26.Batchelder PB, Raley DM. Maximizing the laboratory setting for testing devices and understanding statistical output in pulse oximetry. Anesth Analg. 2007;105(6 Suppl):S85–S94. doi: 10.1213/01.ane.0000268495.35207.ab. [DOI] [PubMed] [Google Scholar]


