Abstract
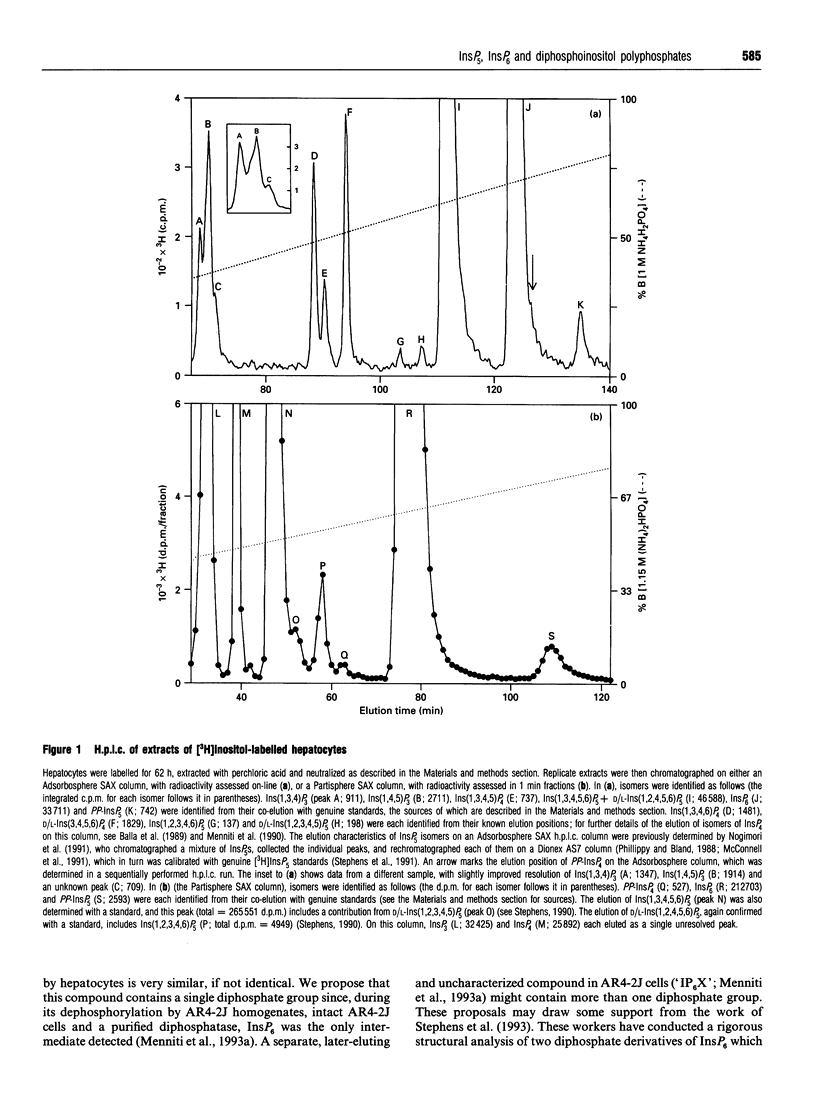
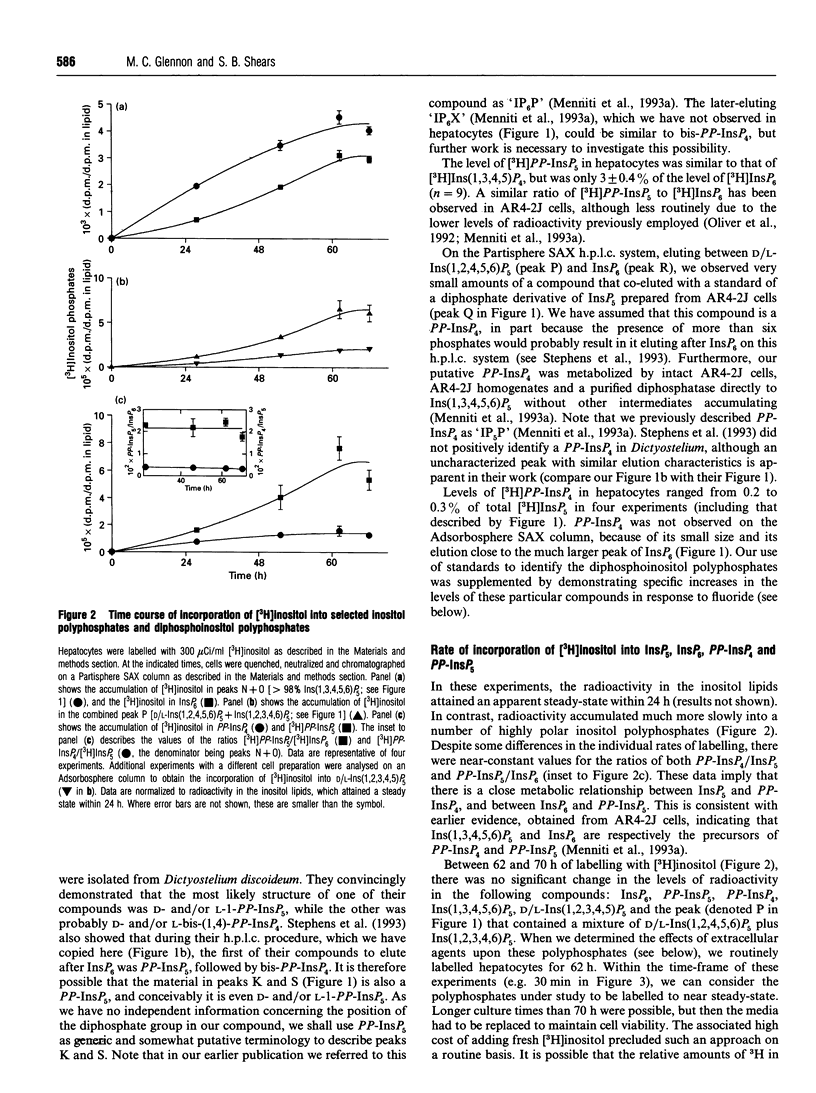
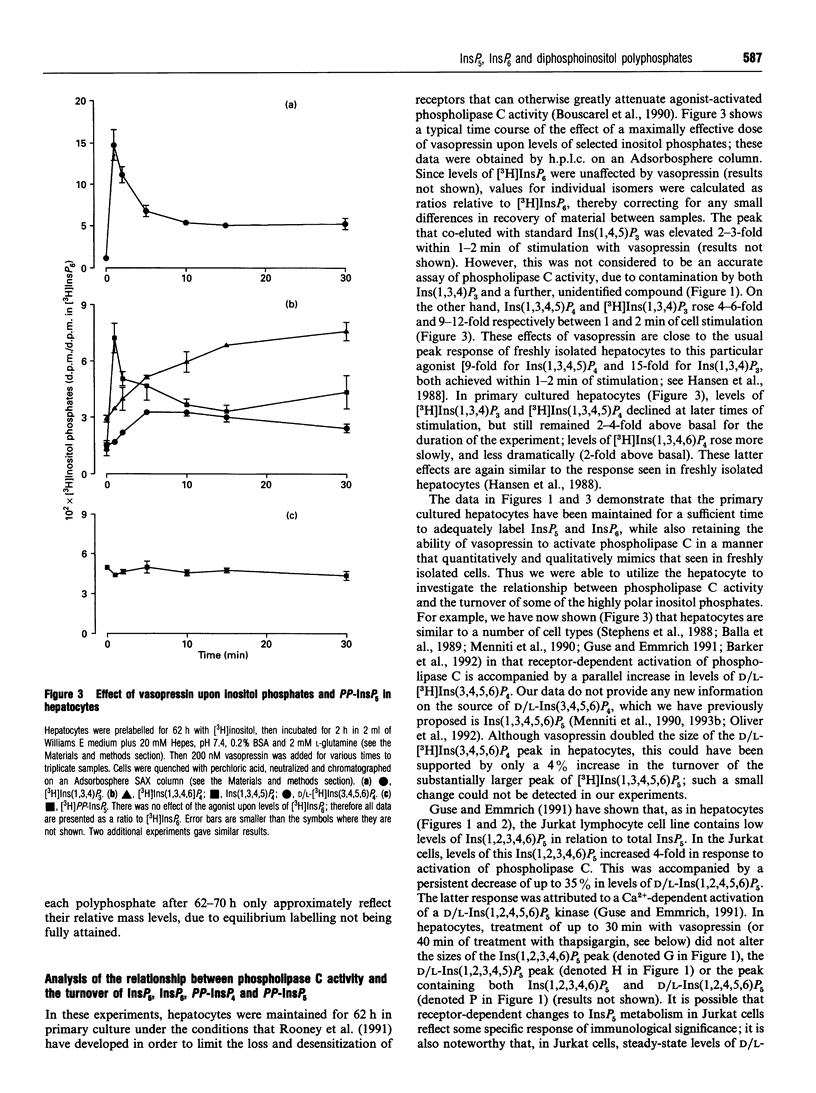
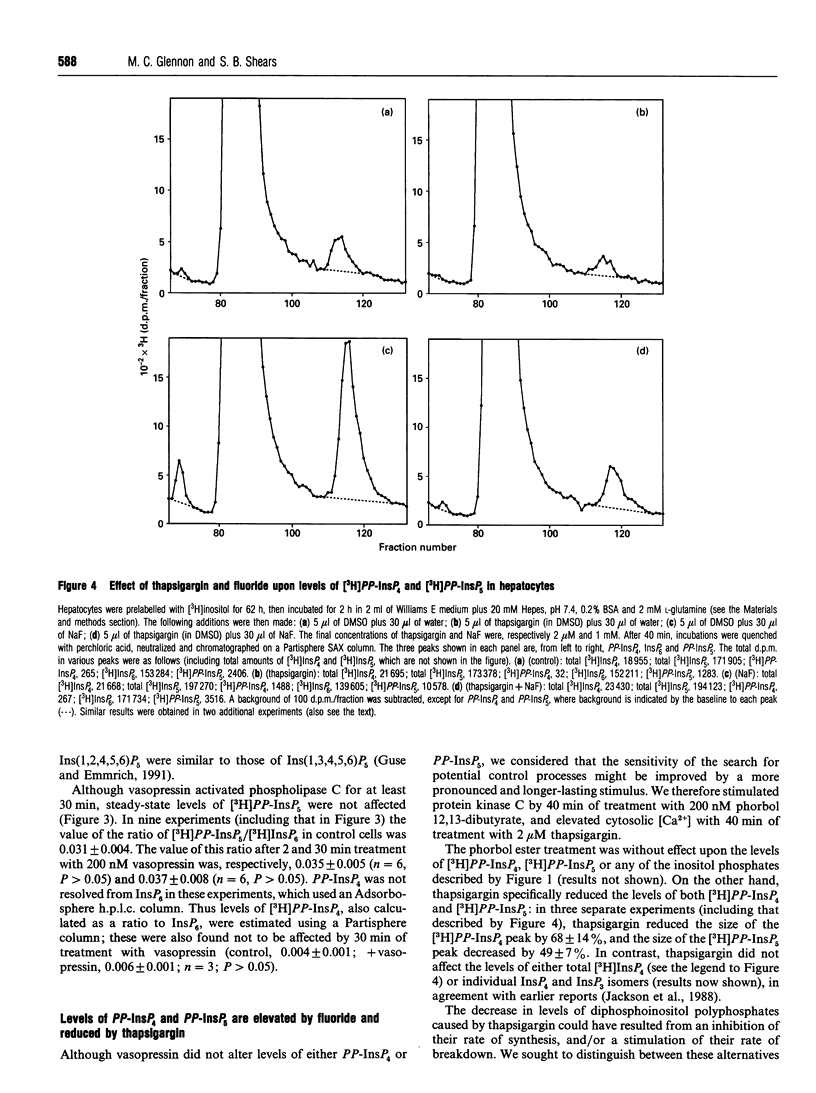
We have used a non-transformed cell model, the primary cultured hepatocyte, to explore the turnover of inositol hexakisphosphate, multiple isomers of inositol pentakisphosphate and two novel diphosphoinositol polyphosphates. All of these compounds gradually accumulated radioactivity throughout a 70 h period of labelling with [3H]inositol. However, a rapid metabolic rate was revealed upon inhibition of diphosphoinositol polyphosphate biphosphatase(s) with 1 mM fluoride for 40 min: this treatment elevated levels of [3H]diphosphoinositol polyphosphates up to 10-fold, indicating that their cellular pools were normally turning over at least 10 times every 40 min. This was accompanied by a turnover of about 10% of the pool of inositol hexakisphosphate. Control experiments established that 200 nM vasopressin brought about a typical activation of phospholipase C in hepatocytes after 62 h of primary culture. This agonist treatment did not affect steady-state levels of [3H]inositol pentakisphosphates, [3H]inositol hexakisphosphate or [3H]diphosphoinositol polyphosphates. However, prolonged treatment of hepatocytes with 2 microM thapsigargin reduced steady-state levels of [3H]diphosphoinositol polyphosphates by 50-70%. This effect of thapsigargin was also observed in the presence of fluoride, indicating that thapsigargin inhibited the rate of synthesis of diphosphoinositol polyphosphates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdullah M., Hughes P. J., Craxton A., Gigg R., Desai T., Marecek J. F., Prestwich G. D., Shears S. B. Purification and characterization of inositol-1,3,4-trisphosphate 5/6-kinase from rat liver using an inositol hexakisphosphate affinity column. J Biol Chem. 1992 Nov 5;267(31):22340–22345. [PubMed] [Google Scholar]
- Balla T., Hunyady L., Baukal A. J., Catt K. J. Structures and metabolism of inositol tetrakisphosphates and inositol pentakisphosphate in bovine adrenal glomerulosa cells. J Biol Chem. 1989 Jun 5;264(16):9386–9390. [PubMed] [Google Scholar]
- Barker C. J., Wong N. S., Maccallum S. M., Hunt P. A., Michell R. H., Kirk C. J. The interrelationships of the inositol phosphates formed in vasopressin-stimulated WRK-1 rat mammary tumour cells. Biochem J. 1992 Sep 1;286(Pt 2):469–474. doi: 10.1042/bj2860469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bird G. S., Rossier M. F., Hughes A. R., Shears S. B., Armstrong D. L., Putney J. W., Jr Activation of Ca2+ entry into acinar cells by a non-phosphorylatable inositol trisphosphate. Nature. 1991 Jul 11;352(6331):162–165. doi: 10.1038/352162a0. [DOI] [PubMed] [Google Scholar]
- Bouscarel B., Augert G., Taylor S. J., Exton J. H. Alterations in vasopressin and angiotensin II receptors and responses during culture of rat liver cells. Biochim Biophys Acta. 1990 Dec 10;1055(3):265–272. doi: 10.1016/0167-4889(90)90042-c. [DOI] [PubMed] [Google Scholar]
- Guse A. H., Emmrich F. T-cell receptor-mediated metabolism of inositol polyphosphates in Jurkat T-lymphocytes. Identification of a D-myo-inositol 1,2,3,4,6-pentakisphosphate-2-phosphomonoesterase activity, a D-myo-inositol 1,3,4,5,6-pentakisphosphate-1/3-phosphatase activity and a D/L-myo-inositol 1,2,4,5,6-pentakisphosphate-1/3-kinase activity. J Biol Chem. 1991 Dec 25;266(36):24498–24502. [PubMed] [Google Scholar]
- Hansen C. A., vom Dahl S., Huddell B., Williamson J. R. Characterization of inositol 1,3,4-trisphosphate phosphorylation in rat liver. FEBS Lett. 1988 Aug 15;236(1):53–56. doi: 10.1016/0014-5793(88)80284-9. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. Is inositol tetrakisphosphate the second messenger that controls Ca2+ entry into cells? Adv Second Messenger Phosphoprotein Res. 1992;26:161–185. [PubMed] [Google Scholar]
- Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly R. R., Stephens L. R., Irvine R. F., Garrison J. C. Effects of transformation with the v-src oncogene on inositol phosphate metabolism in rat-1 fibroblasts. D-myo-inositol 1,4,5,6-tetrakisphosphate is increased in v-src-transformed rat-1 fibroblasts and can be synthesized from D-myo-inositol 1,3,4-trisphosphate in cytosolic extracts. J Biol Chem. 1991 Aug 15;266(23):15144–15153. [PubMed] [Google Scholar]
- Mayr G. W. A novel metal-dye detection system permits picomolar-range h.p.l.c. analysis of inositol polyphosphates from non-radioactively labelled cell or tissue specimens. Biochem J. 1988 Sep 1;254(2):585–591. doi: 10.1042/bj2540585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell F. M., Stephens L. R., Shears S. B. Multiple isomers of inositol pentakisphosphate in Epstein-Barr-virus- transformed (T5-1) B-lymphocytes. Identification of inositol 1,3,4,5,6-pentakisphosphate, D-inositol 1,2,4,5,6-pentakisphosphate and L-inositol 1,2,4,5,6-pentakisphosphate. Biochem J. 1991 Dec 1;280(Pt 2):323–329. doi: 10.1042/bj2800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menniti F. S., Miller R. N., Putney J. W., Jr, Shears S. B. Turnover of inositol polyphosphate pyrophosphates in pancreatoma cells. J Biol Chem. 1993 Feb 25;268(6):3850–3856. [PubMed] [Google Scholar]
- Menniti F. S., Oliver K. G., Nogimori K., Obie J. F., Shears S. B., Putney J. W., Jr Origins of myo-inositol tetrakisphosphates in agonist-stimulated rat pancreatoma cells. Stimulation by bombesin of myo-inositol 1,3,4,5,6-pentakisphosphate breakdown to myo-inositol 3,4,5,6-tetrakisphosphate. J Biol Chem. 1990 Jul 5;265(19):11167–11176. [PubMed] [Google Scholar]
- Menniti F. S., Oliver K. G., Putney J. W., Jr, Shears S. B. Inositol phosphates and cell signaling: new views of InsP5 and InsP6. Trends Biochem Sci. 1993 Feb;18(2):53–56. doi: 10.1016/0968-0004(93)90053-p. [DOI] [PubMed] [Google Scholar]
- Nagelkerke J. F., Dogterom P., De Bont H. J., Mulder G. J. Prolonged high intracellular free calcium concentrations induced by ATP are not immediately cytotoxic in isolated rat hepatocytes. Changes in biochemical parameters implicated in cell toxicity. Biochem J. 1989 Oct 15;263(2):347–353. doi: 10.1042/bj2630347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogimori K., Hughes P. J., Glennon M. C., Hodgson M. E., Putney J. W., Jr, Shears S. B. Purification of an inositol (1,3,4,5)-tetrakisphosphate 3-phosphatase activity from rat liver and the evaluation of its substrate specificity. J Biol Chem. 1991 Sep 5;266(25):16499–16506. [PubMed] [Google Scholar]
- Oliver K. G., Putney J. W., Jr, Obie J. F., Shears S. B. The interconversion of inositol 1,3,4,5,6-pentakisphosphate and inositol tetrakisphosphates in AR4-2J cells. J Biol Chem. 1992 Oct 25;267(30):21528–21534. [PubMed] [Google Scholar]
- Phillippy B. Q., Bland J. M. Gradient ion chromatography of inositol phosphates. Anal Biochem. 1988 Nov 15;175(1):162–166. doi: 10.1016/0003-2697(88)90374-0. [DOI] [PubMed] [Google Scholar]
- Rooney T. A., Renard D. C., Sass E. J., Thomas A. P. Oscillatory cytosolic calcium waves independent of stimulated inositol 1,4,5-trisphosphate formation in hepatocytes. J Biol Chem. 1991 Jul 5;266(19):12272–12282. [PubMed] [Google Scholar]
- Shears S. B. Metabolism of inositol phosphates. Adv Second Messenger Phosphoprotein Res. 1992;26:63–92. [PubMed] [Google Scholar]
- Stanley A. F., Hawkins P. T., Poyner D. R., Hanley M. R. Metabolism of myo-inositol pentakisphosphates in mammalian brain. Biochem Soc Trans. 1992 May;20(2):149S–149S. doi: 10.1042/bst020149s. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Barker C. J., Downes C. P. Synthesis of myo-inositol 1,3,4,5,6-pentakisphosphate from inositol phosphates generated by receptor activation. Biochem J. 1988 Aug 1;253(3):721–733. doi: 10.1042/bj2530721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Stanley A. F., Moore T., Poyner D. R., Morris P. J., Hanley M. R., Kay R. R., Irvine R. F. myo-inositol pentakisphosphates. Structure, biological occurrence and phosphorylation to myo-inositol hexakisphosphate. Biochem J. 1991 Apr 15;275(Pt 2):485–499. doi: 10.1042/bj2750485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L., Radenberg T., Thiel U., Vogel G., Khoo K. H., Dell A., Jackson T. R., Hawkins P. T., Mayr G. W. The detection, purification, structural characterization, and metabolism of diphosphoinositol pentakisphosphate(s) and bisdiphosphoinositol tetrakisphosphate(s). J Biol Chem. 1993 Feb 25;268(6):4009–4015. [PubMed] [Google Scholar]
- Wong N. S., Barker C. J., Morris A. J., Craxton A., Kirk C. J., Michell R. H. The inositol phosphates in WRK1 rat mammary tumour cells. Biochem J. 1992 Sep 1;286(Pt 2):459–468. doi: 10.1042/bj2860459. [DOI] [PMC free article] [PubMed] [Google Scholar]



