TO THE EDITOR:
Acute myeloid leukemia (AML) with myelodysplasia related (MR) gene mutations accounts for 30–35% of all AML cases and is predominantly classified in the poor-risk group. AML-MR had a high incidence in elderly patients, with most of whom were ineligible for intensive chemotherapy. When treated with intensive chemotherapy, AML patients with MR mutations had lower complete remission (CR) rates and inferior survival compared to those without MR mutations [1].
Venetoclax (VEN) plus hypomethylating agents (HMAs) is recommended for newly diagnosed (ND) elderly AML patients or those who were ineligible for intensive chemotherapy [2]. Patients with MR mutations have also been shown to benefit from VEN + HMA regimen. AML patients with spliceosome mutations had favorable overall outcomes with VEN + HMA in another retrospective study [3]. Therefore, genetic aberrations of AML-MR subgroups may help guide treatment decisions for patients who are candidates for both of VEN + HMA and intensive chemotherapy. VEN + HMA and intensive chemotherapy have been compared in AML patients overall [4], but has not been compared in the AML-MR subtype. Here, we retrospectively compared the efficacy of VEN + HMA and intensive chemotherapy in ND AML-MR patients.
We collected data of 188 AML-MR patients aged 18–64 years, who were treated with VEN + HMA or 7 + 3 induction regimen between January 2017 and June 2023 at the First Affiliated Hospital of Soochow University. AML-MR was diagnosed according to the International Consensus classification (ICC) [5]. Age and history of previous hematological malignancy were selected for propensity score matching in a 1:2 ratio using the nearest-neighbor algorithm in the VEN + HMA versus 7 + 3 cohort. A standardized mean difference of less than 0.2 is considered to reduce bias between the two cohorts. Mutations were detected by target next-generation sequencing following our routine protocol [6]. MR genes were categorized into RNA splicing factors (SRSF2, SF3B1, U2AF1, ZRSR2), chromatin-cohesin genes (ASXL1, EZH2, BCOR, STAG2), and transcription factor (RUNX1) according to their functions for further analysis. Patients were classified into the MR-G group, MR-C group, and MR-G/C group if they had only MR gene mutations, only MR cytogenetic abnormalities, or both gene mutations and cytogenetic abnormalities, respectively. Measurable residual disease (MRD) was detected by multi-parameter flow cytometry, with a positive threshold of 0.1%. Treatment responses were assessed according to the ELN 2022 [7]. Composite complete remission (CRc) was calculated as CR plus CR with incomplete recovery of blood cell counts (CRi). The overall response rate (ORR) was calculated as CRc plus morphology-free leukemia status (MLFS). Overall survival (OS) was tabulated from the time of therapy initiation to death or last follow-up and event-free survival (EFS) from the therapy initiation to induction failure, relapse, death, or last follow-up. The study was approved by the Ethical Committee of the First Affiliated Hospital of Soochow University (No. 2024138). All patients provided written informed consent.
Propensity score matching was conducted in all the 188 AML-MR patients. In total, 61 of 70 patients in the VEN + HMA cohort and 103 of 118 patients in the 7 + 3 cohort were paired (Fig. S1). The baseline characteristics of the two cohorts were well-matched (Table 1). Cytogenetic features of the matched patients are shown in Figure S2 and Table S1. Patients in the VEN + HMA cohort received venetoclax combined with either azacitidine (75 mg/m2, subcutaneously, days 1–7) or decitabine (20 mg/m2, intravenously, days 1–5). The dose of venetoclax was started at 100 mg on day 1 and gradually increased over 3 days to reach a target dose of 400 mg on days 3–28. Dose adjustments were made for VEN with concomitant CYP3A4 inhibitors as reported [8]. Patients in the 7 + 3 cohort received intravenous standard-dose of cytarabine with anthracyclines including idarubicin, doxorubicin, mitoxantrone, or homoharringtonine on days 1–3. FLT3 inhibitors may be added in both cohorts for patients with FLT3-ITD (Table S2). Patients who failed the initial induction regimen received re-induction treatment at their physicians’ discretion.
Table 1.
Patients’ characteristic of the matched cohorts.
| Variable | Total (n = 164) | HMA + VEN (n = 61) | 7 + 3 (n = 103) | P-value |
|---|---|---|---|---|
| Median age, years (range) | 46 (18–64) | 47(18–64) | 46 (18–64) | 0.107 |
| Male sex, n (%) | 99 (60) | 40 (66) | 59 (57) | 0.294 |
| Hematological parameters | ||||
| Median WBC, ×109/L (range) | 5.1 (0.5–258.6) | 5.11 (0.5–258.6) | 6.07 (0.77–135.7) | 0.184 |
| Median Hb, g/L (range) | 73 (36–146) | 73 (38–126) | 78 (36–146) | 0.086 |
| Median Plt, ×109/L (range) | 43 (5–303) | 43 (6–218) | 43 (5–303) | 0.780 |
| Median BM blasts, % (range) | 54.5 (20–98.5) | 54.5 (20–98.5) | 55.5 (20–98.5) | 0.932 |
| Disease type, n (%) | ||||
| De novo AML | 157 (96) | 58 (95) | 99 (96) | 1.000 |
| s-AML / t-AML | 7 (4) | 3 (5) | 4 (4) | |
| Cytogenetic abnormalities, n (%) | ||||
| MR-C | 34 (21) | 8 (13) | 26 (25) | 0.064 |
| MR-G | 94 (57) | 39 (64) | 58 (56) | 0.337 |
| MR-G/C | 36 (22) | 17 (28) | 19 (18) | 0.159 |
| Allo-HSCT, n (%) | 95 (58) | 34 (56) | 61 (59) | 0.662 |
| Co-mutations, n (%) | ||||
| Splicing factor | 51 (31) | 24 (39) | 27 (44) | 0.079 |
| U2AF1 | 30 (18) | 17 (28) | 13 (13) | 0.015 |
| SF3B1 | 10 (6) | 2 (3) | 8 (8) | 0.410 |
| SRSF2 | 11 (7) | 4 (7) | 7 (7) | 1.000 |
| ZRSR2 | 3 (2) | 3 (5) | 0 | 0.051 |
| Chromatin-cohesin | 82 (50) | 39 (64) | 43 (42) | 0.006 |
| ASXL1 | 32 (20) | 14 (23) | 18 (17) | 0.392 |
| BCOR | 36 (22) | 19 (31) | 17 (17) | 0.029 |
| EZH2 | 10 (6) | 5 (8) | 5 (5) | 0.598 |
| STAG2 | 17 (10) | 7 (11) | 10 (10) | 0.720 |
| Transcription factor | 59 (36) | 28 (46) | 31 (30) | 0.042 |
| RUNX1 | 59 (36) | 28 (46) | 31 (30) | 0.042 |
WBC White blood cell, Hb Hemoglobin, Plt Platelet, BM bone marrow, s-AML secondary AML, t-AML therapy-related AML, MR-C myelodysplasia-related cytogenetic abnormalities, MR-G myelodysplasia-related gene mutation, MR-G/C both myelodysplasia-related gene mutations and cytogenetic abnormalities, Allo-HSCT allogenetic hematopoietic stem cell transplantation.
After the first course of induction therapy, the ORR and CRc rate of the VEN + HMA cohort were significantly higher than that of the 7 + 3 cohort (75% vs 46%, P < 0.001 for ORR, and 61% vs 32%, P < 0.001 for CRc). Correspondingly, the MRD-negative CR rate was also higher in VEN + HMA cohort than in the 7 + 3 cohort (46% vs 19%, P < 0.001) (Table S3). Analyses based on genetic alterations showed that the ORR was higher for the VEN + HMA regimen than for the 7 + 3 regimen in the MR-G (72% vs 52%, P = 0.049), MR-G/C (88% vs 26%, P = 0.031) groups (Fig. S3A) and patients with one mutation of myelodysplasia genes (86% vs 43%, P = 0.001), with mutations of transcription factor genes (82% vs 45%, P = 0.003) and chromatin-cohesin genes (77% vs 47%, P = 0.005) (Fig. S3B, C).
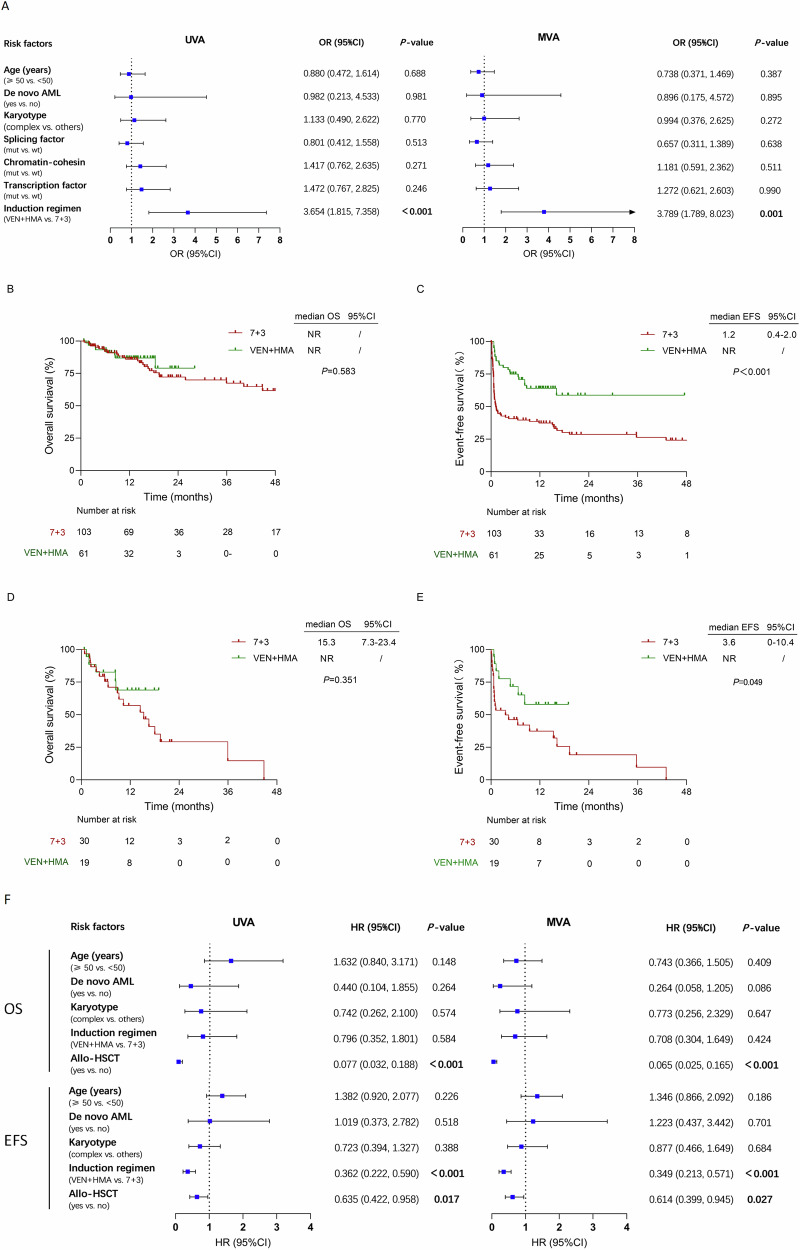
Fifteen patients (15/61, 25%) didn’t achieve ORR after the first cycle of VEN + HMA. Of whom, 11 received a second cycle of VEN + HMA and 5 (45%) achieved CRc. Fifty-six (56/103, 54%) patients didn’t achieve ORR with 7 + 3 regimen. Of whom, 9 switched to VEN + HMA, with 7 (78%) achieved ORR (6 CRc and 1 MLFS). In contrast, 23 patients received a second cycle of 7 + 3, and only 12 (52%) achieved ORR (10 CRc and 2 MLFS). Both the CRc rate (69% vs 52%, P = 0.039) and MRD-negative CR rate (56% vs 36%, P = 0.013) for one or two cycles of VEN + HMA were much higher than that for the 7 + 3 regimen (Table S3). Univariate analysis (UVA) and multivariate analysis (MVA) both revealed that VEN + HMA regimen was the only predictor of a high ORR (OR = 3.654, P < 0.001 for UVA, and OR = 3.789, P = 0.001 for MVA) (Fig. 1A).
Fig. 1. Responses and survival analysis of matched cohorts.
A Univariable and multivariable logistic regression overall response analysis. B, C OS and EFS analysis of the overall 7 + 3 and VEN + HMA cohorts. D, E OS and EFS analysis in patients without allo-HSCT per 7 + 3 or VEN + HMA treatment. F Univariable and multivariable Cox regression survival analysis. (CI confidence interval, OR odds ratio, EFS event-free survival, OS overall survival, VEN + HMA, venetoclax plus hypomethylating agents, NR not reached, UVA univariable analysis, MVA multivariable analysis, Allo-HSCT allogenetic hematopoietic stem cell transplantation, HR hazard ratio).
With a median follow-up of 16.3 (range, 13.6–19.0) months, the median OS were both not reached (NR) (P = 0.583) (Fig. 1B). The median EFS in the VEN + HMA cohort was longer than that in the 7 + 3 cohort (NR vs 1.2 months, P < 0.001) (Fig. 1C). There were no significant differences in OS and EFS among the three MR abnormality groups (Fig. S3D, E). Thirty-four (56%) patients in the VEN + HMA cohort and 61 (59%) patients in the 7 + 3 cohort underwent allogenetic hematopoietic stem cell transplantation (allo-HSCT). In the overall patients, those received allo-HSCT had longer OS (NR vs. 18.0 months, P < 0.001) and EFS (15.5 months vs 4.3 months, P = 0.028) (Fig. S3F, G) than those who were not transplanted. For patients who did not undergo allo-HSCT, 19 (31%) patients in the VEN + HMA cohort received consolidation therapy with VEN + HMA, and 30 (29%) patients in the 7 + 3 cohort received intensive chemotherapy as consolidation. Patients treated with VEN + HMA had a superior EFS (NR vs 3.6 months, P = 0.049) but a similar OS (NR vs 15.3 months, P = 0.351) (Fig. 1D, E) compared with those who were treated with chemotherapy alone. VEN + HMA was shown to be protective factors for prolonged EFS as analyzed by both of UVA (HR = 0.362, P < 0.001) and MVA (HR = 0.349, P < 0.001) (Fig. 1F). In addition, allo-HSCT was demonstrated to be favorable factors for OS and EFS in the overall patients by UVA and MVA (Fig. 1F).
In this study, we screened patients with AML-MR features according to ICC criteria [5]. Since ICC does not have the AML-MR entity, we purposely grouped the AML myelodysplasia-related gene mutations and cytogenetics together as AML-MR. AML with TP53 mutations is grouped as a separate entity apart from AML-MR in ICC criteria due to its distinct clinicopathological and prognostic features [9]. Accordingly, we didn’t include any patients with TP53 mutation in this study.
Genetic characteristics are considered important predictors of treatment response in adults with AML. According to the literature, relapsed or refractory AML patients with mutations in chromatin cohesion genes including ASXL1, BCOR, EZH2, STAG2 demonstrated a CRc rate of 63.6% to VEN plus HMA [10]. A high response rate to VEN + HMA was also found in ND AML patients. In our study, 62% of patients with mutations in chromatin cohesion genes including ASXL1, BCOR, EZH2, STAG2 attained CRc to VEN + HMA, whereas only 30% of patients harboring these mutations achieved CRc when treated with 7 + 3 (Table S3). Patients with ASXL1 mutations achieved a CRc rate of 57% in the VEN + HMA cohort of this study (Table S4). ASXL1 mutant cells showed epigenetic upregulation of BCL2 expression and increased dependence on BCL2 in preclinical studies [11], demonstrating the selective sensitivity of ASXL1 mutant cells to VEN + HMA in patients with myelodysplastic syndrome and excess blasts in a real-world study [12]. Taken together, these data support that the VEN + HMA regimen is appropriate for first-line treatment of patients with chromatin cohesion gene mutations.
Lachowiez et al reported that AML patients with mutations in RNA splicing factors responded to HMA-VEN based first-line therapy at a CRc rate of 79% [3], significantly higher than the 33% observed in our study (Table S3). Partly because nearly half of the patients (46%) were in the low-intermediate risk group. While patients in our study were all high-risk. They also observed that AML patients with U2AF1 mutations showed a lower CRc rate of 57% compared to other splicing factors. In our study, U2AF1 mutations were detected in 30/51 (59%) patients with mutated splicing factors (Table 1), whereas in Lachowiez’s study, U2AF1 mutations were detected in only 17.9% of the patients, which may be an important reason for the difference in therapeutic response between the two studies. In this study, patients with RUNX1 mutation achieved a CRc rate of 68%, significantly higher than the 44% reported in Gangat’s study [13]. The reasons for this difference may be due to the small number of sAML/tAML patients in our VEN + HMA cohort, which was only 5% (3/61).
Liposomal daunorubicin-cytarabine (CPX-351) is the only agent specifically approved for ND AML-MRC [14]. In a retrospective study, AML patients treated with CPX-351 demonstrated similar median OS and early mortality compared to those treated with VEN + HMA, but showed higher rates of febrile neutropenia, infection, and prolonged hospital stay [15]. These data suggest that VEN + HMA may be preferred in AML-MR patients with regard to safety, especially in regions or countries where CPX-351 is unavailable.
In this study, 56% patients in the VEN + HMA cohort and 59% patients in the 7 + 3 cohort eventually received allo-HSCT and had longer survival time than those who did not. These results support that bridging to allo-HSCT after remission with VEN + HMA induction is the treatment paradigm in young AML-MR patients. sAML and tAML were more common in older patients. Due to the retrospective nature of matching studies, patients were relatively young, and a low percentage of sAML and tAML patients were included in this study. The superiority of VEN + HMA compared to the 7 + 3 regimen in older AML-MR patients eligible for intensive chemotherapy warrants validation in prospective studies.
In conclusion, the VEN + HMA regimen showed higher overall and MRD negative response rates and better EFS compared to the 7 + 3 regimen in ND young AML-MR patients in this study. Our data may help guide treatment decisions for ND AML-MR patients who are eligible candidates for both of VEN + HMA and the 7 + 3 regimen.
Supplementary information
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2022YFC2502703), National Natural Science Foundation of China (81970138, 82270165), Jiangsu Province Natural Science Foundation of China (BK20221235), Translational Research Grant of NCRCH (2020ZKMB05), Jiangsu Province “333” Project, Social Development Project of the Science and Technology Department of Jiangsu (BE2021649), Boxi Clinical Research Project (BXLC008) and Gusu Key Medical Talent Program (GSWS2019007).
Author contributions
CLW, YQL and FTL analyzed the data and wrote the manuscript. YHH and HYC performed statistical analyses and data visualization. SMH, KWT, SSG, MW, MJL and ZHW collected and analyzed patient-related data. LZ read the manuscript and gave comments. SLX and HPD designed the study and conducted review and editing. All authors revised and approved the published paper.
Data availability
Data reported in this study are available from the corresponding authors on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Ethical Committee of the First Affiliated Hospital of Soochow University (No. 2024138). All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all participants according to the Declaration of Helsinki.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chao-Ling Wan, Yu-Qing Liu, Fang-Tong Liu.
These authors jointly supervised this work: Zheng Li, Sheng-Li Xue, Hai-Ping Dai.
Contributor Information
Zheng Li, Email: lzheng@suda.edu.cn.
Sheng-Li Xue, Email: slxue@suda.edu.cn.
Hai-Ping Dai, Email: daihaiping8@126.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-024-01130-7.
References
- 1.Tsai XC, Sun KJ, Lo MY, Tien FM, Kuo YY, Tseng MH, et al. Poor prognostic implications of myelodysplasia-related mutations in both older and younger patients with de novo AML. Blood Cancer J. 2023;13:4. 10.1038/s41408-022-00774-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimony S, Stahl M, Stone RM. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98:502–26. 10.1002/ajh.26822 [DOI] [PubMed] [Google Scholar]
- 3.Lachowiez CA, Loghavi S, Furudate K, Montalban-Bravo G, Maiti A, Kadia T, et al. Impact of splicing mutations in acute myeloid leukemia treated with hypomethylating agents combined with venetoclax. Blood Adv. 2021;5:2173–83. 10.1182/bloodadvances.2020004173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu J, Xue S, Wang Y, Dai HP, He X, Hu X, et al. Comparing the efficacy and safety of venetoclax combined with decitabine versus conventional chemotherapy as induction therapy for young adults with newly diagnosed acute myeloid leukemia - interim analysis of a multicenter, randomized, phase 2b trial. Blood. 2023;142:970. 10.1182/blood-2023-181347 [DOI] [Google Scholar]
- 5.Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28. 10.1182/blood.2022015850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q, Cai WZ, Wang R, Zhu MQ, Yan LZ, Yu Y, et al. Integrative genomic and transcriptomic profiling reveals distinct molecular subsets in adult mixed phenotype acute leukemia. Am J Hematol. 2023;98:66–78. 10.1002/ajh.26758 [DOI] [PubMed] [Google Scholar]
- 7.Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombretet H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77. 10.1182/blood.2022016867 [DOI] [PubMed] [Google Scholar]
- 8.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl J Med. 2020;383:617–29. 10.1056/NEJMoa2012971 [DOI] [PubMed] [Google Scholar]
- 9.Zhou Q, Zhao D, Zarif M, Davidson MB, Minden MD, Tierens A, et al. A real-world analysis of clinical outcomes in AML with myelodysplasia-related changes: a comparison of ICC and WHO-HAEM5 criteria. Blood Adv. 2024;8:1760–71. 10.1182/bloodadvances.2023011869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng G, Zhang Y, Yu G, Luo T, Yu S, Xu N, et al. Genetic characteristics predict response to venetoclax plus hypomethylating agents in relapsed or refractory acute myeloid leukemia. J Intern Med. 2023;293:329–39. 10.1111/joim.13581 [DOI] [PubMed] [Google Scholar]
- 11.Rahmani NE, Ramachandra N, Sahu S, Gitego N, Lopez A, Pradhan K, et al. ASXL1 mutations are associated with distinct epigenomic alterations that lead to sensitivity to venetoclax and azacytidine. Blood Cancer J. 2021;11:157. 10.1038/s41408-021-00541-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gangat N, McCullough K, Johnson I, Al-Kali A, Begna KH, Patnaik MM, et al. Real-world experience with venetoclax and hypomethylating agents in myelodysplastic syndromes with excess blasts. 2022;97:E214–E216. [DOI] [PubMed]
- 13.Gangat N, Karrar O, Iftikhar M, McCullough K, Johnson IM, Abdelmagid M, et al. Venetoclax and hypomethylating agent combination therapy in newly diagnosed acute myeloid leukemia: Genotype signatures for response and survival among 301 consecutive patients. Am J Hematol. 2024;99:193–202. 10.1002/ajh.27138 [DOI] [PubMed] [Google Scholar]
- 14.Lancet JE, Uy GL, Newell LF, Lin TL, Ritchie EK, Stuart RK, et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021;8:e481–e491. 10.1016/S2352-3026(21)00134-4 [DOI] [PubMed] [Google Scholar]
- 15.Matthews AH, Perl AE, Luger SM, Loren AW, Gill SI, Porter DL, et al. Real-world effectiveness of CPX-351 vs venetoclax and azacitidine in acute myeloid leukemia. Blood Adv. 2022;6:3997–4005. 10.1182/bloodadvances.2022007265 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported in this study are available from the corresponding authors on reasonable request.