Abstract
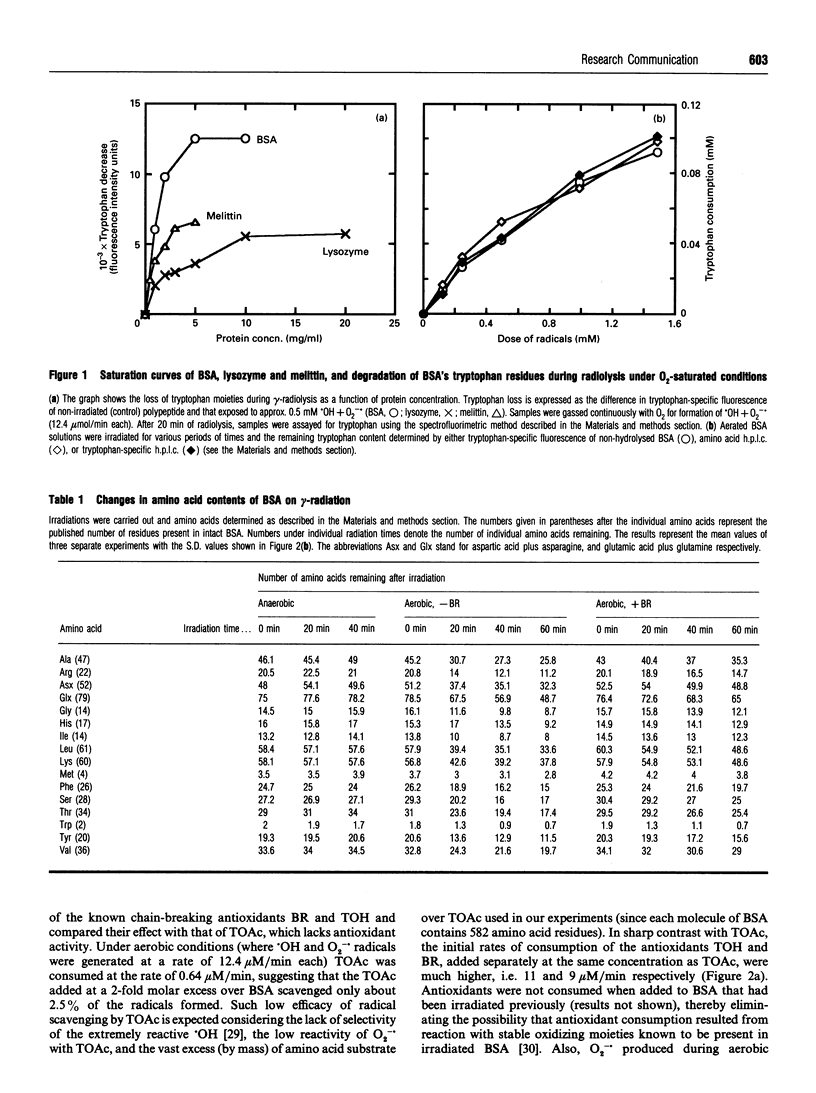
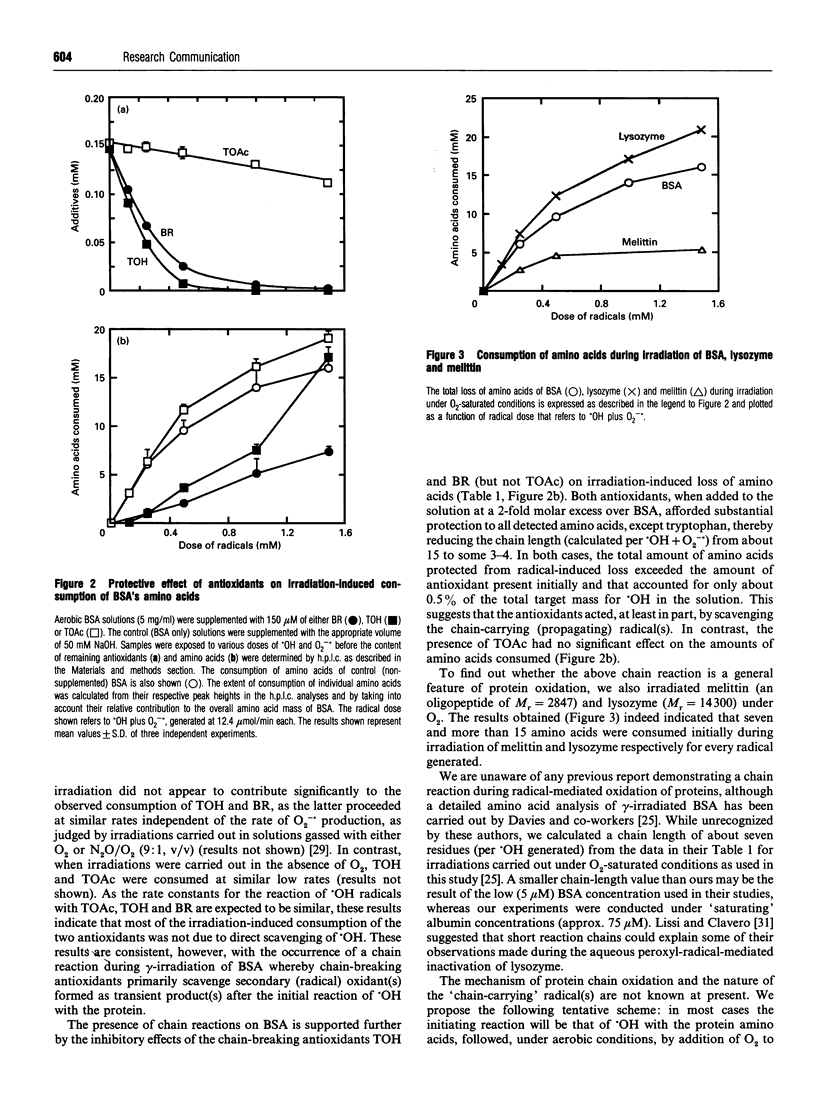
Exposure of proteins to oxygen-centred radicals results in dramatic changes in their structure, stability and function; properties that have been studied in many laboratories from a qualitative viewpoint. To allow a quantitative evaluation, we subjected aerated solutions of BSA to hydroxyl and superoxide anion radicals generated radiolytically under conditions where all radicals formed reacted with the protein (as judged by maximum damage to BSA). We observed that for each radical generated approx. 15 amino acids were consumed initially. Similar results were found with lysozyme and melittin. Such a massive consumption of BSA's amino acids was not observed when irradiations were carried out under anaerobic conditions. When bilirubin or Trolox (a water-soluble analogue of vitamin E) was added at a 2-fold molar excess over BSA, initial consumption of all measured amino acids, except tryptophan, decreased 4-fold. The total mass of amino acids initially protected (from consumption) exceeded the mass of antioxidants consumed by more than a factor of 20. Such protection of amino acids was not observed when the antioxidant-inactive acetyl Trolox was used. These results suggest that radical-mediated oxidation of proteins can proceed via a previously unrecognized chain reaction that may be inhibited by chain-breaking antioxidants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich J. E., Cundall R. B. The radiation-induced inactivation of lysozyme. Int J Radiat Biol Relat Stud Phys Chem Med. 1969;16(4):343–358. doi: 10.1080/09553006914551371. [DOI] [PubMed] [Google Scholar]
- Bowry V. W., Ingold K. U., Stocker R. Vitamin E in human low-density lipoprotein. When and how this antioxidant becomes a pro-oxidant. Biochem J. 1992 Dec 1;288(Pt 2):341–344. doi: 10.1042/bj2880341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner G. R. In the absence of catalytic metals ascorbate does not autoxidize at pH 7: ascorbate as a test for catalytic metals. J Biochem Biophys Methods. 1988 May;16(1):27–40. doi: 10.1016/0165-022x(88)90100-5. [DOI] [PubMed] [Google Scholar]
- Davies K. J., Delsignore M. E., Lin S. W. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J Biol Chem. 1987 Jul 15;262(20):9902–9907. [PubMed] [Google Scholar]
- Dean R. T., Cheeseman K. H. Vitamin E protects proteins against free radical damage in lipid environments. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1277–1282. doi: 10.1016/s0006-291x(87)80271-1. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Hunt J. V., Grant A. J., Yamamoto Y., Niki E. Free radical damage to proteins: the influence of the relative localization of radical generation, antioxidants, and target proteins. Free Radic Biol Med. 1991;11(2):161–168. doi: 10.1016/0891-5849(91)90167-2. [DOI] [PubMed] [Google Scholar]
- Frei B., Stocker R., Ames B. N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner W. S., Miller W. H., 3rd Reverse-phase liquid chromatographic analysis of amino acids after reaction with o-phthalaldehyde. Anal Biochem. 1980 Jan 1;101(1):61–65. doi: 10.1016/0003-2697(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Gebicki S., Gebicki J. M. Formation of peroxides in amino acids and proteins exposed to oxygen free radicals. Biochem J. 1993 Feb 1;289(Pt 3):743–749. doi: 10.1042/bj2890743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. $51 million tax-exempt bond issue funds Health Central's strategic plans. Mod Healthc. 1985 Nov 8;15(23):172–174. [PubMed] [Google Scholar]
- Ito N., Phillips S. E., Stevens C., Ogel Z. B., McPherson M. J., Keen J. N., Yadav K. D., Knowles P. F. Novel thioether bond revealed by a 1.7 A crystal structure of galactose oxidase. Nature. 1991 Mar 7;350(6313):87–90. doi: 10.1038/350087a0. [DOI] [PubMed] [Google Scholar]
- Lissi E. A., Clavero N. Inactivation of lysozyme by alkylperoxyl radicals. Free Radic Res Commun. 1990;10(3):177–184. doi: 10.3109/10715769009149886. [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Catalano C. E. Epoxidation of styrene by hemoglobin and myoglobin. Transfer of oxidizing equivalents to the protein surface. J Biol Chem. 1985 Aug 5;260(16):9265–9271. [PubMed] [Google Scholar]
- Ozaki Y., Reinhard J. F., Jr, Nichol C. A. Cofactor activity of dihydroflavin mononucleotide and tetrahydrobiopterin for murine epididymal indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1106–1111. doi: 10.1016/0006-291x(86)90339-6. [DOI] [PubMed] [Google Scholar]
- Schuessler H., Schilling K. Oxygen effect in the radiolysis of proteins. Part 2. Bovine serum albumin. Int J Radiat Biol Relat Stud Phys Chem Med. 1984 Mar;45(3):267–281. doi: 10.1080/09553008414550381. [DOI] [PubMed] [Google Scholar]
- Simpson J. A., Narita S., Gieseg S., Gebicki S., Gebicki J. M., Dean R. T. Long-lived reactive species on free-radical-damaged proteins. Biochem J. 1992 Mar 15;282(Pt 3):621–624. doi: 10.1042/bj2820621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. L., Eling T. E., Kulmacz R. J., Marnett L. J., Tsai A. Tyrosyl radicals and their role in hydroperoxide-dependent activation and inactivation of prostaglandin endoperoxide synthase. Biochemistry. 1992 Jan 14;31(1):3–7. doi: 10.1021/bi00116a001. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Science. 1992 Aug 28;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Stocker R., Glazer A. N., Ames B. N. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., McDonagh A. F., Glazer A. N., Ames B. N. Antioxidant activities of bile pigments: biliverdin and bilirubin. Methods Enzymol. 1990;186:301–309. doi: 10.1016/0076-6879(90)86123-d. [DOI] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Stubbe J. A. Protein radical involvement in biological catalysis? Annu Rev Biochem. 1989;58:257–285. doi: 10.1146/annurev.bi.58.070189.001353. [DOI] [PubMed] [Google Scholar]
- Wilks A., Ortiz de Montellano P. R. Intramolecular translocation of the protein radical formed in the reaction of recombinant sperm whale myoglobin with H2O2. J Biol Chem. 1992 May 5;267(13):8827–8833. [PubMed] [Google Scholar]


