Abstract
Background and Aim:
Although reverse zoonotic transmission events from humans to domestic cats have been described, there is currently little evidence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) circulation in stray cats. Due to the evidence of natural and experimental infections in cats and the capacity to disseminate the virus among them, this study aimed to identify the SARS-CoV-2 antigen in stray cats from the Federal University of Sergipe in Brazil.
Materials and Methods:
One hundred twenty six stray cats from the university were screened for SARS-CoV-2 antigens by random sampling. Throat swab samples were tested for the virus using rapid antigen detection tests.
Results:
Of the 126 animals tested, 30 (23.60%) were positive for SARS-CoV-2 antigens. To our knowledge, for the first time, this study detected the SARS-CoV-2 antigen in stray cats and confirmed the presence of SARS-CoV-2 infections in Brazil’s stray cat population.
Conclusion:
The detection of SARS-CoV-2 in stray cats poses a risk for infected and healthy animals and possibly for humans who attend the university daily. As a limitation of the study, the small sample size necessitates caution when interpreting the results. This underscores the need for further research in this area to help control diseases in stray animals during potential pandemics. This highlights the need for monitoring and controlling the spread of the virus in stray animal populations.
Keywords: animals, antigen, cats, severe acute respiratory syndrome coronavirus-2
Introduction
The Coronaviridae family, order Nidovirales, includes the single-stranded RNA-enveloped viruses known as Coronavirinae or coronaviruses (CoVs), divided into four distinct genera [1]. Alpha- and beta-CoVs from their respective genera bring about significant respiratory, gastrointestinal, and systemic illnesses in various mammals, including humans [2]. These two genera, Gammacoronavirus and Deltacoronavirus, primarily cause respiratory and enteric infections in avian and porcine species [3]. Since the beginning of the 21st century, humanity has already experienced the emergence of three new CoVs, belonging to the Betacoronavirus genus: Severe acute respiratory syndrome CoV (SARS-CoV) in 2003, Middle East respiratory syndrome CoV in 2012, and SARS-CoV-2 in late 2019 [4]. These epidemics arose from infectious agents crossing the species barrier from wild reservoirs such as horseshoe bats (Rhinolophus affinis), camels (order Camelus), pangolins (order Pholidota), and masked palm civets (Paguma larvata) [5–10]. The origin of SARS-CoV-2 as a zoonotic disease has yet to be definitively determined.
Domestic animals that live in close contact with humans and are infected with SARS-CoV-2, primarily cats and dogs, pose a threat to human health through potential transmission [11]. Infections in cats have been reported worldwide, especially in homes or environments where animals are in close contact with positive and quarantined humans [12]. The first case of natural transmission from humans to a cat occurred in Belgium, 1 week after its owner tested positive for SARS-CoV-2 [13]. The role of stray, feral, and shelter cats has attracted little research [14]. The infection rate of SARS-CoV-2 in stray cats is lower than in owned cats. A recent study by Sirakov et al. [15] demonstrated that using a Human Leukocyte Antigen – DR isotype (HLA-DR), by method enzyme-linked immunosorbent assay (ELISA) for detecting anti-SARS-CoV-2 antibodies in cats revealed a higher seroprevalence in stray cats compared to domestic cats in Bulgaria, with 83.33% positive results in stray cats versus 41.18% in domestic cats. Stray animals can acquire infectious diseases through contact with infected animals or humans and contaminated environments such as sewage and varied surfaces [14, 16–18]. A study reported natural and experimental infections in cats [19]. Both types of exposure led to subclinical and symptomatic disease in these animals [20]. Cats exhibit respiratory ailments in symptomatic diseases with coughing and sneezing, eye and nasal discharge, lethargy, anorexia, vomiting, and diarrhea [13, 21]. 70–100-day-old cats are most vulnerable to severe disease and fatalities [21]. The length of cats’ infection and viral shedding ranged from a few days to several weeks in various investigations [14].
There are three relations commonly researched with regard to SARS-CoV-2: Human-to-cat transmission, cat-to-cat transmission, and the relation of cats to other animal species. Cats, in addition to being susceptible to infections, are capable of spreading the disease among them [22]. A study found a high seroprevalence of anti-SARS-CoV-2 antibodies in stray cats, indicating significant intra-species transmission [15]. Both clinically affected and asymptomatic, Cats have been documented to disperse viral particles from their oral and nasal secretions, thereby facilitating direct or indirect transmission to other cats and even non-feline species [19, 23, 24]. While naïve cats are susceptible to contracting the disease from infected ones, SARS-CoV-2 infections induce an immune response in cats, which provides protection against re-infection [24, 25]. Domestic and farm animals primarily contract illnesses from humans [26]. While it has been highly reported that humans are responsible for infections in domestic and stray cats, the role of cats in the transmission of the disease to humans has not been confirmed [27]. Although domestic cats are more susceptible, infections have been reported in abandoned and stray animals, which can act as SARS-CoV-2 carriers and spreaders [28]. Oude Munnink et al. [29] showed that the virus can be transmitted between humans and animals and back to humans, as evidenced at a mink farm in the Netherlands.
In Brazil, the first case of SARS-CoV-2 in a domestic cat was reported in late 2020 [30]. Serological data suggest that not only pets owned by households with COVID-19 cases but also stray animals are being exposed to SARS-CoV-2 during the COVID-19 pandemic [31]. Studies on the occurrence of infections in stray animals are scarce but extremely important in the scope of One Health, as cats are one of the most popular pets and often live in close contact with humans [32]. Methods for detecting SARS-CoV-2 in cats include molecular techniques, such as conventional nested polymerase chain reaction (PCR) based on the SARS-CoV-2 N gene, and serological and immunoenzymatic methods, such as HLA-DR ELISAand multi-species ELISA kits [15, 33].
Therefore, it is clear that cats are potential hosts for SARS-CoV-2 infections while also being capable of disseminating the disease to healthy individuals. Identifying infections in stray animals is essential for collecting epidemiological data and implementing control programs. The aim of this study was to introduce an adaptive method for the rapid detection of SARS-CoV-2 antigens in stray cats at a Brazilian university.
Materials and Methods
Ethical approval
This study was approved by the Ethics Committee on the use of animals at the Federal University of Sergipe (AEC 4666041220).
Study period and location
This cross-sectional study was conducted during October and November 2021 at the Federal University of Sergipe, located in the northeastern state of Sergipe, Brazil. The university has a total area of 192,000 m2 and approximately 300,000 members, including students, professors, and technicians (https://en.ufs.br/pagina/8167).
The cats freely roam through the university but generally live in specific groups and colonies. The university’s social projects are responsible for building structures (colonies) where the animals feed and spend the night. These are distributed throughout the university, and even with their presence, it is common to see cats in places frequented by students and university staff.
Sampling and testing
The animals were selected with no distinction of sex, age, or physical/health condition and were captured from colonies and nearby areas. The captures and collection of secretions from the oropharyngeal mucosa of cats were carried out in the afternoon (02:00 p.m.–05:00 p.m.), a period when the cats had not yet been fed and demonstrated more interest in human presence, facilitating their capture.
After capturing and adequately containing the animals, rapid antigen detection was performed using point-of-care tests adapted for animal use. Oropharyngeal samples were collected for the detection of the SARS-CoV-2 antigen using an immunofluorescence assay (Eco F COVID-19 Ag kit with Eco Reader®, Eco Diagnostica, Brazil). This test was chosen for its high sensitivity (99.00%) and specificity (100.00%) compared to reverse transcription polymerase chain reaction (RT-PCR), based on prior validation in animals [34]. The immunofluorescence method is superior to traditional immunochromatographic tests because the reading is not visual but performed using a device that provides a semi-quantitative value. This value estimates the quantity of antigen present in the sample, which is then interpreted against a cutoff value [35].
The Eco F COVID-19 Ag® test (Eco Diagnostica Ltd, Brazil) is reported by the manufacturer to have sensitivities of 91.78% and specificities of 96.70%, with no cross-reactions observed with other CoVs, influenza, adenovirus, respiratory syncytial virus, and rhinovirus. This assay qualitatively detects the SARS-CoV-2 N protein and can detect the Omicron (B.1.1.529) variant without affecting sensitivity and specificity [36]. The biological samples were collected from throat swabs, and the results were released within 15 min, recorded in a spreadsheet, and the animals cataloged to avoid repeated collections from the same animals. The animals were observed by veterinarians for any symptoms, which were duly recorded.
Statistical analysis and data visualization
All information (location, sex, behavior, obvious clinical condition, and rapid test result) and photographs of each cat were organized in spreadsheets. To verify a possible correlation between variables and the result of the rapid antigen detection, Fisher’s exact test and Pearson’s Chi-square test were applied using the software R version 4.1.0 (R Core Team, Vienna, Austria). We considered a p < 0.05 for significant results. In addition, we used the Circos® graph (Martin Krzywinski, Vancouver, Canada) (http://circos.ca/), to visualize the quantitative clinical conditions of the animals.
Results
A total of 126 cats were tested for SARS-CoV-2 antigen, with 79 (62.20%) females and 48 (37.80%) males. Among them, 30 (23.60%) had a positive diagnosis, including 19 (63.30%) females and 11 (36.60%) males. It was possible to observe (through clinical observation) that a total of 26 (21.60%), 7 (5.50%), and 5 (3.90%) cats presented with nasal discharge, dyspnea, and cough and/or sneezing, respectively. In addition, through palpation, it was possible to observe that 5 (6.40%) of the analyzed females were pregnant (Figures-1 and 2, Table-1).
Figure-1.
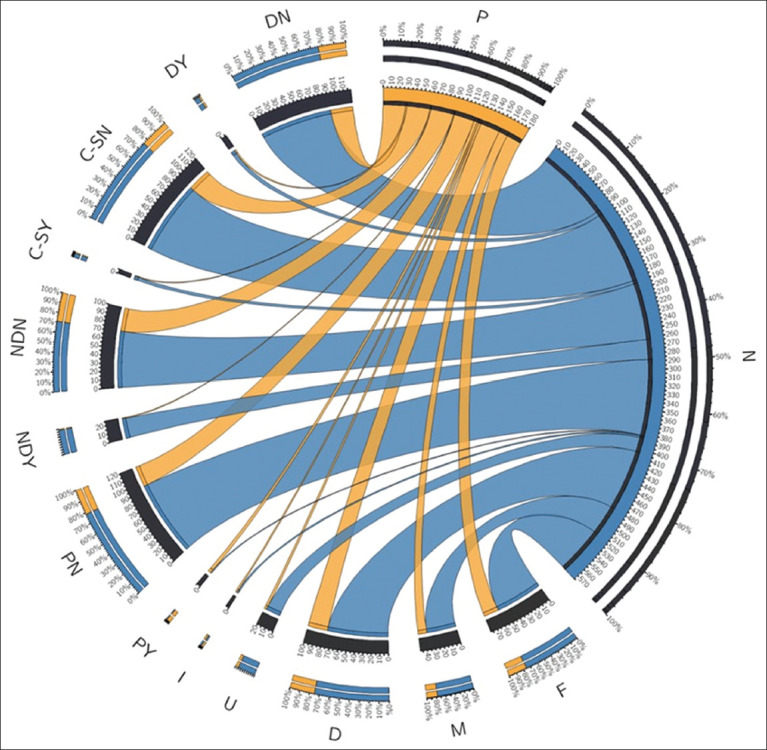
Graphical representation of the clinical conditions and quantitative results of the animals studied. The graph contains the quantitative results of the infected cats (P=Positive and N=Negative). F (P = 19; N = 59) and M (P = 11; N = 37) represent female and male sex, respectively (D=Docile; P = 25; N = 76), (U=Unsociable; P = 3; N = 18), and (I=Indifferent; P = 2; N = 2) represent feline behavior, only participants who knew the brands of vaccines they had taken. (PY=Confirmed pregnancy; P = 4; N = 1) and (PN=No pregnancy; P = 26; N = 95) represent pregnant or not pregnant females. Regarding the cats that had nasal discharge, we represented NDY (P = 1; N = 25) as cats with nasal discharge and NDN (P = 29; N=71) as cats without nasal discharge. For cats that presented with cough/sneeze, we represented them as C-SY (P = 1; N = 4) and those without Cough/Sneeze as C-SN (P = 29; N = 92). In addition, we assessed dyspnea, considering DY (P = 2; N = 5) as the cats that had dyspnea and DN (P = 28; N = 91) that did not.
Figure-2.

Overview of the methodology and results obtained with stray cats by the Federal University of Sergipe.
Table-1.
Statistical analysis of the studied animals.
| Characteristics | Positive, n (%) | Negative, n (%) | p-value | RR | CI 95% | ARR | CI 95% |
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 30 (23.80) | 96 (76.20) | ||||||
| Sex, n (%) | |||||||
| Female | 19 (63.30) | 59 (61.50) | 1.000F | 1.06 | 0.55–2.04 | ||
| Male | 11 (36.70) | 37 (38.50) | |||||
| Behavior, n (%) | |||||||
| Docile | 25 (83.30) | 76 (79.20) | 0.279Q | 0.49 | 0.17–1.40 | ||
| Unsociable | 3 (10.00) | 18 (18.80) | 0.29 | 0.07–1.20 | |||
| Indifferent | 2 (6.70) | 2 (2.10) | |||||
| Pregnancy, n (%) | |||||||
| Yes | 4 (13.30) | 1 (1.00) | 0.011F | 3.72 | 2.14–6.49 | 3.70 | 1.80–7.59 |
| No | 26 (86.70) | 95 (99.00) | |||||
| Nasal discharge, n (%) | |||||||
| Yes | 1 (3.30) | 25 (26.00) | 0.008F | 0.13 | 0.02–0.93 | 0.13 | 0.02–0.78 |
| No | 29 (96.70) | 71 (74.00) | |||||
| Cough/sneeze, n (%) | |||||||
| Yes | 1 (3.30) | 4 (4.20) | 1.000F | 0.83 | 0.14–4.96 | ||
| No | 29 (96.70) | 92 (95.80) | |||||
| Dyspnea, n (%) | |||||||
| Yes | 2 (6.70) | 5 (5.20) | 0.671F | 1.21 | 0.36–4.09 | ||
| No | 28 (93.30) | 91 (94.80) |
n=Absolute frequency, %=Relative frequency,
=Fisher’s exact test,
=Pearson’s Chi-squared test, RR=Relative risk, ARR=Adjusted relative risk, CI 95%=Confidence interval of 95%
Table-1 also represents the results of the statistical analysis that demonstrated, by means of Fisher’s exact test, an association between the presence of infections (positive test result) with pregnancy (χ2[1] = 9.062, p < 0.05) and nasal secretion (χ2[1] = 7.197, p < 0.05), considering a 95% confidence interval.
Discussion
A total of 30/126 (23.80%) animals were tested positive for the SARS-CoV-2 antigen. Many studies have sought to report natural infections in cats worldwide with a wide variation in prevalence: 0.00% [27], 3.51% [33], and 14.70% [37]. This variation can be related to the exposure of animals to infected humans, which is directly related to the human infection rates of each country and internal measures such as quarantine and lockdowns. Even though the cats analyzed in the present study are stray, it is likely that the cases observed at the university emerged from human infection, probably from students and employees who are responsible for the health care and feeding of the cats. Humans primarily transmit SARS-CoV-2 to cats, particularly in quarantine and domestic isolation cases [26]. In docile animals, the infection ratio is higher due to their closer contact with humans compared to wild animals. Our earlier research [34] supports these findings by identifying immunoglobulin (Ig)M and IgG antibodies to SARS-CoV-2 and investigating potential cross-reactivity with feline CoVs and parvovirus. Moreover, the analysis of antigens in negative stool samples further confirmed the presence of the infection, aligning with the results of this study.
20.60% (26/126) of studied cats exhibited nasal discharge, 5.50% (7/126) presented with dyspnea, and 3.90% (5/126) coughed or sneezed, complicating treatment due to their wild behavior and restricting resources from social projects. The study revealed a positive correlation between nasal discharge in cats, pregnancy, and infections. Respiratory symptoms have been reported in numerous cat studies [14]. Given the limited number of tested animals and the small sample size of positive animals with clinical symptoms, the ratio of positive animals and nasal discharge should be interpreted with great caution. Multiple animals should undergo new testing procedures.
No studies have yet investigated the link between cat pregnancy and SARS-CoV-2 susceptibility. 5 females (6.40%) were found to be both pregnant and positive for SARS-CoV-2 antigens (Figures-1 and 2, Table-1). Older and domestic cats have lower infection rates for respiratory pathogens compared to younger and stray cats, as observed in a study in Bulgaria [15, 37]. Animals with pre-existing immunosuppression conditions are more likely to contract SARS-CoV-2 infections due to their weakened immune response. For the 1st time, Villanueva-Saz et al. [27] documented infections in stray cats from Spain. Animals with inadequate health, housing, and food conditions, and concurrent infections with Toxoplasma gondii, Leishmania infantum, or feline immunodeficiency virus, are more susceptible to SARS-CoV-2 infections. In addition, co-infections and cross-reactivity from other pathogens presenting similar symptoms, such as Mycoplasma, Chlamydia, and Feline Herpesvirus, should be considered when diagnosing SARS-CoV-2 in cats [38]. The environment and climate significantly influence the transmission of the virus. According to studies [15, 39], climate conditions in different regions impact the spread of SARS-CoV-2.
Based on limited research utilizing rapid antigen detection (RAD) tests, some authors have successfully isolated viruses and identified viral RNA from the oral and/or throat swabs of infected cats [24, 25, 40, 41]. Although there is a lack of studies based on RAD tests, many authors were able to perform viral isolation and/or viral RNA identification from oral and/or throat swabs of infected cats [24, 25, 40, 41]. The detection of SARS-CoV-2 viral particles in oral and throat secretions of animals allows for the identification of infections through RAD tests. RT-PCR, while being the gold standard test for SARS-CoV-2 detection, offers advantages such as quick results, technical simplicity, and lower cost compared to other diagnostic tests [42]. RAD tests are ideal for making quick diagnostic decision in field conditions.
The RAD sensitivity and specificity for SARS-CoV-2 depend significantly on factors like brand and experimental design (https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays). The sensitivity of detecting diseases using nasal or nasopharyngeal swabs and comparing it with RT-PCR varies greatly (0.00%–94.00%) depending on disease stage, but specificity remains high (>97.00%). The World Health Organization recommends antigen-detecting rapid diagnostic tests (Ag-RDTs) with a minimum performance of ≥80.00% sensitivity and ≥97.00% specificity, per the standard set by the reference assay (https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-SARS-COV-2infection-using-rapid-immunoassays). In samples with high viral loads, such as those from the initial stages of infection, rapid diagnostic tests (RDTs) are more effective.
In addition, it is important to emphasize that the Coronaviridae family has many genera that affect a wide variety of animals, including the feline enteric coronavirus (FECV) and the feline infectious peritonitis coronavirus (FIPV), both of which are responsible for acute diseases in cats [43]. No serological reaction has been observed between FECV/FIPV and SARS-CoV-2 [27, 28, 44]. Cross-reactivity is not an issue in the context of antigen detection using the RAD test, as it targets unique SARS-CoV-2 nucleoproteins.
Several outbreaks of SARS-CoV-2 infection were marked by numerous positive animal colonies. Bosco-Lauth et al. [24] experimentally infected cats and confirmed direct transmission between them. The study used viral isolation and qRT-PCR to confirm that animals in experimentally and direct contact with infected groups shed viral particles in their oral and nasal secretions for 5–7 days following infection. Shi et al. [22] demonstrated the susceptibility of cats to SARS-CoV-2 infections and reinforced the transmission between animals by respiratory droplets. On the other hand, many colonies had no cases of cats infected by SARS-CoV-2.
This result can be explained by the distribution of animals across the university. Even though cats are stray and can freely roam through the campus, they tend to be close together or in specific colonies, limiting the spread of disease among them. Cats can develop antibodies against SARS-CoV-2, which protect them from reinfections following either natural or experimental infections [43, 45].
Conclusion
Based on RDA test results, this study confirms that stray cats from the Federal University of Sergipe have been infected with SARS-CoV-2. These findings underscore the infection’s occurrence in stray animals and heighten concerns about its prevalence. Although the study has limitations, such as the small sample size and the uneven number of cats with respect to their characteristics and conditions, these issues underscore the need for caution in interpreting the results. These limitations also highlight the importance of conducting further research with larger, more uniformly categorized samples. The detection of SARS-CoV-2 in stray cats emphasizes the critical need for ongoing research and monitoring, including studies with larger sample sizes, more uniform categorization of animal conditions, and the use of diverse diagnostic methods. Additionally, it is important to investigate the transmission dynamics of SARS-CoV-2 across different animal populations and their interactions with humans to better understand and manage the virus’s spread.
Data Availability
The supplementary data can be made available by the corresponding author upon request.
Authors’ Contributions
RSS, DABL, TLB, and POMS: Conceived and designed the study. EEDS, PCJ, JBS, RSS, MSB, PHMM, DMRRS, and CRP: Performed the study. DABL, EEDS, PCJ, PHMM, DMRRS, RSS, MSB, and LPB: Analyzed and interpreted the data. DABL, RSS, LPB, and CRP: Performed statistical analysis. DABL, RSS, LPB, and CRP: Wrote the manuscript. RSS, MSB, AGG, LAMS, CRP, and LPB: Revised the manuscript. AGG, LAMS, CRP, and LPB: Supervised the study. LPB: Project administration. All authors have read, reviewed, and approved the final manuscript.
Acknowledgments
We thank the Federal University of Sergipe and the Department of Veterinary Medicine of UFS for providing necessary facilities for the study. The authors did not receive any funds for this study.
Footnotes
We thank the Federal University of Sergipe and the Department of Veterinary Medicine of UFS for providing necessary facilities for the study. The authors did not receive any funds for this study.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes?A review of coronaviruses and ocular implications in humans and animals. Ocul. Immunol. Inflamm. 2020;28(3):391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaudreault N.N, Trujillo J.D, Carossino M, Meekins D.A, Morozov I, Madden D.W, Indran S.V, Bold D, Balaraman V, Kwon T, Artiaga B.L, Cool K, García-Sastre A, Ma W, Wilson W.C, Henningson J, Balasuriya U.B.R, Richt J.A. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg. Microbes Infect. 2020;9(1):2322–2332. doi: 10.1080/22221751.2020.1833687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boley P.A, Alhamo M.A, Lossie G, Yadav K.K, Vasquez-Lee M, Saif L.J, Kenney S.P. Porcine delta coronavirus infection and transmission in poultry, United States1. Emerg. Infect. Dis. 2020;26(2):255–265. doi: 10.3201/eid2602.190346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tu Y.F, Chien C.S, Yarmishyn A.A, Lin Y.Y, Luo Y.H, Lin Y.T, Lai W.Y, Yang D.M, Chou S.J, Yang Y.P, Wang M.L, Chiou S.H. A review of SARS-CoV-2 and the ongoing clinical trials. Int. J. Mol. Sci. 2020;21(7):2657. doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer B, Juhasz J, Barua R, Das Gupta A, Hakimuddin F, Corman V.M, Müller M.A, Wernery U, Drosten C, Nagy P. Time course of MERS-CoV infection and immunity in dromedary camels. Emerg. Infect. Dis. 2016;22(12):2171–2173. doi: 10.3201/eid2212.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang N, Li S.Y, Yang X.L, Huang H.M, Zhang Y.J, Guo H, Luo C.M, Miller M, Zhu G, Chmura A.A, Hagan E, Zhou J.H, Zhang Y.Z, Wang L.F, Daszak P, Shi Z.L. Serological evidence of bat SARS-related coronavirus infection in humans, China. Virol. Sin. 2018;33(1):104–107. doi: 10.1007/s12250-018-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luk H.K.H, Li X, Fung J, Lau S.K.P, Woo P.C.Y. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect. Genet. Evol. 2019;71:21–30. doi: 10.1016/j.meegid.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdel-Moneim A.S, Abdelwhab E.M. Evidence for SARS-CoV-2 infection of animal hosts. Pathogens. 2020;9(7):529. doi: 10.3390/pathogens9070529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau S.K.P, Luk H.K.H, Wong A.C.P, Li K.S.M, Zhu L, He Z, Fung J, Chan T.T.Y, Fung K.S.C, Woo P.C.Y. Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020;26(7):1542–1547. doi: 10.3201/eid2607.200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao K, Zhai J, Feng Y, Zhou N, Zhang X, Zou J.J, Li N, Guo Y, Li X, Shen X, Zhang Z, Shu F, Huang W, Li Y, Zhang Z, Chen R.A, Wu Y.J, Peng S.M, Huang M, Xie W.J, Cai Q.H, Hou F.H, Chen W, Xiao L, Shen Y. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature. 2020;583(7815):286–289. doi: 10.1038/s41586-020-2313-x. [DOI] [PubMed] [Google Scholar]
- 11.Decaro N, Balboni A, Bertolotti L, Martino P.A, Mazzei M, Mira F, Pagnini U. SARS-CoV-2 infection in dogs and cats:Facts and speculations. Front. Vet. Sci. 2021;8:619207. doi: 10.3389/fvets.2021.619207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreutz L.C, Flores E.F, Cargnelutti J, Henzel A, Anzilero D, Brum M.C.S, Lima M, Torres F, Franco D, Oliveira S.A.M, Silva A.M. SARS-CoV-2/COVID/19 in companion animals [SARS-CoV-2/COVID/19 em animais de companhia] Ars Vet. 2021;37(1):1. [Google Scholar]
- 13.Garigliany M, Van Laere A.S, Clercx C, Giet D, Escriou N, Huon C, van der Werf S, Eloit M, Desmecht D. SARS-CoV-2 natural transmission from human to cat, Belgium, March 2020. Emerg. Infect. Dis. 2020;26(12):3069–3071. doi: 10.3201/eid2612.202223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doliff R, Martens P. Cats and SARS-CoV-2:A scoping review. Animals (Basel) 2022;12(11):1413. doi: 10.3390/ani12111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sirakov I, Rusenova N, Rusenov A, Gergova R, Strateva T. Human ELISA detects anti-SARS-CoV-2 antibodies in cats:Seroprevalence and risk factors for virus spread in domestic and stray cats in Bulgaria. Vet. Sci. 2023;10(1):42. doi: 10.3390/vetsci10010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prado T, Fumian T.M, Mannarino C.F, Maranhão A.G, Siqueira M.M, Miagostovich M.P. Preliminary results of SARS-CoV-2 detection in sewerage system in Niterói municipality, Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2020;115:e200196. doi: 10.1590/0074-02760200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrahão J.S, Sacchetto L, Rezende I.M, Rodrigues R.A.L, Crispim A.P.C, Moura C, Mendonça D.C, Reis E, Souza F, Oliveira G.F.G, Domingos I, de Miranda Boratto P.V, Silva P.H.B, Queiroz V.F, Machado T.B, Andrade L.A.F, Lourenço K.L, Silva T, Oliveira G.P, de Souza Alves V, Alves P.A, Kroon E.G, de Souza Trindade G, Drumond B.P. Detection of SARS-CoV-2 RNA on public surfaces in a densely populated urban area of Brazil:A potential tool for monitoring the circulation of infected patients. Sci. Total Environ. 2021;766:142645. doi: 10.1016/j.scitotenv.2020.142645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sit T.H.C, Brackman C.J, Ip S.M, Tam K.W.S, Law P.Y.T, To E.M.W, Yu V.Y.T, Sims L.D, Tsang D.N.C, Chu D.K.W, Perera R.A.P.M, Poon L.L.M, Peiris M. Infection of dogs with SARS-CoV-2. Nature. 2020;586(7831):776–778. doi: 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hossain M.G, Javed A, Akter S, Saha S. SARS-CoV-2 host diversity:An update of natural infections and experimental evidence. J. Microbiol. Immunol. Infect. 2021;54(2):175–181. doi: 10.1016/j.jmii.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hobbs E.C, Reid T.J. Animals and SARS-CoV-2:Species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transbound. Emerg. Dis. 2021;68(4):1850–1867. doi: 10.1111/tbed.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman A, Smith D, Ghai R.R, Wallace R.M, Torchetti M.K, Loiacono C, Murrell L.S, Carpenter A, Moroff S, Rooney J.A, Barton Behravesh C. First reported cases of SARS-CoV-2 infection in companion animals - New York, March-April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(23):710–713. doi: 10.15585/mmwr.mm6923e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, Zhao Y, Liu P, Liang L, Cui P, Wang J, Zhang X, Guan Y, Tan W, Wu G, Chen H, Bu Z. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368(6494):1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halfmann P.J, Hatta M, Chiba S, Maemura T, Fan S, Takeda M, Kinoshita N, Hattori S.I, Sakai-Tagawa Y, Iwatsuki-Horimoto K, Imai M, Kawaoka Y. Transmission of SARS-CoV-2 in domestic cats. N. Engl. J. Med. 383(6):592–594. doi: 10.1056/NEJMc2013400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosco-Lauth A.M, Hartwig A.E, Porter S.M, Gordy P.W, Nehring M, Byas A.D, VandeWoude S, Ragan I.K, Maison R.M, Bowen R.A. Experimental infection of domestic dogs and cats with SARS-CoV-2:Pathogenesis, transmission, and response to re-exposure in cats. Proc. Natl. Acad. Sci. U S A. 2020;117(42):26382–26388. doi: 10.1073/pnas.2013102117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaudreault N.N, Carossino M, Morozov I, Trujillo J.D, Meekins D.A, Madden D.W, Cool K, Artiaga B.L, McDowell C, Bold D, Balaraman V, Kwon T, Ma W, Henningson J, Wilson D.W, Wilson W.C, Balasuriya U.B.R, García-Sastre A, Richt J.A. Experimental re-infected cats do not transmit SARS-CoV-2. Emerg. Microbes Infect. 2021;10(1):638–650. doi: 10.1080/22221751.2021.1902753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manzini S, Rodrigues N.J.L, Bertozzo T.V, Aires I.N, Lucheis S.B. SARS-CoV-2:Its relation to animals and potential zoonotic disease [SARS-COV-2:Sua relação com os animais e potencial doença zoonótica] Vet. Zootec. 2021;28:1–13. [Google Scholar]
- 27.Villanueva-Saz S, Giner J, Tobajas A.P, Pérez M.D, González-Ramírez A.M, Macías-León J, González A, Verde M, Yzuel A, Hurtado-Guerrero R, Pardo J, Santiago L, Paño-Pardo J.R, Ruíz H, Lacasta D.M, Sánchez L, Marteles D, Gracia A.P, Fernández A. Serological evidence of SARS-CoV-2 and co-infections in stray cats in Spain. Transbound. Emerg. Dis. 2022;69(3):1056–1064. doi: 10.1111/tbed.14062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spada E, Vitale F, Bruno F, Castelli G, Reale S, Perego R, Baggiani L, Proverbio D. A pre- and during pandemic survey of sars-Cov-2 infection in stray colony and shelter cats from a high endemic area of Northern Italy. Viruses. 2021;13(4):618. doi: 10.3390/v13040618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oude Munnink B.B, Sikkema R.S, Nieuwenhuijse D.F, Molenaar R.J, Munger E, Molenkamp R, van der Spek A, Tolsma P, Rietveld A, Brouwer M, Bouwmeester-Vincken N, Harders F, Hakze-van der Honing R, Wegdam-Blans M.C.A, Bouwstra R.J, GeurtsvanKessel C, van der Eijk A.A, Velkers F.C, Smit L.A.M, Stegeman A, van der Poel W.H.M, Koopmans M.P.G. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2021;371(6525):172–177. doi: 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Andrade J.F, de Lima Cruz I.R, de Sampaio F.M.S, da Silva C.G.L, Lopes e Silva M.R, Gadelha M.S.V. SARS-CoV-2 Investigation in dogs and cats:Case reports in the literature [pesquisa de SARS-COV-2 em cães e gatos:Relatos de casos na literatura] Braz. J. Dev. 2021;7(5):45198–45209. [Google Scholar]
- 31.Dias H.G, Resck M.E.B, Caldas G.C, Resck A.F, da Silva N.V, Dos Santos A.M.V, Sousa T.D.C, Ogrzewalska M.H, Siqueira M.M, Pauvolid-Corrêa A, Dos Santos F.B. Neutralizing antibodies for SARS-CoV-2 in stray animals from Rio de Janeiro, Brazil. PLoS One. 2021;16(3):e0248578. doi: 10.1371/journal.pone.0248578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaves T.S.S, Bellei N.C.J. SARS-COV-2, SARS-CoV-2, the new coronavirus. Rev. Med. 2020;99(1):i–iv. [Google Scholar]
- 33.Zhang Q, Zhang H, Gao J, Huang K, Yang Y, Hui X, He X, Li C, Gong W, Zhang Y, Zhao Y, Peng C, Gao X, Chen H, Zou Z, Shi Z.L, Jin M. A serological survey of SARS-CoV-2 in cat in Wuhan. Emerg. Microbes Infect. 2020;9(1):2013–2019. doi: 10.1080/22221751.2020.1817796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guimarães A.G, Lee D.A.B, Meira-Santos P.O, Bezerra T.L, Pinto Borges L.P. Can pets have COVID-19:Incidence and seroprevalence in symptomatic animals seen in a private clinic. Sci. Plena. 2022;18(6):066101. [Google Scholar]
- 35.Matsuda E.M, De Almeida S.M, de Oliveira I.P, Colpas D.R, Carmo A.M.D.S, Brígido L.F.M. Field evaluation of COVID-19 antigen tests versus RNA based detection:Potential lower sensitivity compensated by immediate results, technical simplicity, and low cost. J. Med. Virol. 2021;93(7):4405–4410. doi: 10.1002/jmv.26985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ECO F COVID-19 Ag. ECO Diagnóstica. [Retrieved on 13-06-2024]. Available from: https://ecodiagnostica.com.br/diagnostico-rapido/eco-f-covid-19-ag .
- 37.Stranieri A, Lauzi S, Giordano A, Galimberti L, Ratti G, Decaro N, Brioschi F, Lelli D, Gabba S, Amarachi N.L, Lorusso E, Moreno A, Trogu T, Paltrinieri S. Absence of SARS-CoV-2 RNA and anti-SARS-CoV-2 antibodies in stray cats. Transbound. Emerg. Dis. 2022;69(4):2089–2095. doi: 10.1111/tbed.14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sirakov I, Popova-Ilinkina R, Ivanova D, Rusenova N, Mladenov H, Mihova K, Mitov I. Development of nested PCR for SARS-CoV-2 detection and its application for diagnosis of active infection in cats. Vet. Sci. 2022;9(6):272. doi: 10.3390/vetsci9060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sirakov I, Stankova P, Bakalov D, Mirani Y, Bardarska L, Paraskova G, Popov I, Alexandrova A, Dimitrov G, Mizgova G, Kalvatchev N, Gergova R. Retrospective analysis of the spread of SARS-CoV-2 in the mediterranean part of Bulgaria, during the first wave of the pandemic. J. Pure Appl. Microbiol. 2024;18(1):438–450. [Google Scholar]
- 40.Liu H, Wang L.L, Zhao S.J, Kwak-Kim J, Mor G, Liao A.H. Why are pregnant women susceptible to COVID-19?An immunological viewpoint. J. Reprod. Immunol. 2020;139:103122. doi: 10.1016/j.jri.2020.103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamer S.A, Pauvolid-Corrêa A, Zecca I.B, Davila E, Auckland L.D, Roundy C.M, Tang W, Torchetti M.K, Killian M.L, Jenkins-Moore M, et al. SARS-CoV-2 Infections and viral isolations among serially tested cats and dogs in households with infected owners in Texas, USA. Viruses. 2021;13:938. doi: 10.3390/v13050938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neira V, Brito B, Agüero B, Berrios F, Valdés V, Gutierrez A, Ariyama N, Espinoza P, Retamal P, Holmes E.C, Gonzalez-Reiche A.S, Khan Z, van de Guchte A, Dutta J, Miorin L, Kehrer T, Galarce N, Almonacid L.I, Levican J, van Bakel H, García-Sastre A, Medina R.A. A household case evidences shorter shedding of SARS-CoV-2 in naturally infected cats compared to their human owners. Emerg. Microbes Infect. 2021;10(1):376–383. doi: 10.1080/22221751.2020.1863132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuda E.M, de Campos I.B, de Oliveira I.P, Colpas D.R, Dos Santos Carmo A.M, de Macedo Brígido L.F. Field evaluation of COVID-19 antigen tests versus RNA based detection:Potential lower sensitivity compensated by immediate results, technical simplicity, and low cost. J. Med. Virol. 2021;93(7):4405–4410. doi: 10.1002/jmv.26985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tekes G, Thiel H.J. Feline coronaviruses:Pathogenesis of feline infectious peritonitis. Adv. Virus Res. 2016;96:193–218. doi: 10.1016/bs.aivir.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michelitsch A, Hoffmann D, Wernike K, Beer M. Occurrence of antibodies against SARS-CoV-2 in the domestic cat population of Germany. Vaccines (Basel) 2020;8(4):772. doi: 10.3390/vaccines8040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The supplementary data can be made available by the corresponding author upon request.


