Abstract
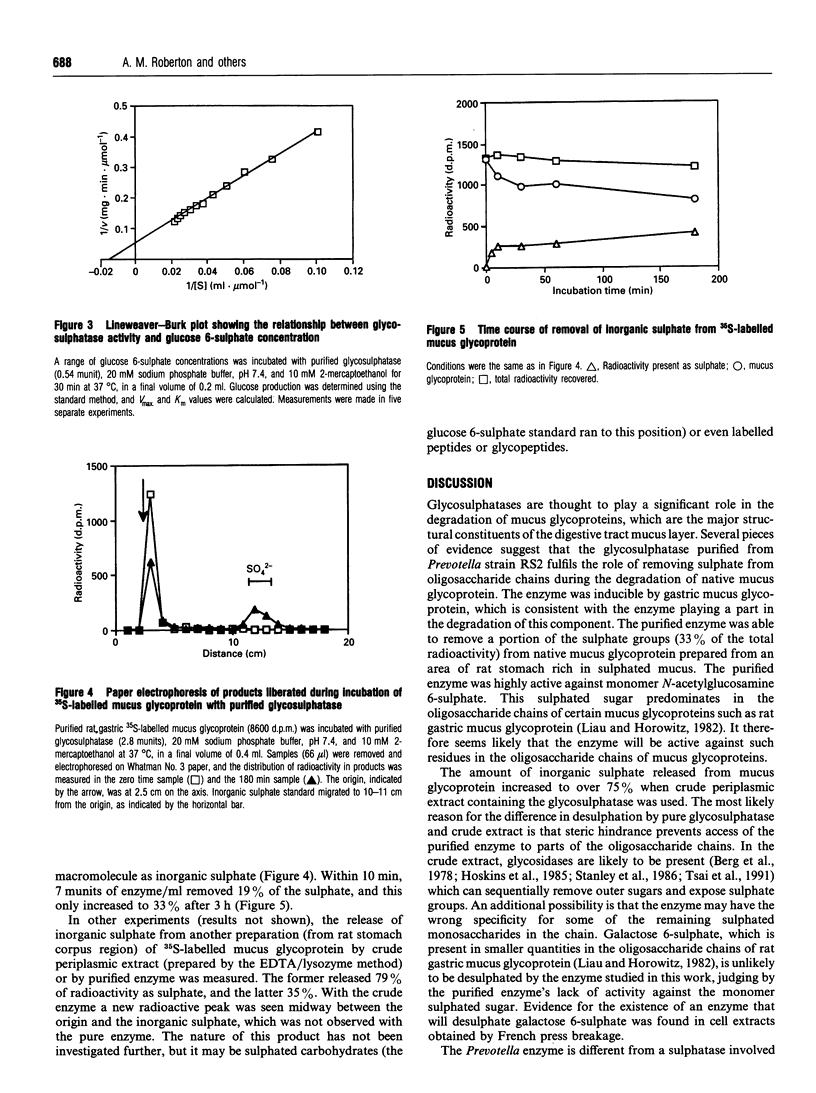
A novel glycosulphatase has been purified from a mucus glycopeptide-degrading Prevotella from the colon. The purified enzyme removed inorganic [35S]sulphate from 35S-labelled native rat gastric mucus glycoprotein. Desulphation of mucus glycoprotein was initially rapid (19% complete after 10 min) but then plateaued, reaching only 33% after 3 h. Crude periplasmic extracts could remove 79% of the radioactivity as inorganic sulphate. These results suggest that steric hindrance may limit the access of the purified glycosulphatase to the mucus glycoprotein oligosaccharide chains in the absence of glycosidases, and/or that the enzyme may have the wrong specificity for some of the remaining sulphated sugars in the chains. The apparent molecular mass of the enzyme was 111 kDa as judged from gel exclusion chromatography, and it appeared to be composed of two identical subunits. The enzyme was localized in the periplasm of the bacterium, and using pig gastric mucus glycopeptide as a growth substrate markedly increased enzyme levels. Enzymic activity increased at the end of the growth phase. The substrate specificity of the enzyme was tested against low-molecular-mass sulphated molecules. The monosaccharides glucose 6-sulphate and N-acetylglucosamine 6-sulphate were rapidly desulphated, the latter being the major sulphated sugar in some mucus glycoproteins. Lactose 6-sulphate, galactose 6-sulphate, sulphated steroids and unsaturated disaccharide sulphate breakdown products from chondroitin sulphate were not desulphated. Glycosulphatases which can remove sulphate from mucus glycoproteins may play an important role in the degradation of highly sulphated mucus glycoproteins in the digestive tract, and could modify the effectiveness of mucus glycoproteins in mucosal protection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashor M. M. Dispersion and disruption of tissues. Methods Enzymol. 1979;58:119–131. doi: 10.1016/s0076-6879(79)58130-0. [DOI] [PubMed] [Google Scholar]
- Berg J. O., Nord C. E., Wadström T. Formation of glycosidases in batch and continuous culture of Bacteroides fragilis. Appl Environ Microbiol. 1978 Feb;35(2):269–273. doi: 10.1128/aem.35.2.269-273.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe M. I. Mucins in the human gastrointestinal epithelium: a review. Invest Cell Pathol. 1979 Jul-Sep;2(3):195–216. [PubMed] [Google Scholar]
- Goso Y., Hotta K. Types of oligosaccharide sulphation, depending on mucus glycoprotein source, corpus or antral, in rat stomach. Biochem J. 1989 Dec 15;264(3):805–812. doi: 10.1042/bj2640805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Agustines M., McKee W. B., Boulding E. T., Kriaris M., Niedermeyer G. Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J Clin Invest. 1985 Mar;75(3):944–953. doi: 10.1172/JCI111795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdret N., Ramphal R., Scharfman A., Perini J. M., Filliat M., Lamblin G., Roussel P. Evidence for the in vivo degradation of human respiratory mucins during Pseudomonas aeruginosa infection. Biochim Biophys Acta. 1989 Jul 21;992(1):96–105. doi: 10.1016/0304-4165(89)90055-x. [DOI] [PubMed] [Google Scholar]
- Joseph J. P., Dusza J. P., Bernstein S. Steroid conjugates I. The use of sulfamic acid for the preparation of steroid sulfates. Steroids. 1966 Jun;7(6):577–587. doi: 10.1016/0039-128x(66)90145-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liau Y. H., Horowitz M. I. Incorporation in vitro of [3H]glucosamine or [3H]glucose and [35S]SO42- into rat gastric mucosa. Presence of N-acetylhexosamine mono- and disulfates and galactose monosulfate in glycoprotein. J Biol Chem. 1982 May 10;257(9):4709–4718. [PubMed] [Google Scholar]
- Mian N., Anderson C. E., Kent P. W. Effect of O-sulphate groups in lactose and N-acetylneuraminyl-lactose on their enzymic hydrolysis. Biochem J. 1979 Aug 1;181(2):387–399. doi: 10.1042/bj1810387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty V. L., Piotrowski J., Morita M., Slomiany A., Slomiany B. L. Inhibition of Helicobacter pylori glycosulfatase activity toward gastric sulfomucin by nitecapone. Biochem Int. 1992 May;26(6):1091–1099. [PubMed] [Google Scholar]
- Piotrowski J., Slomiany A., Murty V. L., Fekete Z., Slomiany B. L. Inhibition of Helicobacter pylori colonization by sulfated gastric mucin. Biochem Int. 1991 Jul;24(4):749–756. [PubMed] [Google Scholar]
- Rhodes J. M., Black R. R., Gallimore R., Savage A. Histochemical demonstration of desialation and desulphation of normal and inflammatory bowel disease rectal mucus by faecal extracts. Gut. 1985 Dec;26(12):1312–1318. doi: 10.1136/gut.26.12.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton A. M., Rabel B., Harding C. A., Tasman-Jones C., Harris P. J., Lee S. P. Use of the ileal conduit as a model for studying human small intestinal mucus glycoprotein secretion. Am J Physiol. 1991 Nov;261(5 Pt 1):G728–G734. doi: 10.1152/ajpgi.1991.261.5.G728. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Salyers A. A., O'Brien M. Cellular location of enzymes involved in chondroitin sulfate breakdown by Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):772–780. doi: 10.1128/jb.143.2.772-780.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley R. A., Ram S. P., Wilkinson R. K., Roberton A. M. Degradation of pig gastric and colonic mucins by bacteria isolated from the pig colon. Appl Environ Microbiol. 1986 May;51(5):1104–1109. doi: 10.1128/aem.51.5.1104-1109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H. H., Sunderland D., Gibson G. R., Hart C. A., Rhodes J. M. A novel mucin sulphatase from human faeces: its identification, purification and characterization. Clin Sci (Lond) 1992 Apr;82(4):447–454. doi: 10.1042/cs0820447. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]



