Abstract
Glutamate semialdehyde aminotransferase (glutamate-1-semialdehyde 2,1-aminomutase; EC 5.4.3.8) was converted into its pyridoxaldimine form by exhaustive replacement of endogenous pyridoxamine phosphate with pyridoxal phosphate. The isomerization of glutamate 1-semialdehyde to 5-aminolaevulinate by this form of the enzyme followed an accelerating time course which indicated that the enzyme initially had no activity but was converted into the active pyridoxamine phosphate form in an exponential process characterized by a rate constant (k) of 0.027 s-1. The pyridoxaldimine form of the enzyme was converted rapidly into the pyridoxamine form by (S)-4-aminohex-5-enoate and much more slowly by 4-aminobutyrate. The steady-state velocity of the enzyme increased in a markedly non-linear fashion with increasing enzyme concentration, indicating that the extent of dissociation of an intermediate in the reaction to free diaminovalerate and the pyridoxaldimine form of the enzyme depends upon the concentration of the enzyme.
Full text
PDF

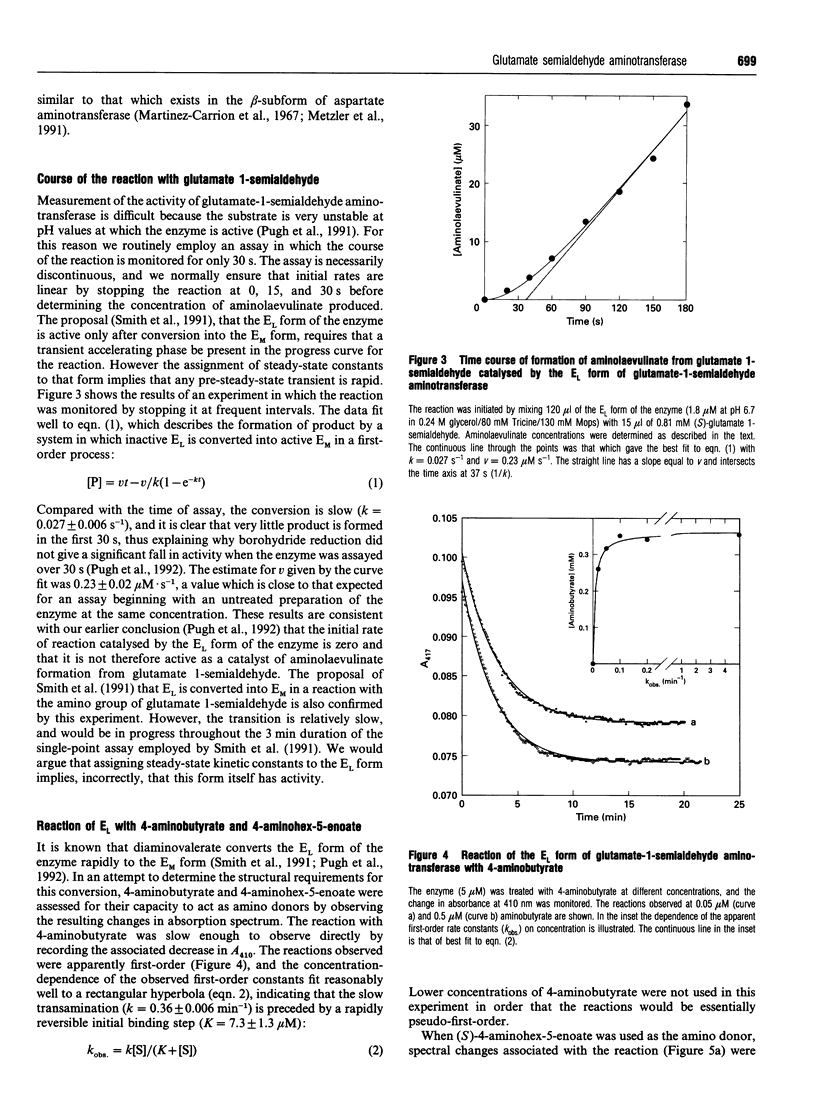

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dunathan H. C. Conformation and reaction specificity in pyridoxal phosphate enzymes. Proc Natl Acad Sci U S A. 1966 Apr;55(4):712–716. doi: 10.1073/pnas.55.4.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm B., Bull A., Welinder K. G., Gough S. P., Kannangara C. G. Purification and partial amino acid sequence of the glutamate 1-semialdehyde aminotransferase of barley and synechococcus. Carlsberg Res Commun. 1989;54(2):67–79. doi: 10.1007/BF02907586. [DOI] [PubMed] [Google Scholar]
- Hoober J. K., Kahn A., Ash D. E., Gough S., Kannangara C. G. Biosynthesis of delta-aminolevulinate in greening barley leaves. IX. Structure of the substrate, mode of gabaculine inhibition, and the catalytic mechanism of glutamate 1-semialdehyde aminotransferase. Carlsberg Res Commun. 1988;53(1):11–25. doi: 10.1007/BF02908411. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Gough S. P., Bruyant P., Hoober J. K., Kahn A., von Wettstein D. tRNA(Glu) as a cofactor in delta-aminolevulinate biosynthesis: steps that regulate chlorophyll synthesis. Trends Biochem Sci. 1988 Apr;13(4):139–143. doi: 10.1016/0968-0004(88)90071-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Carrion M., Turano C., Chiancone E., Bossa F., Giartosio A., Riva F., Fasella P. Isolation and characterization of multiple forms of glutamate-asparate aminotransferase from pig heart. J Biol Chem. 1967 May 25;242(10):2397–2409. [PubMed] [Google Scholar]
- Nair S. P., Harwood J. L., John R. A. Direct identification and quantification of the cofactor in glutamate semialdehyde aminotransferase from pea leaves. FEBS Lett. 1991 May 20;283(1):4–6. doi: 10.1016/0014-5793(91)80540-j. [DOI] [PubMed] [Google Scholar]
- Pugh C. E., Harwood J. L., John R. A. Mechanism of glutamate semialdehyde aminotransferase. Roles of diamino- and dioxo-intermediates in the synthesis of aminolevulinate. J Biol Chem. 1992 Jan 25;267(3):1584–1588. [PubMed] [Google Scholar]
- Pugh C. E., Nair S. P., Harwood J. L., John R. A. Conditions for the assay of glutamate semialdehyde aminotransferase that overcome the problem of substrate instability. Anal Biochem. 1991 Oct;198(1):43–46. doi: 10.1016/0003-2697(91)90503-l. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Kannangara C. G., Grimm B., von Wettstein D. Characterization of glutamate-1-semialdehyde aminotransferase of Synechococcus. Steady-state kinetic analysis. Eur J Biochem. 1991 Dec 18;202(3):749–757. doi: 10.1111/j.1432-1033.1991.tb16429.x. [DOI] [PubMed] [Google Scholar]



