Abstract
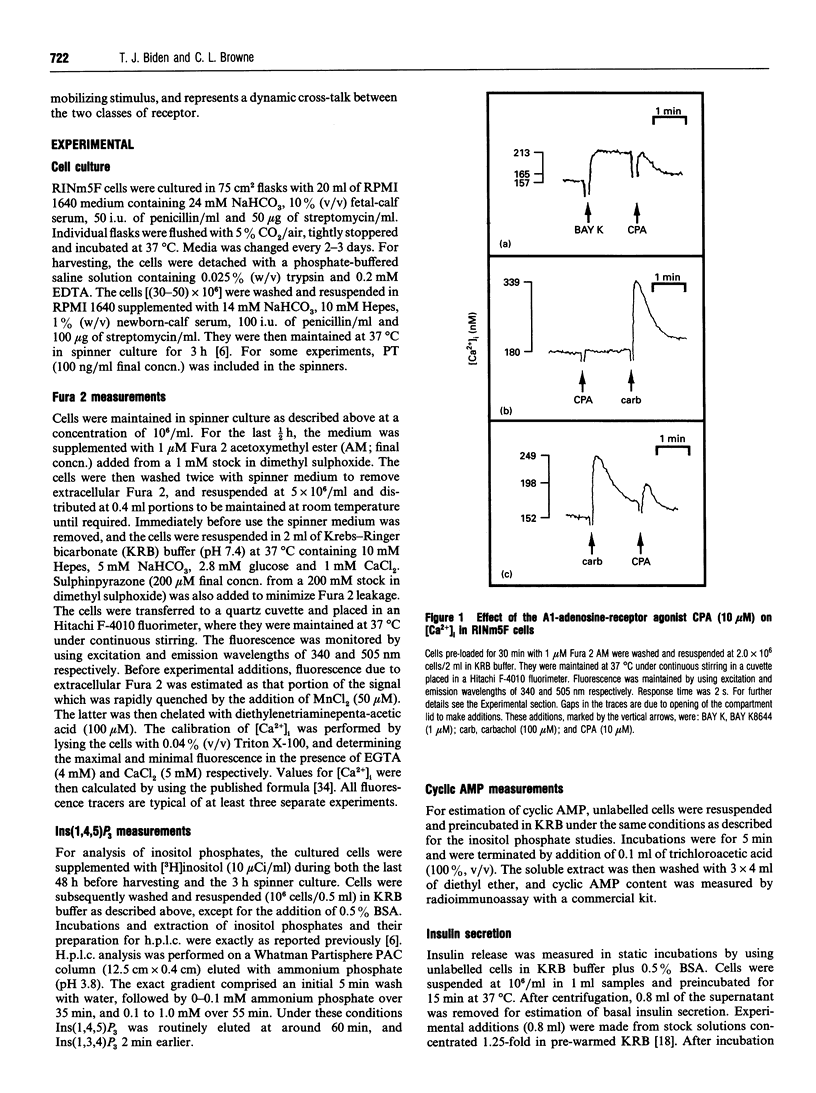
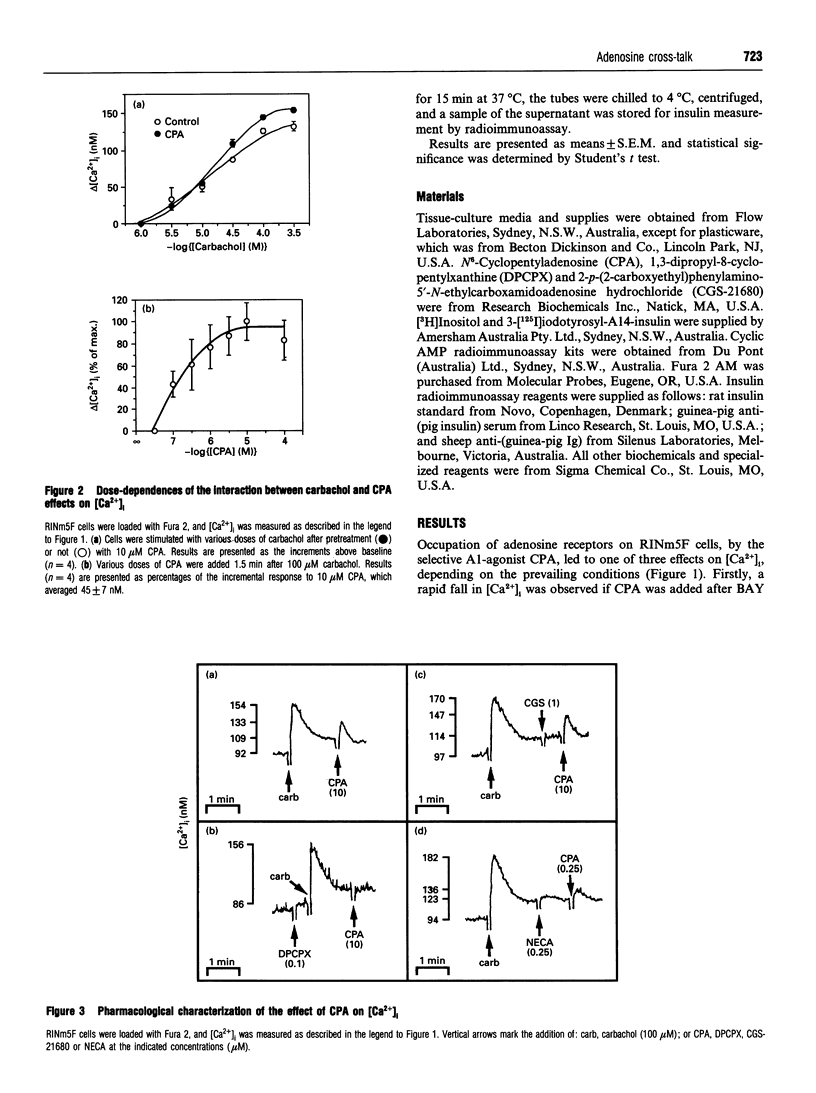
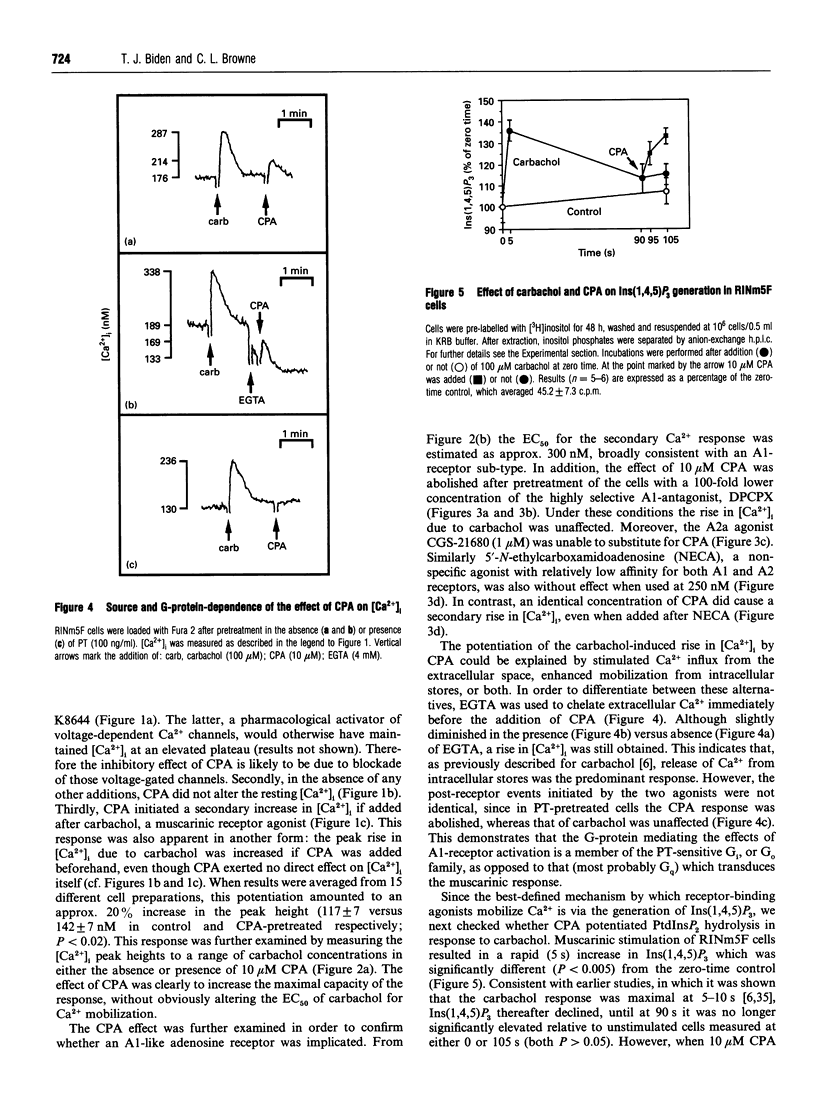
The effects of A1-adenosine-receptor occupation on Ca2+ handling in the insulin-secreting RINm5F cell line were investigated. The selective A1-agonist N6-cyclopentyladenosine (CPA) had no effect itself on the cytosolic free Ca2+ concentration in cells loaded with Fura 2. However, CPA (1) attenuated the rise due to activation of voltage-gated Ca2+ channels with Bay K 8644, and (2) caused a secondary increase (EC50 approx. 300 nM) if added after the primary Ca(2+)-mobilizing agonists vasopressin or carbamoylcholine (carbachol). Prior addition of CPA (10 microM) also potentiated (by approx. 20%) the subsequent Ca2+ peak due to maximal (100 microM) carbachol, but did not alter the EC50 of the carbachol response. Detailed analysis of the secondary rise in Ca2+ revealed further features. First, it was due to mobilization from intracellular stores, since it persisted in the absence of extracellular Ca2+. Second, it was associated with a rapid (5-15 s) increase in phospholipase C (PLC) activity, as measured by h.p.l.c. analysis of Ins(1,4,5)P3. This increase was only apparent after prior stimulation with carbachol. Third, and unlike the response to carbachol, it was mediated by a pertussis-toxin-sensitive G-protein. Fourth, it was not secondary to a decrease in cyclic AMP. Fifth, it was absolutely dependent on continued occupation of the primary receptor, since it was abolished if carbachol was displaced with the antagonist atropine. This implies a dynamic cross-talk between the two receptor coupling systems, rather than covalent modification as a result of the prior activation of PLC. Sixth, it was not associated with any desensitization of the ability of CPA to inhibit forskolin-stimulated adenylate cyclase activity. Glyceraldehyde (10 mM)-induced insulin secretion was also potently inhibited by CPA > 10 nM, but the secretory response to 100 microM carbachol was unaffected up to 10 microM. The results suggest that, in vivo, adenosine would inhibit secretion due to carbohydrate nutrients much more effectively than that due to stimuli which activate PLC.
Full text
PDF
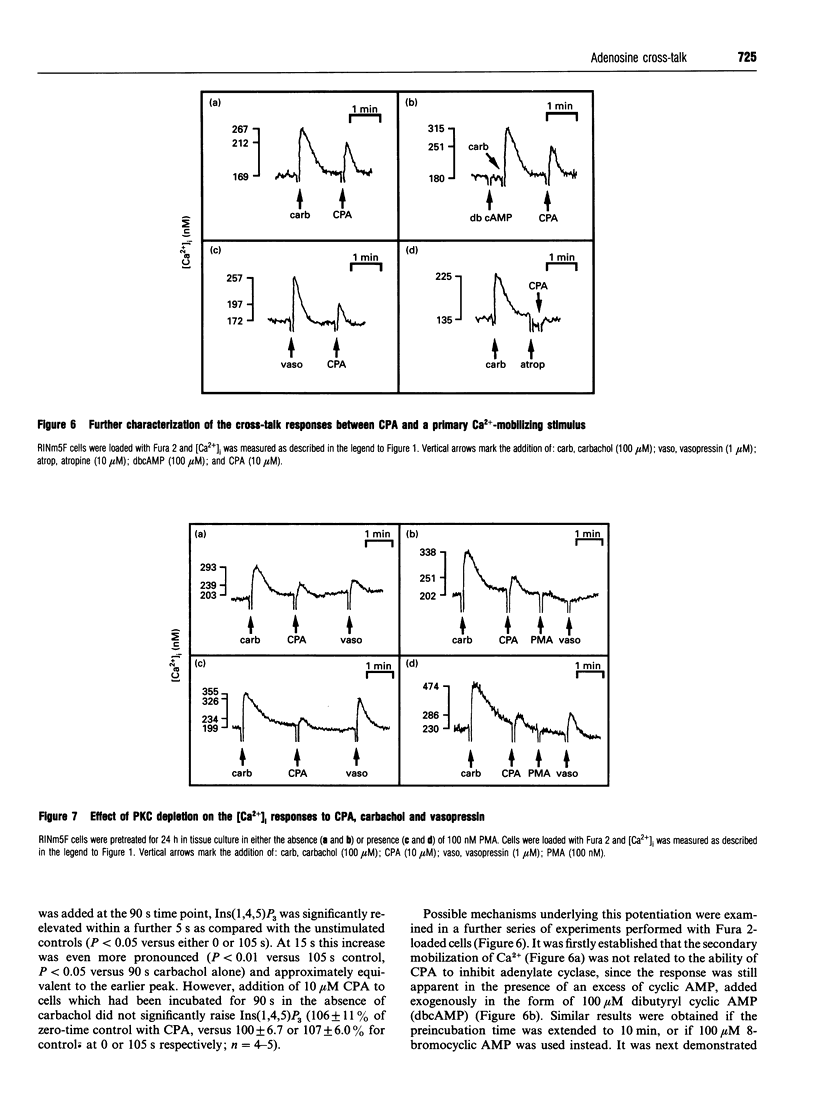
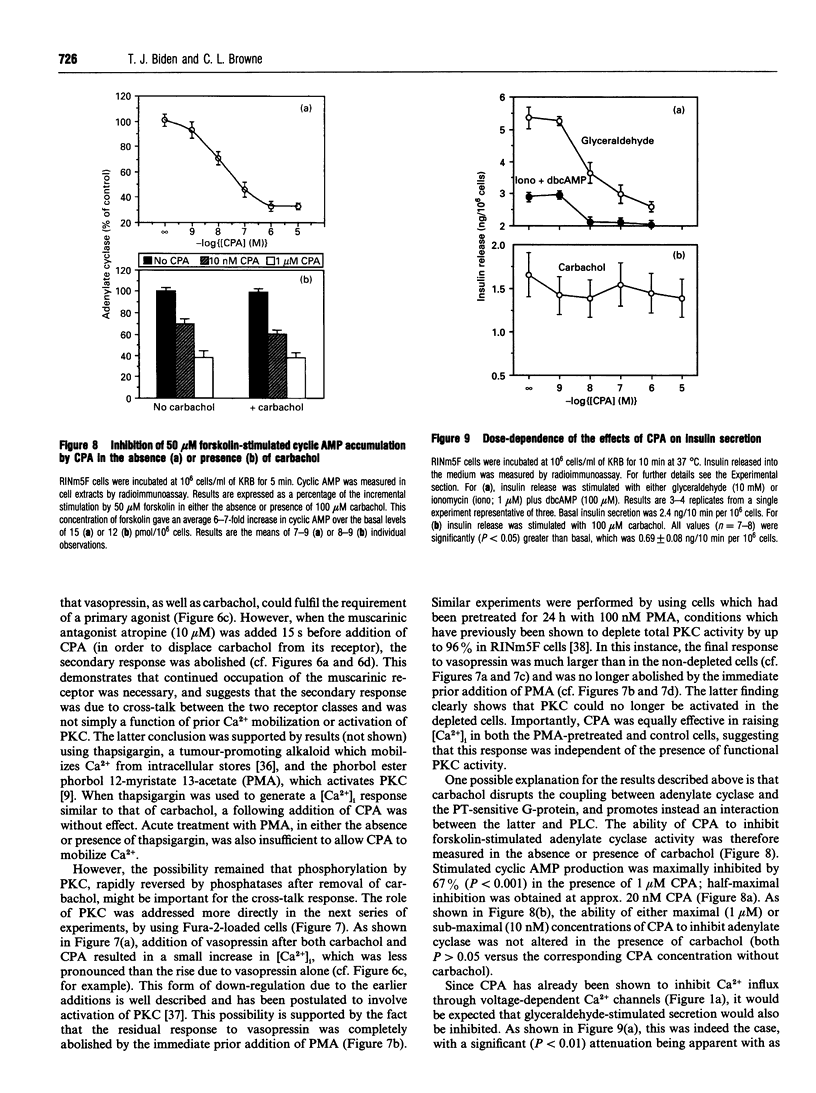


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali H., Cunha-Melo J. R., Saul W. F., Beaven M. A. Activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen-stimulated RBL-2H3 cells. Evidence for a novel adenosine receptor. J Biol Chem. 1990 Jan 15;265(2):745–753. [PubMed] [Google Scholar]
- Amiranoff B., Lorinet A. M., Lagny-Pourmir I., Laburthe M. Mechanism of galanin-inhibited insulin release. Occurrence of a pertussis-toxin-sensitive inhibition of adenylate cyclase. Eur J Biochem. 1988 Oct 15;177(1):147–152. doi: 10.1111/j.1432-1033.1988.tb14355.x. [DOI] [PubMed] [Google Scholar]
- Arend L. J., Handler J. S., Rhim J. S., Gusovsky F., Spielman W. S. Adenosine-sensitive phosphoinositide turnover in a newly established renal cell line. Am J Physiol. 1989 Jun;256(6 Pt 2):F1067–F1074. doi: 10.1152/ajprenal.1989.256.6.F1067. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bertrand G., Nenquin M., Henquin J. C. Comparison of the inhibition of insulin release by activation of adenosine and alpha 2-adrenergic receptors in rat beta-cells. Biochem J. 1989 Apr 1;259(1):223–228. doi: 10.1042/bj2590223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Ca2+ regulates the inositol tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells. J Biol Chem. 1986 Sep 15;261(26):11931–11934. [PubMed] [Google Scholar]
- Boyer J. L., Waldo G. L., Evans T., Northup J. K., Downes C. P., Harden T. K. Modification of AlF-4- and receptor-stimulated phospholipase C activity by G-protein beta gamma subunits. J Biol Chem. 1989 Aug 15;264(23):13917–13922. [PubMed] [Google Scholar]
- Burr I. M., Slonim A. E., Sharp R. Interactions of acetylcholine and epinephrine on the dynamics of insulin release in vitro. J Clin Invest. 1976 Jul;58(1):230–239. doi: 10.1172/JCI108454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. L., Taylor K. W. Effects of adenosine, 2-deoxyadenosine and N6-phenylisopropyladenosine on rat islet function and metabolism. Biochem J. 1982 Jun 15;204(3):689–696. doi: 10.1042/bj2040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M., Hou C., Sidiropoulos D., Stock J. B., Jakobs K. H., Gierschik P. Stimulation of phospholipase C by guanine-nucleotide-binding protein beta gamma subunits. Eur J Biochem. 1992 Jun 15;206(3):821–831. doi: 10.1111/j.1432-1033.1992.tb16990.x. [DOI] [PubMed] [Google Scholar]
- Delahunty T. M., Cronin M. J., Linden J. Regulation of GH3-cell function via adenosine A1 receptors. Inhibition of prolactin release, cyclic AMP production and inositol phosphate generation. Biochem J. 1988 Oct 1;255(1):69–77. doi: 10.1042/bj2550069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Forda S. R., Scott R. H. Calcium-dependent currents in cultured rat dorsal root ganglion neurones are inhibited by an adenosine analogue. J Physiol. 1986 Apr;373:47–61. doi: 10.1113/jphysiol.1986.sp016034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosset M., Schmid-Antomarchi H., de Weille J. R., Lazdunski M. Somatostatin activates glibenclamide-sensitive and ATP-regulated K+ channels in insulinoma cells via a G-protein. FEBS Lett. 1988 Dec 19;242(1):94–96. doi: 10.1016/0014-5793(88)80992-x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hill S. J., Kendall D. A. Studies on the adenosine-receptor mediating the augmentation of histamine-induced inositol phospholipid hydrolysis in guinea-pig cerebral cortex. Br J Pharmacol. 1987 Jul;91(3):661–669. doi: 10.1111/j.1476-5381.1987.tb11260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillaire-Buys D., Bertrand G., Gross R., Loubatières-Mariani M. M. Evidence for an inhibitory A1 subtype adenosine receptor on pancreatic insulin-secreting cells. Eur J Pharmacol. 1987 Apr 7;136(1):109–112. doi: 10.1016/0014-2999(87)90786-2. [DOI] [PubMed] [Google Scholar]
- Hollingsworth E. B., De la Cruz R. A., Daly J. W. Accumulations of inositol phosphates and cyclic AMP in brain slices: synergistic interactions of histamine and 2-chloroadenosine. Eur J Pharmacol. 1986 Mar 11;122(1):45–50. doi: 10.1016/0014-2999(86)90156-1. [DOI] [PubMed] [Google Scholar]
- Houslay M. D. 'Crosstalk': a pivotal role for protein kinase C in modulating relationships between signal transduction pathways. Eur J Biochem. 1991 Jan 1;195(1):9–27. doi: 10.1111/j.1432-1033.1991.tb15671.x. [DOI] [PubMed] [Google Scholar]
- Hsu W. H., Xiang H. D., Rajan A. S., Boyd A. E., 3rd Activation of alpha 2-adrenergic receptors decreases Ca2+ influx to inhibit insulin secretion in a hamster beta-cell line: an action mediated by a guanosine triphosphate-binding protein. Endocrinology. 1991 Feb;128(2):958–964. doi: 10.1210/endo-128-2-958. [DOI] [PubMed] [Google Scholar]
- Hsu W. H., Xiang H. D., Rajan A. S., Kunze D. L., Boyd A. E., 3rd Somatostatin inhibits insulin secretion by a G-protein-mediated decrease in Ca2+ entry through voltage-dependent Ca2+ channels in the beta cell. J Biol Chem. 1991 Jan 15;266(2):837–843. [PubMed] [Google Scholar]
- Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall D. A., Hill S. J. Adenosine inhibition of histamine-stimulated inositol phospholipid hydrolysis in mouse cerebral cortex. J Neurochem. 1988 Feb;50(2):497–502. doi: 10.1111/j.1471-4159.1988.tb02939.x. [DOI] [PubMed] [Google Scholar]
- Li G. D., Regazzi R., Ullrich S., Pralong W. F., Wollheim C. B. Potentiation of stimulus-induced insulin secretion in protein kinase C-deficient RINm5F cells. Biochem J. 1990 Dec 15;272(3):637–645. doi: 10.1042/bj2720637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J. Structure and function of A1 adenosine receptors. FASEB J. 1991 Sep;5(12):2668–2676. doi: 10.1096/fasebj.5.12.1916091. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Schwabe U., Cristalli G., Vittori S., Grifantini M. 2-Chloro-N6-cyclopentyladenosine: a highly selective agonist at A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1988 Jun;337(6):687–689. doi: 10.1007/BF00175797. [DOI] [PubMed] [Google Scholar]
- Morgan N. G., Rumford G. M., Montague W. Studies on the role of inositol trisphosphate in the regulation of insulin secretion from isolated rat islets of Langerhans. Biochem J. 1985 Jun 15;228(3):713–718. doi: 10.1042/bj2280713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Arkhammar P., Rorsman P., Berggren P. O. Suppression of insulin release by galanin and somatostatin is mediated by a G-protein. An effect involving repolarization and reduction in cytoplasmic free Ca2+ concentration. J Biol Chem. 1989 Jan 15;264(2):973–980. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Okajima F., Sato K., Sho K., Kondo Y. Stimulation of adenosine receptor enhances alpha 1-adrenergic receptor-mediated activation of phospholipase C and Ca2+ mobilization in a pertussis toxin-sensitive manner in FRTL-5 thyroid cells. FEBS Lett. 1989 May 8;248(1-2):145–149. doi: 10.1016/0014-5793(89)80450-8. [DOI] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F. M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987 Oct;67(4):1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Bokvist K., Ammälä C., Arkhammar P., Berggren P. O., Larsson O., Wåhlander K. Activation by adrenaline of a low-conductance G protein-dependent K+ channel in mouse pancreatic B cells. Nature. 1991 Jan 3;349(6304):77–79. doi: 10.1038/349077a0. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Hescheler J., Offermanns S., Spicher K., Hinsch K. D., Klinz F. J., Codina J., Birnbaumer L., Gausepohl H., Frank R. Involvement of pertussis toxin-sensitive G-proteins in the hormonal inhibition of dihydropyridine-sensitive Ca2+ currents in an insulin-secreting cell line (RINm5F). J Biol Chem. 1991 Sep 25;266(27):18025–18033. [PubMed] [Google Scholar]
- Sharp G. W., Le Marchand-Brustel Y., Yada T., Russo L. L., Bliss C. R., Cormont M., Monge L., Van Obberghen E. Galanin can inhibit insulin release by a mechanism other than membrane hyperpolarization or inhibition of adenylate cyclase. J Biol Chem. 1989 May 5;264(13):7302–7309. [PubMed] [Google Scholar]
- Sharp G. W. The adenylate cyclase-cyclic AMP system in islets of Langerhans and its role in the control of insulin release. Diabetologia. 1979 May;16(5):287–296. doi: 10.1007/BF01223617. [DOI] [PubMed] [Google Scholar]
- Sho K. M., Okajima F., Abdul Majid M., Kondo Y. Reciprocal modulation of thyrotropin actions by P1-purinergic agonists in FRTL-5 thyroid cells. Inhibition of cAMP pathway and stimulation of phospholipase C-Ca2+ pathway. J Biol Chem. 1991 Jul 5;266(19):12180–12184. [PubMed] [Google Scholar]
- Stiles G. L. Adenosine receptors. J Biol Chem. 1992 Apr 5;267(10):6451–6454. [PubMed] [Google Scholar]
- Taylor S. J., Chae H. Z., Rhee S. G., Exton J. H. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991 Apr 11;350(6318):516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- Ullrich S., Wollheim C. B. GTP-dependent inhibition of insulin secretion by epinephrine in permeabilized RINm5F cells. Lack of correlation between insulin secretion and cyclic AMP levels. J Biol Chem. 1988 Jun 25;263(18):8615–8620. [PubMed] [Google Scholar]
- Ullrich S., Wollheim C. B. Islet cyclic AMP levels are not lowered during alpha 2-adrenergic inhibition of insulin release. J Biol Chem. 1984 Apr 10;259(7):4111–4115. [PubMed] [Google Scholar]
- White T. E., Dickenson J. M., Alexander S. P., Hill S. J. Adenosine A1-receptor stimulation of inositol phospholipid hydrolysis and calcium mobilisation in DDT1 MF-2 cells. Br J Pharmacol. 1992 May;106(1):215–221. doi: 10.1111/j.1476-5381.1992.tb14317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim C. B., Biden T. J. Second messenger function of inositol 1,4,5-trisphosphate. Early changes in inositol phosphates, cytosolic Ca2+, and insulin release in carbamylcholine-stimulated RINm5F cells. J Biol Chem. 1986 Jun 25;261(18):8314–8319. [PubMed] [Google Scholar]
- Wollheim C. B., Biden T. J. Signal transduction in insulin secretion: comparison between fuel stimuli and receptor agonists. Ann N Y Acad Sci. 1986;488:317–333. doi: 10.1111/j.1749-6632.1986.tb46568.x. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- Zawalich W. S. Synergistic impact of cholecystokinin and gastric inhibitory polypeptide on the regulation of insulin secretion. Metabolism. 1988 Aug;37(8):778–781. doi: 10.1016/0026-0495(88)90014-5. [DOI] [PubMed] [Google Scholar]