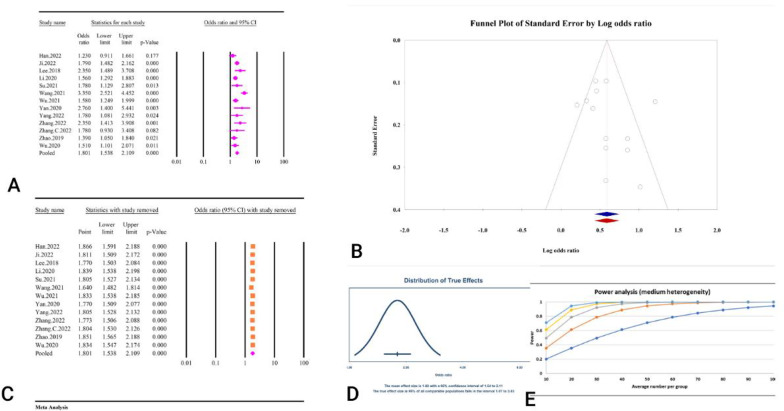
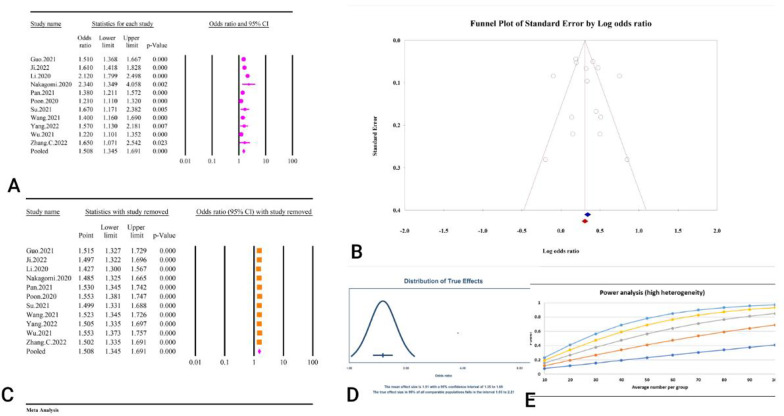
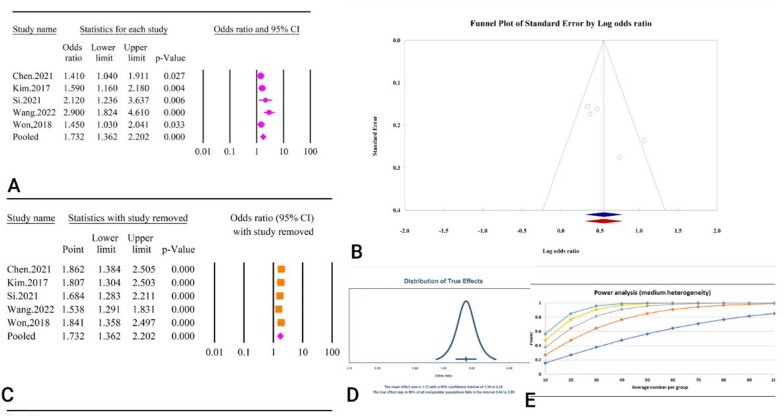
Abstract
Objective
The present umbrella review aims to collate and summarize the findings from previous meta-analyses on the Triglyceride and Glucose (TyG) Index, providing insights to clinicians, researchers, and policymakers regarding the usefulness of this biomarker in various clinical settings.
Methods
A comprehensive search was conducted in PubMed, Scopus, and Web of Science up to April 14, 2024, without language restrictions. The AMSTAR2 checklist assessed the methodological quality of the included meta-analyses. Statistical analyses were performed using Comprehensive Meta-Analysis (CMA) software.
Results
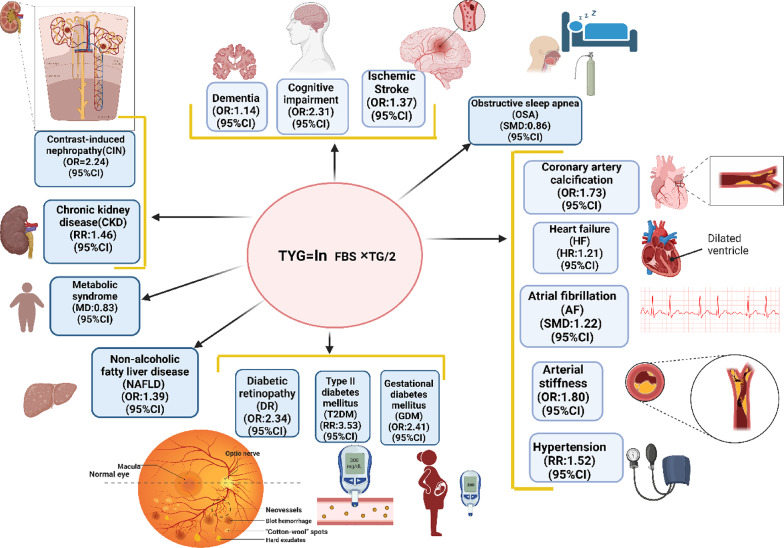
A total of 32 studies were finally included. The results revealed significant associations between the TyG index and various health outcomes. For kidney outcomes, a high TyG index was significantly associated with an increased risk of contrast-induced nephropathy (CIN) (OR = 2.24, 95% CI: 1.82–2.77) and chronic kidney disease (CKD) (RR = 1.46, 95% CI: 1.32–1.63). High TyG index was significantly associated with an increased risk of type 2 diabetes mellitus (T2DM) (RR = 3.53, 95% CI: 2.74–4.54), gestational diabetes mellitus (GDM) (OR = 2.41, 95% CI: 1.48–3.91), and diabetic retinopathy (DR) (OR = 2.34, 95% CI: 1.31–4.19). Regarding metabolic diseases, the TyG index was significantly higher in patients with obstructive sleep apnea (OSA) (SMD = 0.86, 95% CI: 0.57–1.15), metabolic syndrome (MD = 0.83, 95% CI: 0.74–0.93), and non-alcoholic fatty liver disease (NAFLD) (OR = 2.36, 95% CI: 1.88–2.97) compared to those without these conditions. In cerebrovascular diseases, a higher TyG index was significantly associated with an increased risk of dementia (OR = 1.14, 95% CI: 1.12–1.16), cognitive impairment (OR = 2.31, 95% CI: 1.38–3.86), and ischemic stroke (OR = 1.37, 95% CI: 1.22–1.54). For cardiovascular outcomes, the TyG index showed significant associations with an increased risk of heart failure (HF) (HR = 1.21, 95% CI: 1.12–1.30), atrial fibrillation (AF) (SMD = 1.22, 95% CI: 0.57–1.87), and hypertension (HTN) (RR = 1.52, 95% CI: 1.25–1.85).
Conclusion
The TyG index is a promising biomarker for screening and predicting various medical conditions, particularly those related to insulin resistance and metabolic disorders. However, the heterogeneity and methodological quality of the included studies suggest the need for further high-quality research to confirm these findings and refine the clinical utility of the TyG index.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02392-y.
Keywords: Triglyceride and glucose (TyG) index, TyG, Meta-analysis, Umbrella review, Cardiovascular outcomes, Insulin resistance, Clinical significance
Introduction
In the field of medical diagnostics, finding affordable yet valid biomarkers is crucial for efficient disease detection and treatment [1, 2]. One such biomarker that has gained significant attention is the Triglyceride and Glucose (TyG) Index due to its potential as a cost-effective diagnostic tool for various medical conditions [3, 4]. The TyG Index is derived from fasting triglyceride and glucose levels, and is known for its simplicity of calculation [5]. It is believed to reflect underlying insulin resistance, which is a key factor in many metabolic disorders [6].
The TyG Index holds promise as a surrogate marker for insulin resistance and metabolic dysregulation. Its calculation— natural logarithm of glucose times triglycerides divided by 2 —renders it a practical and accessible metric in clinical settings [7, 8]. This index underscores the intricate interplay between triglyceride and glucose metabolism, offering insights into metabolic disturbances implicated in various medical conditions [9–12].
So far, numerous original studies have been undertaken to assess the diagnostic effectiveness of the TyG index across diverse medical conditions. This growing body of research has led to the publication of several meta-analyses examining the diagnostic and prognostic potential of the TyG index in conditions such as cardiovascular diseases, neurological disorders, endocrine ailments, and renal conditions [13–16].
While there has been a recent increase in the number of meta-analyses examining the diagnostic ability of the TyG index, there remains substantial heterogeneity and controversies among these studies. Moreover, the reported findings from these meta-analyses may vary in epidemiological robustness due to differences in their quality. Consequently, there is a need for a comprehensive umbrella review to consolidate the outcomes of previous meta-analyses, evaluate their quality, delineate current findings, and provide current andup-to-dateevidence-based insights into this biomarker.
This umbrella review aims to collate and summarize the findings from previous meta-analyses on the TyG Index. The goal is to provide meaningful insights to clinicians, researchers, and policymakers regarding the usefulness of this biomarker in various clinical settings.
Trend of TyG index research
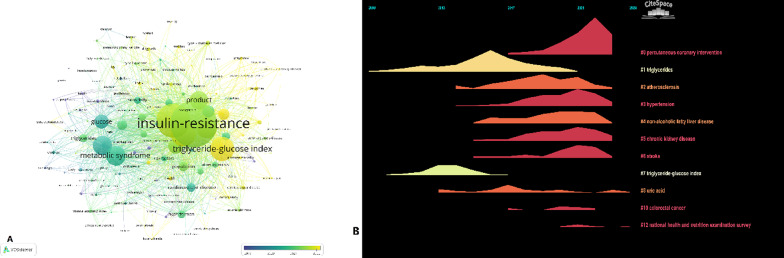
The results of the bibliometric analysis revealed the increasing interest and growing research focus on the TyG index. As shown in Fig. 1A, the overlay visualization indicated that among metabolic-related keywords, the TyG index is a relatively new term (shown in yellow), which is closely related to the keyword “insulin resistance”. Furthermore, the timeline analysis demonstrated that most research topics peaked after 2021, with Percutaneous coronary intervention (PCI), hypertension (HTN), and chronic kidney disease (CKD) being the most prominent trend areas (Fig. 1B).
Fig. 1.
Research trend of triglyceride glucose index. A Overlay visualization B Timeline visualization
Methods
We adhered to the Cochrane Handbook for Systematic Reviews’ guidelines in conducting our umbrella study, which is a methodical investigation encompassing several meta-analyses [17]. The PRISMA standards were utilized when reporting the findings [18]. Using the registration number CRD42024532457, the study protocol was preregistered in PROSPERO.
Search strategy
To uncover meta-analyses exploring the diagnostic and predictive capability of the TyG index across various contexts, two researchers devised an extensive search strategy. This strategy was employed to search three sources (PubMed, Scopus, and Web of Science) until April 14, 2024. The search terms encompassed phrases such as “Triglyceride-glucose index,” “Triacylglycerol glucose index,” “Triglyceride glucose,” “Meta-Analysis,” and “Systematic Review.” Language restrictions were not imposed. Discrepancies were resolved by a third researcher when disagreements arose. EndNote X20 was utilized for the organization and management of identified studies. The specific search strategies for each database are delineated in Table S1. To ensure the accuracy of the search methodology, the assistance of two information specialists was enlisted. Furthermore, a manual review of citations from pertinent studies was conducted.
Study selection and eligibility criteria
Two researchers independently conducted the study selection process. In cases of disagreement, resolutions were reached through correspondence involving a third researcher. Meta-analyses included in the study were required to meet specific criteria: they had to assess the TyG index as an indicator for the target disease and focus on a particular underlying condition within the study population. Original research, case reports, case series, commentaries, editorials, and narrative reviews were excluded from consideration.
Quality assessment
The AMSTAR2 checklist was implemented to determine the methodological quality of the meta-analyses. Two impartial reviewers appraised each study using the 16 questions on this checklist, selecting “yes,” “no,” or “partial yes” for each item. A third researcher was consulted in order to settle any disputes. The meta-analyses were subsequently separated into four groups: high-quality, moderate quality, low-quality, and critically low-quality [19].
Data extraction
The data extracted from the meta-analyses encompassed various details such as the primary author’s name, publication year, journal, number of original studies included, total sample size, effect size with a 95% confidence interval of the original included studies, and the checklist utilized for assessing the original studies’ quality.
To ensure the extraction comprehensiveness, the primary and the corresponding authors were contacted to address any missing information. Two researchers collaborated to gather data and consulted a third researcher to resolve any disagreements.
Statistical analyses
Comprehensive meta-analysis (CMA) software version 4 was utilized for the analysis of the current umbrella review. Furthermore, to construct summary receiver operating characteristic (SROC) charts for the TyG index across different medical conditions, STATA version 18 was employed. The results of original studies within each meta-analysis were obtained to conduct the analysis. In instances where multiple meta-analyses were available for a single outcome, original studies were identified from each meta-analysis. Following the elimination of duplicates, our meta-analysis was carried out using the results of the remaining original studies.
Between-study heterogeneity was assessed using both I2 statistics and Cochrane’s Q test. Significance in heterogeneity was determined if I2 exceeded 50% and the P-value was found to be less than 0.1. In cases where significant heterogeneity was observed, the random effects model was applied, while in instances of non-significant heterogeneity, the fixed effects model was employed.
For every outcome, power tests were performed to check the suitability of sample sizes. Additionally, CMA software was used to establish prediction intervals (PI) for each result. The epidemiological strength of the results was evaluated using GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) assessment conducted with GRADE profiler software.
To assess publication bias, visual inspection of funnel plots was conducted, along with Egger’s regression test, Begg’s regression test, and trim and fill analysis [20, 21].
Results
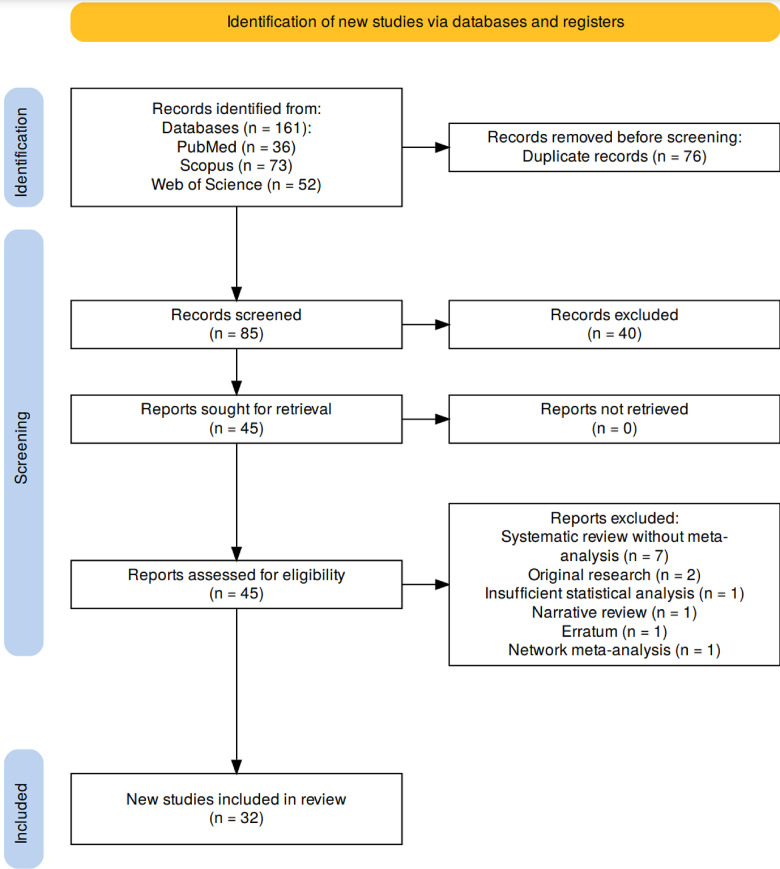
In the first stage of the investigation, a combined total of 161 research articles were identified, sourced from PubMed (36), Scopus (73), and Web of Science (52). After removing 76 duplicate articles and reviewing the titles and abstracts of the remaining papers, 45 studies advanced to a comprehensive evaluation of their full texts. Thirteen studies were excluded at this phase of which four were from China [12, 22–24], two were from the USA [25, 26] and one study was for each of Indonesia [27], Mexico [28], Brazil [29], Iran [30], Egypt [31], Sweden [32], and Poland [33]). Ultimately, following this thorough assessment, 32 meta-analyses met the criteria. A visual representation of the study selection process is presented in Fig. 2.
Fig. 2.
Study selection process
Characteristics of included studies
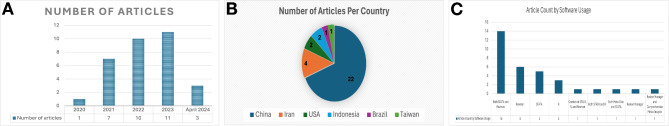
Table 1 provides a comprehensive summary of the 32 studies included in our meta-umbrella analysis, covering publications from 2020 to 2024 (Fig. 3A). The majority of the studies originated from China (22 articles) [13, 15, 16, 34–52] followed by Iran (4 articles) [53–56], the USA (2 articles) [57, 58], Indonesia (2 articles) [27, 59] and one each from Brazil [60] and Taiwan [61](Fig. 3B). These studies encompassed a wide range of sample sizes, from 1,610 to a substantial 11,944,688 participants, and varied in scope, analyzing between 4 and 41 individual studies.
Table 1.
Characteristics of included studies
| First author, Year of publication | Published journal | Searched databased | Date of search | Outcome | Number of included studies | Total sample size | Model of analysis | Quality of assessment checklist | Registration ID | Statistical software | Publication bias assessment | Country | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yan, 2022 [37] | Cardiovascular Diabetology | PubMed, EMBASE, Cochrane Library, Web of Science and Medline databases | From inception up to March 2022 | Cerebrovascular disease | 29 | 11,944,688 | Random‒effects model | NOS | NR | Revman | Visual inspection of funnel plots, Egger’s test, and trim-and-fill test | China | Critically low |
| Chang, 2023 [61] | Frontiers in Endocrinology | Medline, Cochrane, and Embase | From inception up to August 3, 2023 | Contrast induced nephropathy | 5 | 3,518 | NR | QUADAS-2 | PROSPERO (CRD42023452257) | STATA and Revman | Deeks’ funnel plot asymmetry test | Taiwan | Low |
| Sajdeya, 2022 [57] | Current Problems in Cardiology | PubMed, Embase, and Web of Science databases | From inception up to April 30, 2022 | Arterial Stiffness | 9 | 37,780 | Random‒effects model | NOS | NR | Revman | NA (not assessed) | USA | Critically low |
| Zhou, 2023 [38] | Frontiers in Endocrinology | PubMed, Embase, Web of Science, and China National Knowledge Infrastructure (CNKI) | From inception up to June 2023 | Diabetic Retinopathy | 10 | 13,716 | Random effects model for heterogenic and fixed effect model for homogenic results | NOS and AHRQ |
PROSPERO (CRD42023432747) |
STATA, R, and Revman | Visual inspection of funnel plots, cut-and-fill methods and Egger’s regression test | China | High |
| Khalaj, 2023 [53] | Cardiovascular Diabetology | PubMed, Embase, Scopus, and Web of Science | From inception up to June 16, 2023 | Heart failure | 30 | 772,809 | Random‒effects model | NOS |
PROSPERO (CRD42023437470) |
R | NA | Iran | High |
| Beran, 2022 [58] | Journal of Clinical Medicine | PubMed, Embase, and Web of Science | From inception up to 25 March 2022 | Non-alcoholic fatty liver disease | 17 | 121,975 | Random‒effects model | NOS | NR | Review Manager and Comprehensive Meta-Analysis | NA | USA | High |
| Ling, 2023 [39] | Frontiers Endocrinology | Cochrane Library, PubMed, and Embase | From inception up to July 18, 2022 | Non-alcoholic fatty liver disease | 12 | 105,365 | Random‒effects model | JBI and NOS |
PROSPERO (CRD42022347813) |
STATA and Revman | Visual inspection of funnel plots, Egger’s test, and Begg’s test | China | Moderate |
| Behnoush, 2024 [54] | Lipids in Health and Disease | PubMed, Scopus, the Web of Science, and Embase | From inception up to September 6, 2023 | Obstructive sleep apnea | 10 | 16,726 | Random‒effects model | NOS |
PROSPERO (CRD42023460518) |
R | Visual inspection of funnel plots, Egger’s test, and Begg’s test | Iran | Low |
| Pranata, 2021 [27] | Endocrine | PubMed, Scopus, and Embase | From inception up to February 9, 2021 | Type 2 diabetes mellitus | 14 | 270,229 | Random‒effects model | NOS | NR | STATA | Visual inspection of funnel plots, Egger’s test and trim and fill analysis | Indonesia | High |
| Silva, 2020 [60] | Primary Care Diabetes | PubMed, Cochrane, Scopus, and Lilacs | From inception up to October 1, 2019. | Type 2 diabetes mellitus | 13 | 70,380 | Random‒effects model | NOS |
PROSPERO (CRD42018114496) |
Revman | Visual inspection of funnel plots | Brazil | Moderate |
| Nabipoorashrafi, 2022 [55] | Nutrition, Metabolism & Cardiovascular Diseases | PubMed, Scopus, and Web of Science | From inception up to February 2022 | Metabolic syndrome | 13 | 49,325 | Random effects model for heterogenic and fixed effect model for homogenic results | QUADAS-2 |
PROSPERO (CRD42022316209) |
STATA and Revman | Visual inspection of funnel plots, Egger’s test and Deeks’ funnel plot asymmetry test | Iran | Low |
| Song, 2022 [40] | Gynecological Endocrinology | PubMed, Embase, China National Knowledge Infrastructure, and Wan Fang | From inception up to January 16, 2021 | Gestational Diabetes mellitus | 5 | 382,213 | Random‒effects model | NOS | NR | STATA and Revman | Visual inspection of funnel plots, and Egger’s test | China | High |
| Liu, 2021 [41] | Endocrine Connections | PubMed, Web of Science, Cochrane Library, and Embase | From inception up to March 1, 2021 | Gestational Diabetes mellitus | 4 | 1,610 | Random‒effects model | NOS | NR | STATA and Revman | Visual inspection of funnel plots, and Egger’s test | China | High |
| Azarboo, 2024 [56] | European Journal of Medical Research | PubMed, Embase, Scopus, and Web of Science | From inception up to September 2023 | Atrial fibrillation | 9 | 6,171 | Random‒effects model | NOS |
PROSPERO (CRD42023456875) |
R | Visual inspection of funnel plots, Egger’s test and trim and fill analysis | Iran | Low |
| Sun, 2024 [42] | ESC Heart Failure | Embase, PubMed, Cochrane Library, and Web of Science | From inception up to March 3, 2023 | Post percutaneous coronary intervention major adverse cardiovascular events | 9 | 10,164 | Random‒effects model | NOS |
PROSPERO (CRD42023387411) |
Revman | NA | China | Low |
| Wang, 2023 [43] | Frontiers in Aging Neuroscience | PubMed, Cochrane Library, and Embase | From inception up to February 2023 | Cognitive impairment and dementia | 10 | 8,443,279 | Random‒effects model | JBI and NOS |
PROSPERO (CRD42023388028) |
STATA and Revman | NA | China | High |
| Wang, 2021 [44] | Frontiers in Cardiovascular Medicine | PubMed, Embase, Web of Science, Wan fang data, and Chinese National Knowledge Infrastructure | From inception up to March 10, 2021 | Hypertension | 8 | 200,044 | Random‒effects model | NOS | NR | STATA and Revman | Visual inspection of funnel plots, Egger’s test, and Begg’s test | China | High |
| Liu, 2023 [45] | Cardiovascular Diabetology | Embase, Cochrane Library, and PubMed | From inception up to September 9, 2022 | Arterial stiffness and coronary artery calcification | 26 | 87,307 | Random‒effects model | JBI and NOS |
PROSPERO (CRD42022360981) |
STATA and Revman | Visual inspection of funnel plots, Egger’s test, and Begg’s test | China | High |
| Zhong, 2023 [46] | Medicine | PubMed, Embase, and Scopus | From inception up to July 31, 2022 | Arterial stiffness | 13 | 48,332 | Random‒effects model | NOS |
PROSPERO (CRD42022330963) |
STATA and Revman | Visual inspection of funnel plots, and Egger’s test | China | Low |
| Yang, 2023 [15] | Cardiovascular Diabetology | Cochrane, Embase, Medline, Web of Science, PubMed | From inception up to April 4, 2022 | Ischemic stroke | 18 | 592,635 | Random‒effects model | NOS and AHRQ |
INPLASY (INPLASY2022110145) |
STATA | Visual inspection of funnel plots, clip-and-complement method, Egger’s test, and Begg’s test | China | Low |
| Liao, 2022 [47] | Frontiers in Neurology | PubMed, Cochrane Library, and Embase | From inception up to December 16, 2021 | Stroke | 8 | 5,804,215 | Random‒effects model | NOS | NR | STATA and Revman | Visual inspection of funnel plots | China | High |
| Feng, 2022 [48] | Hormone and Metabolic Research | PubMed, Embase, Cochrane Library and Web of science. | From inception up to October 26, 2021 | Stroke | 11 | 5,721,077 | Random effects model for heterogenic and fixed effect model for homogenic results | NOS | NR | STATA and Revman | Egger’s test, and Begg’s test | China | High |
| Ma, 2022 [49] | Hormone and Metabolic Research | PubMed, Embase, Web of Science, Wan fang, and China National Knowledge Infrastructure | From the inception up to February 20, 2022 | Stroke | 6 | 34,076 | Random‒effects model | NOS | NR | STATA and Revman | Visual inspection of funnel plots and Egger’s test | China | High |
| Ding, 2021 [50] | Cardiovascular Diabetology | PubMed and Embase | From the inception up to January 5, 2021 | Cardiovascular diseases | 8 | 5,731,294 | Random‒effects model | NOS | NR | STATA and Revman | Visual inspection of funnel plots and Egger’s test | China | Low |
| Liang, 2023 [13] | Cardiovascular Diabetology | PubMed, Embase, The Cochrane Library, and Web of Science | From the inception up to May 1, 2023 | Adverse events in patients with acute coronary syndrome | 41 | 65,974 | Random effects model for heterogenic and fixed effect model for homogenic results | NOS |
PROSPERO (CRD42023422917) |
Revman | Visual inspection of funnel plots | China | Low |
| Ren, 2023 [16] | Nutrition, Metabolism and Cardiovascular Diseases | PubMed and Web of Science | From inception to March 2023 | Chronic kidney diseases | 13 | 80,777 | NR | NOS | NR | STATA | Egger’s test, and Begg’s test | China | Critically low |
| Wang, 2022 [51] | Nutrients | PubMed, Medline, Web of Science, Embase and the Cochrane Library | From inception to April 23,2022 | Metabolic dysfunction-associated steatotic liver disease | 20 | 165,725 | Random‒effects model | QUADAS-2 | NR | Meta-Disc and STATA | Deeks’ funnel plot asymmetry test | China | High |
| Li, 2021 [52] | Hormone and Metabolic Research | PubMed, Embase, and Web of Science | From inception up to January 18, 2021 | Adverse events in patients with acute coronary syndrome | 8 | 19,611 | Random‒effects model | NOS | NR | STATA and Revman | Visual inspection of funnel plots and Egger’s test | China | High |
| Akbar, 2021 [59] | Nutrition, Metabolism and Cardiovascular Diseases | PubMed, Scopus, and Embase | From inception up to February 7, 2021 | Adverse events in patients with acute coronary syndrome | 4 | 13,684 | Random‒effects model | NOS |
PROSPERO (CRD42021235765) |
STATA | Funnel plots and Egger’s test | Indonesia | High |
| Xu, 2023 [36] | Frontiers in Cardiovascular Medicine | CNKI, VIP, Wan Fang, CBM, PubMed, Embase, Web of Science, and Cochrane Library | From inception up to May 10, 2023 | Hypertension | 22 | 668,486 | Random effects model for heterogenic and fixed effect model for homogenic results | AHRQ and NOS |
PROSPERO (CRD42023425836) |
STATA and R | Visual inspection of funnel plots, Egger’s test, Begg’s test and trim and fill methods | China | Low |
| Luo, 2021 [35] | Frontiers in Cardiovascular Medicine | Scopus, PubMed, Embase, and Web of Science | From inception up to October 5, 2021 | Adverse events in patients with acute coronary syndrome | 12 | 28,795 | Random effects model | NOS |
PROSPERO (CRD42021228521) |
STATA | Egger’s test, and Begg’s test | China | Low |
| Liu, 2022 [34] | Cardiovascular Diabetology | PubMed, Cochrane Library and Embase | From inception to April 16, 2022 | Cardiovascular diseases | 12 | 6,354,990 | Random effects model | NOS |
PROSPERO (CRD42022296235) |
Revman | Visual inspection of funnel plots | China | Low |
AHQR: Agency for Healthcare Research and Quality, JBI: Joanna Briggs Institute, NA: Not assessed, NOS: Newcastle-Ottawa scale Studies, NR: Not reported, QUADS: Quality Assessment of Diagnostic Accuracy
Fig. 3.
Summary of study characteristics. A Distribution of studies by country: Annual publication trends of articles. C Usage of statistical software and analysis tools across the studies
Included studies assessed the TyG index using the following calculation: Ln [fasting triglycerides (mg/dl) × fasting glucose (mg/dl)/2]. The outcomes of the studies included in our meta-umbrella analysis covered a diverse range of medical conditions. Four studies focused on adverse events in patients with acute coronary syndrome (ACS) [13, 35, 52, 59], while arterial stiffness was analyzed in three studies [45, 46, 57]. Hypertension (HTN) and stroke were the subjects of two [36, 44] and three studies [47–49], respectively. Non-alcoholic fatty liver disease (NAFLD) [39, 58], type 2 diabetes mellitus [27, 60], and gestational diabetes mellitus [40, 41] were each addressed in two studies. Cardiovascular diseases were examined in two other studies [34, 50]. The research scope also included single studies investigating cerebrovascular disease [37], contrast induced nephropathy (CIN) [61], diabetic retinopathy (DR) [38], heart failure (HF) [53], obstructive sleep apnea (OSA) [54], metabolic syndrome [55], atrial fibrillation (AF) [56], post percutaneous coronary intervention (PCI) major adverse cardiovascular events [42], cognitive impairment and dementia [43], and coronary artery calcification [45], ischemic stroke [15], chronic kidney disease (CKD) [16], and metabolic dysfunction-associated steatosis liver disease (MAFLD) [51].
Additionally, protocol registration details revealed that 17 studies had their protocols registered in PROSPERO [13, 34–36, 38, 39, 42, 43, 45, 46, 53–56, 59–61], one in INPLASY [15], and 14 were not registered [16, 27, 37, 40, 41, 44, 47–52, 57, 58].
In our included studies, a variety of statistical tools and review management software were employed to ensure robust data analysis. Fourteen studies utilized both STATA and Revman [39–41, 43–50, 52, 55, 61], six used Revman alone [13, 34, 37, 42, 57, 60], and another five employed STATA independently [15, 16, 27, 35, 59]. Three studies chose R software [53, 54, 56] (Fig. 3C).
Quality assessments were conducted using several checklists, with the Newcastle-Ottawa Scale (NOS) being the most prevalent, used in 29 articles [13, 15, 16, 27, 34–50, 52–54, 56–60]. Other methods included the Quality Assessment of Diagnostic Accuracy checklist (QUADS) [51, 55, 61], the Agency for Healthcare Research and Quality standards (AHQR) [15, 36, 38], and the Joanna Briggs Institute (JBI) checklist [39, 43, 45].
Search strategies varied among the studies. PubMed/Medline was the most frequently accessed database, with searches conducted in 32 studies [13, 15, 16, 27, 34–61]. EMBASE followed closely, being searched in 29 studies [13, 15, 27, 34–54, 56–59, 61]. The Cochrane Library was utilized in 15 studies [13, 15, 34, 36, 37, 39, 41–43, 45, 47, 48, 51, 60, 61], while Web of Science was searched in 20 studies [13, 15, 16, 35–38, 41, 42, 44, 48, 49, 51–58]. Scopus was used for literature searches in 9 studies [27, 35, 46, 53–56, 59, 60], and more region-specific databases like China National Knowledge Infrastructure (CNKI) [36, 38, 40, 44, 49] and Wan Fang [36, 40, 44, 49] were searched in 5 and 4 studies, respectively. Less commonly, Lilacs [60], VIP [36], and CBM [36] were each searched in one study.
Quality assessment
Among the included studies, 15 were assessed as high quality [27, 38, 40, 41, 43–45, 47–49, 51–53, 58, 59], two were of moderate quality [39, 60], and 12 were determined to have low quality [13, 15, 34–36, 42, 46, 50, 54–56, 61]. Furthermore, the AMSTAR2 evaluation determined that three of the meta-analyses had critically low methodological quality [16, 37, 57] (Table 1; Fig. 4).
Fig. 4.
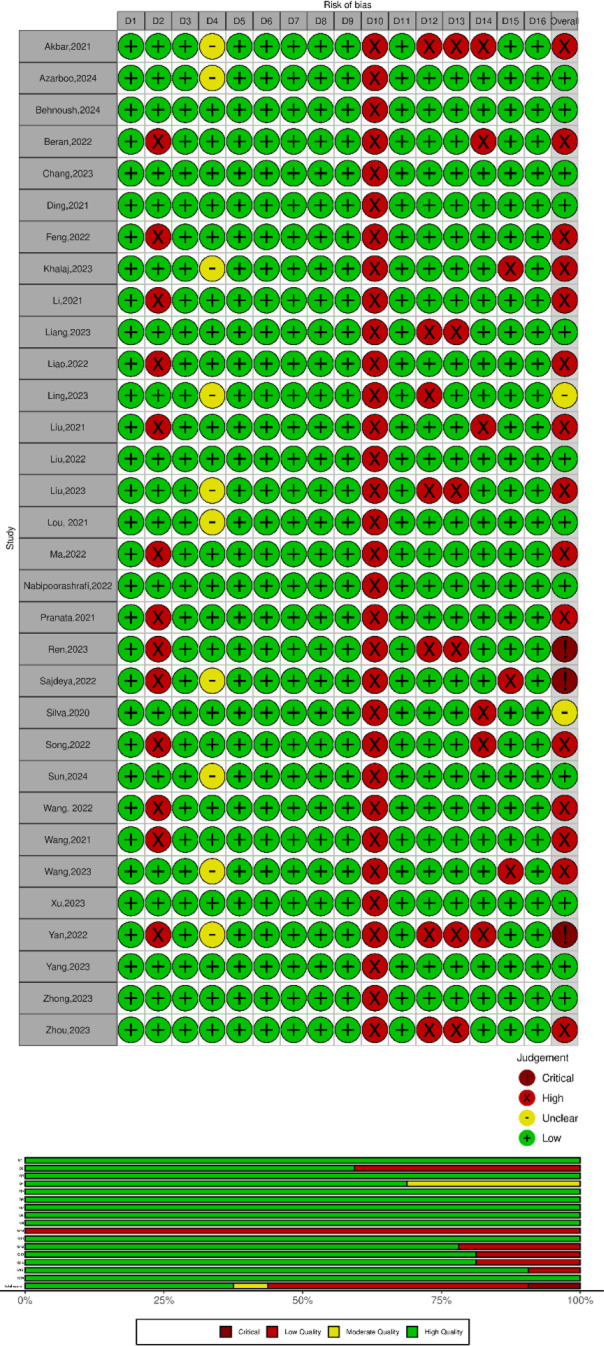
Quality assessment of included studies based on AMSTAR 2 appraisal tool
Results of meta-analysis
TyG index and kidney outcomes
The correlation between TyG index and CIN
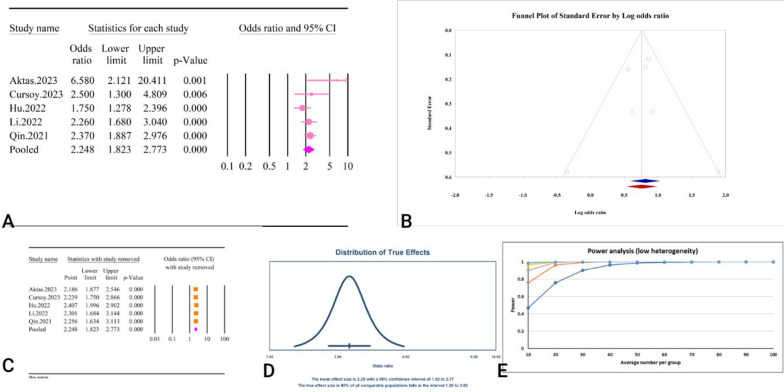
A total of five studies, comprising a combined sample size of 3518 individuals, investigated the relationship between the TyG index and CIN following coronary interventions (Table 2). The analysis revealed that a high TyG index was significantly associated with CIN (OR = 2.24, 95%CI:1.82–2.77, P < 0.01) (Fig. 5A). The studies demonstrated low heterogeneity (I2 = 35.42%, P = 0.18), and no significant publication bias was detected based on Egger’s and Begg’s tests (P = 0.33 and P = 0.80, respectively). Additionally, the trim and fill analysis, which included two imputed studies, adjusted the OR to 2.13 (95% CI: 1.70–2.67) (Fig. 5B). The results of the sensitivity analysis indicated that the total effect size remained stable and did not show any significant change after the sequential deletion of each study (Fig. 5C). Moreover, the prediction interval for the relationship between TyG and CIN ranged from 1.29 to 3.92(Fig. 5D). The results of the power analysis indicated high statistical power (1–β = 1), suggesting the sample size was adequate (Fig. 5E). The epidemiological strength of the association between TyG and CIN was low based on the GRADE criteria (Table 3).
Table 2.
Results of the meta-analyses of the correlation between TyG index and various health outcomes
| Variable | Effect size | Prediction interval | Heterogeneity | Egger’s regression test P value | Begg’s regression test P value | Number of imputes studies in trim and fill analysis | Trim and Fill analysis | Power analysis | ||
|---|---|---|---|---|---|---|---|---|---|---|
| CIN | OR = 2.24, 95% CI: 1.82–2.77, P < 0.01 | 1.29–3.92 | 35.42%, P = 0.18 | 0.33 | 0.80 | 2 | OR = 2.13, 95% CI: 1.70–2.67 | 1−b = 1 | ||
| Diabetic Retinopathy | Categorical | OR = 2.34, 95% CI: 1.31–4.19, P < 0.01 | 0.31-17.82 |
89.70%, P < 0.01 |
P < 0.01 | P < 0.01 | 2 | OR = 1.79, 95% CI: 1.06–3.03 | 1−b = 1 | |
| Continuous | OR = 1.48, 95% CI: 1.11–1.97, P < 0.01 | 0.59–3.72 |
83.46%, P < 0.01 |
0.01 | 0.25 | 2 | OR = 1.33, 95% CI: 1.03–1.71 | 1−b = 1 | ||
| Obstructive sleep apnea | SMD = 0.86, 95% CI: 0.57–1.15, P < 0.01 | -1.89-3.61 | 36.74%, P = 0.20 | 0.36 | 0.29 | 0 | 0.86, 95% CI: 0.57–1.15 | 1−b = 1 | ||
| Gestational diabetes mellitus | OR = 2.41, 95% CI: 1.48–3.91, P < 0.01 | 0.61–9.47 | 59.48%, P = 0.03 | 0.15 | 0.13 | 2 | OR = 1.83, 95% CI: 1.11–3.01 | 1−b = 1 | ||
| Metabolic syndrome | MD = 0.83, 95% CI: 0.74–0.93, P < 0.01 | 0.47–1.20 | 98.18%, P < 0.01 | 0.43 | 0.76 | 0 | MD = 0.83, 95% CI: 0.74–0.93 | 1−b = 1 | ||
| Nonalcoholic fatty liver disease | Categorical | OR = 6.00, 95% CI: 4.12–8.74, P < 0.01 | 1.64–21.98 | 91.80%, P < 0.01 | 0.89 | 0.75 | 0 | OR = 6.00, 95% CI: 4.12–8.73 | 1−b = 1 | |
| continuous | OR = 2.36, 95% CI: 1.88–2.97, P < 0.01 | 0.99–5.68 | 98.13%, P < 0.01 | 0 < 0.01 | 0.24 | 0 | OR = 2.36, 95% CI: 1.88–2.97 | 1−b = 1 | ||
| Chronic kidney disease | RR = 1.46, 95% CI: 1.32–1.63, P < 0.01 | 1.06–2.03 | 50.44%, P = 0.01 | 0 < 0.01 | 0 < 0.01 | 6 | RR = 1.37, 95% CI: 1.24–1.53 | − | ||
| Type 2 diabetes mellitus | Risk ratio | RR = 3.53, 95%CI:2.74–4.54, P < 0.01 | 1.36–9.16 | 83.70%, P < 0.01 | 0.79 | 0.19 | 5 | RR = 2.93, 95% CI: 2.25–3.82 | − | |
| Hazard ratio | HR = 2.60, 95% CI: 2.19–3.09, P < 0.01 | 1.33–5.12 | 79.05%, P < 0.01 | 0 < 0.01 | 0.067 | 8 | 2.09, 95% CI: 1.77–2.46 | − | ||
| Dementia | Categorical | OR = 1. 14, 95% CI: 1.12–1.16, P < 0.01 | LN | 0.00%, P = 0.32 | LN | LN | LN | LN | 1−b = 1 | |
| Continuous | OR = 1. 37, 95% CI: 1.03–1.82, P = 0.03 | LN | 0.00%, P = 0.77 | LN | LN | LN | LN | 1−b = 1 | ||
| Cognitive impairment | Categorical | OR = 2.31, 95% CI: 1.38–3.86, P < 0.01 | 0.37–14.26 | 84.75%, P < 0.01 | < 0.01 | 0.46 | 2 | OR = 1.67, 95% CI: 1.05–2.64 | 1−b = 1 | |
| Continuous | OR = 3.38, 95% CI: 1.67–6.83, P < 0.01 | 0.16–72.58 | 81.56%, P < 0.01 | 0.84 | 0.73 | 0 | OR = 3.38, 95% CI: 1.67–6.83 | 1−b = 1 | ||
| Ischemic stroke | Ischemic stroke association | OR = 1. 37, 95% CI: 1.22–1.54, P < 0.01 | 0.99–1.91 | 74.18%, P < 0.01 | 0.04 | 0.90 | 1 | OR = 1. 35, 95% CI: 1.20–1.52 | 1−b = 1 | |
| Ischemic stroke recurrence | OR = 1. 50, 95% CI: 1.19–1.89, P < 0.01 | 0.75–2.99 | 56.22%, P = 0.05 | 0.02 | 0.08 | 2 | OR = 1. 33, 95% CI: 1.04–1.70, | 1−b = 1 | ||
| Ischemic stroke poor functional outcomes | OR = 1. 36, 95% CI: 1.10–1.69, P < 0.01 | 0.74–2.54 | 71.40%, P < 0.01 | < 0.01 | 0.02 | 2 | OR = 1. 25, 95% CI: 1.01–1.54 | 1−b = 1 | ||
| Ischemic stroke mortality | OR = 1. 40, 95% CI: 1.14–1.71, P < 0.01 | 0.76–2.57 | 70.70%, P < 0.01 | 0.19 | 0.70 | 0 | OR = 1. 40, 95% CI: 1.14–1.71 | 1−b = 1 | ||
| Ischemic stroke neurological worsening | OR = 1. 33, 95% CI: 1.09–1.63, P < 0.01 | LN | 76.57%, P = 0.03 | LN | LN | LN | LN | 1−b = 0.99 | ||
| Heart Failure | Categorical | HR = 1.21, 95% CI: 1.12–1.30, P < 0.01 | 1.04–1.41 | 21.43%, P = 0.26 | 0.29 | 0.76 | 1 | HR = 1.19, 95% CI: 1.08–1.31 | − | |
| Continues | HR = 1.16, 95% CI: 1.11–1.21, P < 0.01 | 0.86–1.57 | 0.64%, P = 0.36 | 0.40 | 1 | 1 | HR = 1.15, 95% CI: 1.09–1.21 | − | ||
| Atrial fibrillation | SMD: 1.22, 95% CI: 0.57–1.87, P < 0.01 | -1.18-3.63 | 98.04%, P < 0.01 | 0.64 | 1 | 0 | SMD: 1.22, 95% CI: 0.57–1.87 | 1−b = 1 | ||
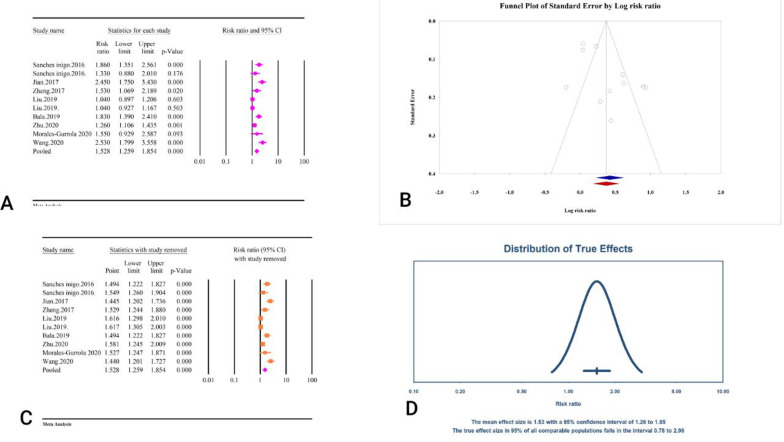
| Hypertension | RR = 1. 52, 95% CI: 1.25–1.85, P < 0.01 | 0.78–2.98 | 85.67%, P < 0.01 | < 0.01 | 0.28 | 1 | RR = 1. 44, 95% CI: 1.20–1.74 | − | ||
| Atrial stiffness | Categorical | OR = 1. 80, 95% CI: 1.53–2.10, P < 0.01 | 1.07–3.03 | 66.26%, P < 0.01 | 0.35 | 0.20 | 0 | OR = 1. 80, 95% CI: 1.53–2.10 | 1−b = 1 | |
| Continuous | OR = 1. 50, 95% CI: 1.34–1.69, P < 0.01 | 1.03–2.21 | 81.66%, P < 0.01 | 0.10 | 0.16 | 4 | OR = 1. 39, 95% CI: 1.23–1.56 | 1−b = 1 | ||
| Coronary artery calcification | Continuous | OR = 1. 73, 95% CI: 1.36–2.20, P < 0.01 | 0.84–3.59 | 50.81%, P = 0.08 | 0.08 | 0.22 | 0 | OR = 1. 73, 95% CI: 1.36–2.20 | 1−b = 1 | |
| Categorical | OR = 1. 65, 95% CI: 1.50–1.82, P < 0.01 | 1.51–1.83 | 0.00%, P = 0.72 | 0.21 | 1 | 0 | OR = 1. 65, 95% CI: 1.50–1.82 | 1−b = 1 | ||
| Post percutaneous coronary intervention events | Post-PCI major cardiovascular outcomes | Categorical | HR = 2.03, 95% CI: 1.64–2.51, P < 0.01 | 1.01–4.11 | 77.09%, P < 0.01 | 0.05 | 0.25 | 0 | HR = 2.03, 95% CI: 1.64–2.51 | − |
| Continuous | HR = 1.81, 95%CI:1.34–2.46, P < 0.01 | 0.58–5.75 | 91.72%, P < 0.01 | 0.02 | 0.02 | 0 | HR = 1.81, 95% CI: 1.34–2.46 | − | ||
| Post-PCI all-cause mortality | Categorical | HR = 1.92, 95% CI: 1.35–2.74, P < 0.01 | 0.01-16638.72 | 50.91%, P = 0.13 | 0.15 | 1 | 2 | HR = 2.18, 95% CI: 0.97–4.87 | − | |
| Continuous | HR = 0.94, 95% CI: 0.28–3.12, P = 0.92 | 0.01-975086.18 | 73.95%, P = 0.02 | 0.09 | 1 | 0 | HR = 0.94, 95% CI: 0.28–3.12 | − | ||
| Post-PCI revascularization | Categorical | HR = 2.61, 95% CI: 1.46–4.65, P < 0.01 | 0.01–2851.01 | 83.56%, P < 0.01 | 0.58 | 1 | 0 | HR = 2.61, 95% CI: 1.46–4.65 | − | |
| Continuous | HR = 2.05, 95% CI: 1.20–3.50, P < 0.01 | 0.01-1552.36 | 89.78%, P < 0.01 | 0.20 | 1 | 0 | HR = 2.05, 95% CI: 1.20–3.50 | − | ||
| Post-PCI non-fetal MI | Categorical | HR = 2.02, 95% CI: 1.31–3.10, P < 0.01 | 1.32–3.10 | 00.00%, P = 0.96 | 0.57 | 0.29 | 0 | HR = 2.02, 95% CI: 1.31–3.10 | − | |
| Continuous | HR = 2.56, 95% CI: 1.49 4.400, P < 0.01 | 0.01-1013.95 | 63.41%, P = 0.06 | 0.12 | 1 | 0 | HR = 2.56, 95% CI: 1.49 4.400 | − | ||
Fig. 5.
The association between TyG index and CIN.A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval E Power analysis
Table 3.
GRADE assessment of the correlation of TyG index with various outcomes
| GRADE assessment | |||||||
|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Quality |
| CIN | |||||||
| 5 | Observational studies | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None |
⊕⊕OO LOW |
| Arterial stiffness | |||||||
| 13 | Observational studies | Serious | Serious | No serious indirectness | No serious imprecision | None |
⊕OOO VERY LOW |
| Diabetic retinopathy | |||||||
| 10 | Observational studies | No serious risk of bias | Serious | No serious indirectness | No serious imprecision |
Reporting bias Strong association |
⊕OOO VERY LOW |
| Heart failure (categorical) | |||||||
| 7 | Observational studies | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None |
⊕⊕OO LOW |
| Non-alcoholic fatty liver disease (categorical) | |||||||
| 12 | Observational studies | No serious risk of bias | Very serious | No serious indirectness | No serious imprecision | Strong association |
⊕OOO VERY LOW |
| Obstructive sleep apnea | |||||||
| 3 | Observational studies | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None |
⊕⊕OO LOW |
| Type 2 diabetes mellitus | |||||||
| 12 | Observational studies | No serious risk of bias | Serious | No serious indirectness | No serious imprecision | Strong association |
⊕⊕OO LOW |
| Metabolic syndrome | |||||||
| 13 | Observational studies | Serious | Serious | No serious indirectness | No serious imprecision | Very strong association |
⊕⊕OO LOW |
| Gestational diabetess mellitus | |||||||
| 6 | Observational studies | No serious risk of bias | Serious | No serious indirectness | No serious imprecision | Strong association |
⊕⊕OO LOW |
| Atrial fibrillation | |||||||
| 6 | Observational studies | No serious risk of bias | Very serious | No serious indirectness | No serious imprecision | Very strong association |
⊕⊕OO LOW |
| Post-PCI major cardiovascular outcomes | |||||||
| 9 | Observational studies | No serious risk of bias | Serious | No serious indirectness | No serious imprecision | Strong association |
⊕⊕OO LOW |
| Post-PCI all-cause mortality | |||||||
| 4 | Observational studies | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None |
⊕⊕OO LOW |
| Post-PCI revascularization | |||||||
| 3 | Observational studies | No serious risk of bias | Very serious | No serious indirectness | No serious imprecision | Strong association |
⊕OOO VERY LOW |
| Post-PCI non-fetal MI | |||||||
| 3 | Observational studies | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | Strong association |
⊕⊕⊕O MODERATE |
| Cognitive impairment | |||||||
| 7 | Observational studies | No serious risk of bias | Very serious | No serious indirectness | No serious imprecision | Strong association |
⊕OOO VERY LOW |
| Hypertension | |||||||
| 7 | Observational studies | No serious risk of bias | Very serious | No serious indirectness | No serious imprecision | None |
⊕OOO VERY LOW |
| Coronary artery calcification | |||||||
| 5 | Observational studies | Serious | No serious inconsistency | No serious indirectness | No serious imprecision | None |
⊕OOO VERY LOW |
| Ischemic stroke | |||||||
| 8 | Observational studies | No serious risk of bias | Serious | No serious indirectness | No serious imprecision | None |
⊕OOO VERY LOW |
| Ischemic stroke recurrence | |||||||
| 5 | Observational studies | No serious risk of bias | Serious | No serious indirectness | No serious imprecision | None |
⊕OOO VERY LOW |
| Ischemic stroke mortality | |||||||
| 6 | Observational studies | No serious risk of bias | Serious | No serious indirectness | No serious imprecision | None |
⊕OOO VERY LOW |
| Ischemic stroke poor functional outcomes | |||||||
| 6 | Observational studies | No serious risk of bias | Serious | No serious indirectness | No serious imprecision | Reporting bias |
⊕OOO VERY LOW |
| Chronic kidney disease | |||||||
| 13 | Observational studies | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | Reporting bias |
⊕OOO VERY LOW |
The correlation between TyG index and CKD
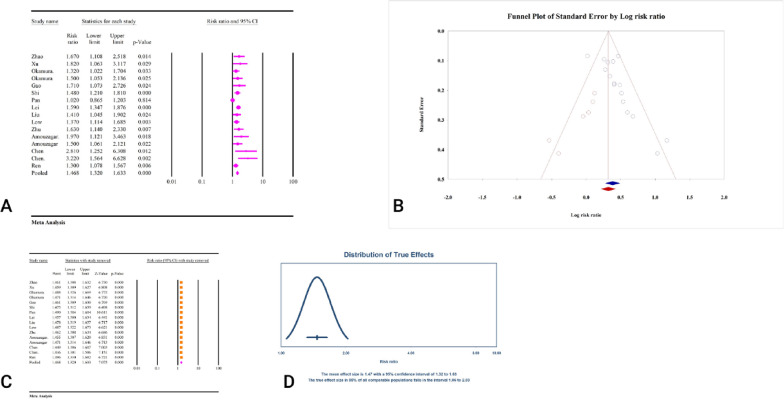
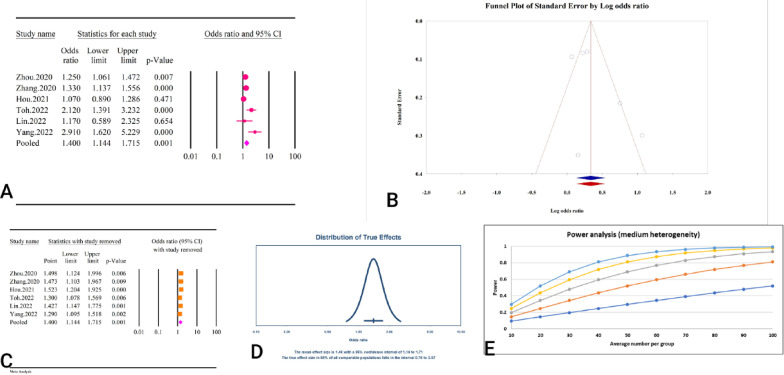
A total of 13 studies, comprising a combined sample size of 70,279 individuals, investigated the relationship between the TyG index and CKD (Table 2). The analysis revealed that a high TyG index was significantly associated with CKD (RR = 1.46, 95%CI: 1.32–1.63, P < 0.01) (Fig. 6A). The studies demonstrated moderate heterogeneity (I2 = 50.44%, P = 0.01), and significant publication bias was detected based on Egger’s and Begg’s tests (P < 0.01). Additionally, the trim and fill analysis, which included six imputed studies, adjusted the RR to 1.37 (95% CI: 1.24–1.53) (Fig. 6B). The results of the sensitivity analysis indicated that the total effect size remained stable and did not show any significant change after the sequential deletion of each study (Fig. 6C). Moreover, the prediction interval for the relationship between TyG and CKD ranged from 1.06 to 2.03 (Fig. 6D). The calculation of power analysis was not possible because the results were reported as relative risk. The epidemiological strength of the association between TyG index and CKD was very low based on GRADE criteria (Table 3).
Fig. 6.
The association between TyG index and CKD. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval
TyG index and diabetes mellitus
The correlation between TyG index and T2DM
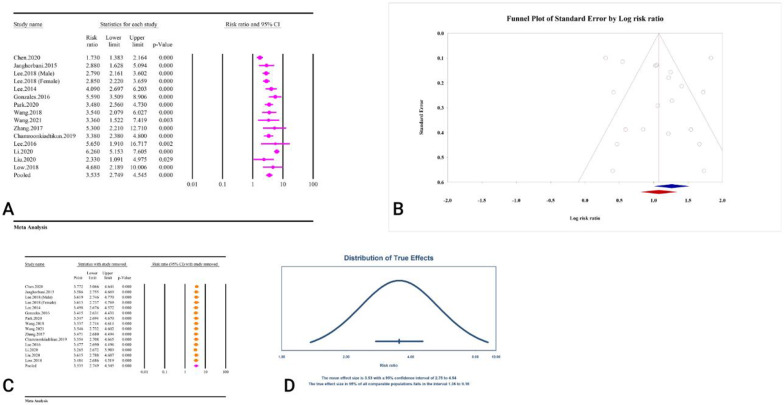
Among the included studies assessing the relationship between the TyG index and T2DM, two statistical measures were reported: risk ratio (RR) and hazard ratio (HR) (Table 2). The TyG index was significantly associated with T2DM when reported as a risk ratio (RR = 3.53, 95% CI: 2.74–4.54, P < 0.01) (Fig. 7A). This association was accompanied by significant heterogeneity (I2 = 83.70%, P < 0.01). No significant publication bias was found based on Egger’s regression test (P = 0.79) and Begg’s regression test (P = 0.19). The results of the trim-and-fill analysis with five imputed studies yielded an RR of 2.93 (95% CI: 2.25–3.82) (Fig. 7B). The sensitivity analysis results indicate that the total effect size remained stable, showing no significant change after sequentially deleting each study (Fig. 7C). The prediction interval was 1.36–9.16 (Fig. 7D). The calculation of power analysis was not possible because the results were reported as relative risks.
Fig. 7.
The association between TyG index and T2DM reported as risk ratio. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
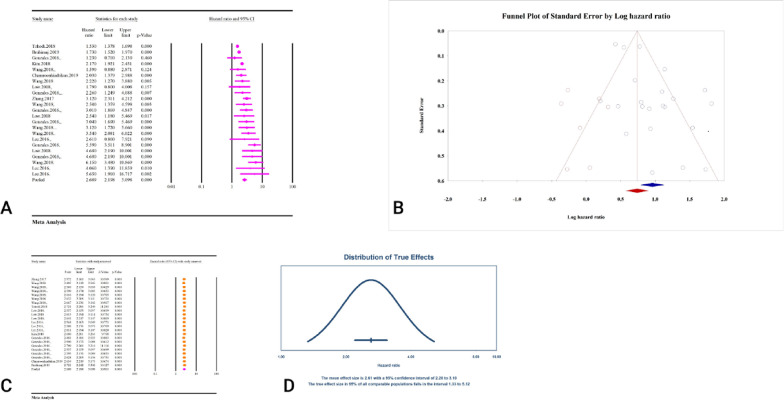
The association between the TyG index and T2DM was significant when calculated as a hazard ratio (HR = 2.60, 95% CI: 2.19–3.09, P < 0.01) (Fig. 8A). Significant heterogeneity was observed (I2 = 79.05%, P < 0.01). Although the Eggers regression test showed significant publication bias (P < 0.01), no significant publication bias was found based on the Beggs regression test (P = 0.06). The results of the Trim and Fill analysis with 8 studies yielded an HR of 2.09, with a 95% CI of 1.77–2.46 (Fig. 8B). Sensitivity analyses showed no effect of each study on the pooled effect (Fig. 8C). The result of the prediction interval was 1.33–5.12 (Fig. 8D). The epidemiological strength of the association between TyG index and T2DM was low based on GRADE criteria (Table 3).
Fig. 8.
The association between TyG index and T2DM reported as hazard ratio. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
The correlation between TyG index and GDM
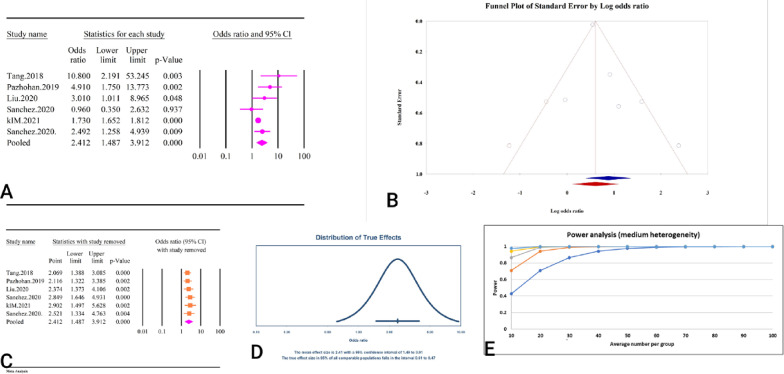
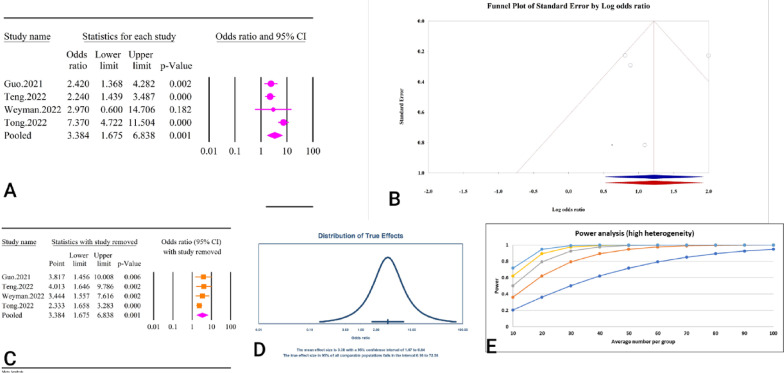
A total of 6 studies, comprising a combined sample size of 18,038 individuals, investigated the relationship between the TyG index and GDM (Table 2). The analysis revealed that a high TyG index was significantly associated with GDM (OR = 2.41, 95% CI: 1.48–3.91, P < 0.01) (Fig. 9A). These studies demonstrated high heterogeneity (59.48%, P = 0.03), with no significant publication bias detected based on Egger’s and Begg’s tests (P = 0.15 and P = 0.13, respectively). Additionally, the trim and fill analysis, which included two imputed studies, adjusted OR to 1.83 (95% CI: 1.11–3.01) (Fig. 9B). The sensitivity analysis indicated that the total effect size remained stable without significant change after the sequential deletion of each study (Fig. 9C). Moreover, the prediction interval for the TyG and GDM relationship ranged from 0.61 to 9.47 (Fig. 9D). The power analysis indicated high statistical power (1−β = 1), suggesting adequate sample size (Fig. 9E). The epidemiological strength of the relationship between TyG index and GDM was accompanied by low evidence (Table 3).
Fig. 9.
The association between TyG index and GDM. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
The correlation between TyG index and DR
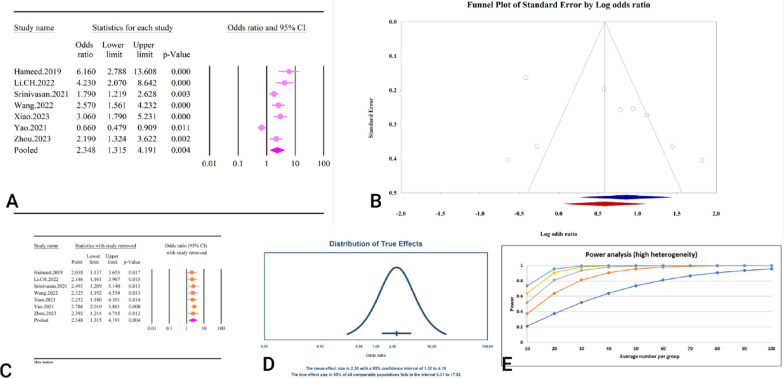
A total of 10 studies comprising 13,716 individuals assessed the relationship between TyG and DR (Table 2). The included meta-analyses assessed the TyG as both a continuous and categorical variable.
The analysis revealed that a high TyG index was significantly associated with DR (OR = 2.34, 95% CI: 1.31–4.19, P < 0.01) as a categorical variable (Fig. 10A). These studies demonstrated high heterogeneity (I²=89.7%, P < 0.01), with significant publication bias detected based on Egger’s and Begg’s tests (P = < 0.01). Additionally, the trim and fill analysis, which included two imputed studies, adjusted the OR to 1.79 (95% CI: 1.06–3.03) (Fig. 10B). The sensitivity analysis showed that the total effect size remained stable without significant change after sequentially deleting each study (Fig. 10C). Moreover, the prediction interval for the TyG as a categorical variable and DR relationship ranged from 0.31 to 17.82 (Fig. 10D). The power analysis indicated high statistical power (1−β = 1), suggesting adequate sample size (Fig. 10E).
Fig. 10.
The association between TyG index and DR as a categorical variable. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
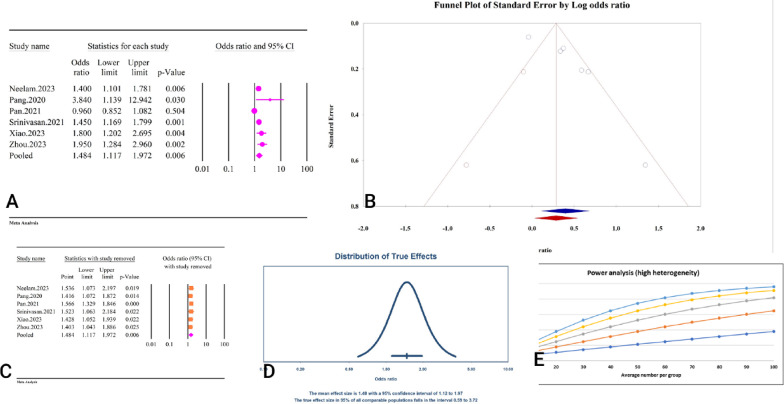
The study found a significant association between TyG and DR when TyG was considered as a continuous variable (OR = 1.48, 95% CI: 1.11–1.97, p < 0.01) (Fig. 11A). This relationship was accompanied by substantial heterogeneity (I2 = 83.4%, p < 0.01). While the Egger’s regression test indicated the presence of significant publication bias (p < 0.01), the Begg’s regression test did not find evidence of publication bias (p = 0.25). The results of the trim and fill analysis, which imputed two additional studies, yielded an adjusted odds ratio of 1.33 (95% CI: 1.03–1.71) (Fig. 11B). The sensitivity analysis demonstrated that the overall effect size remained stable without significant changes after sequentially removing each study (Fig. 11C). Furthermore, the prediction interval for the relationship between TyG as a continuous variable and DR ranged from 0.59 to 3.72 (Fig. 11D). The power analysis indicated a high statistical power, suggesting that the sample size was adequate to detect the observed effect (Fig. 11E). The epidemiological strength between TyG and DR was very low regarding GRADE criteria (Table 3).
Fig. 11.
The association between TyG index and DR as a continuous variable. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
TyG index and metabolic diseases
The correlation between TyG index and OSA
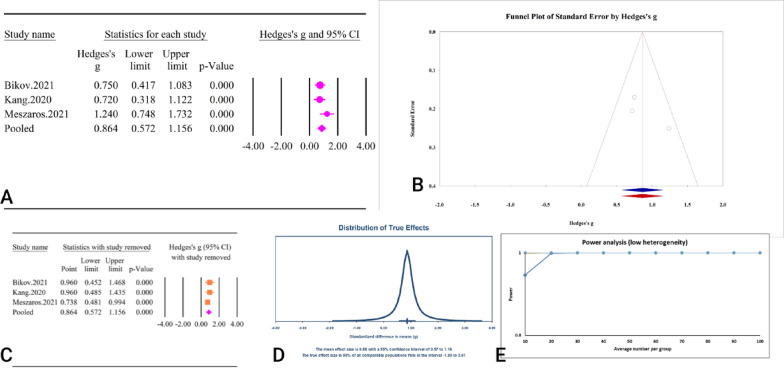
A collective effort from 3 studies, involving a combined sample size of 437 individuals, examined the relationship between the TyG index and OSA (Table 2). The analysis revealed a significant association between a heightened TyG index and OSA (SMD = 0.86, 95% CI: 0.57–1.15, P < 0.01) (Fig. 12A). The results showed minimal heterogeneity (36.74%, P = 0.20), with no noticeable publication bias evident through both Egger’s and Begg’s tests (P = 0.36 and P = 0.29). Additionally, the trim and fill analysis, free of any imputed studies, tuned the standardized SMD to 0.86 (95% CI: 0.57–1.15) (Fig. 12B). The sensitivity analysis indicated that the total effect size remained stable without significant change after the sequential deletion of each study (Fig. 12C). Noteworthy also is the prediction interval for the TyG and OSA relationship, spanning from − 1.89 to 3.61 (Fig. 12D). Furthermore, the power analysis reflected a commendable statistical power (1−β = 1), underscoring the adequacy of the sample size for reliable outcomes (Fig. 12E). The epidemiological strength of the association between TyG index and OSA was low based on GRADE criteria (Table 3).
Fig. 12.
The association between TyG index and OSA. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
The correlation between TyG index and metabolic syndrome
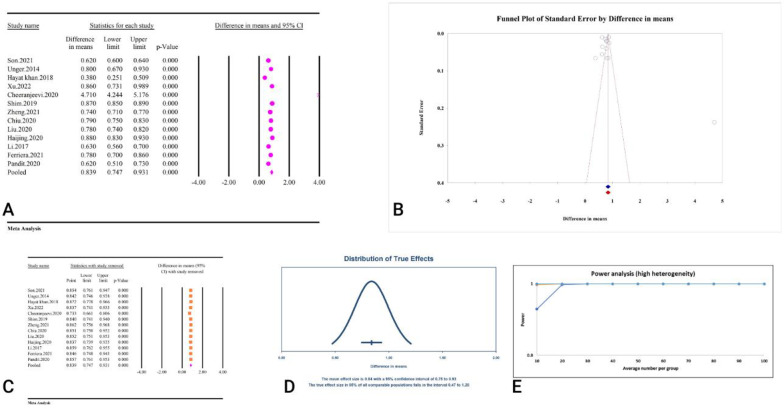
A total of 13 studies, comprising a combined sample size of 49,325 individuals, investigated the relationship between the TyG index and metabolic syndrome (Table 2). The analysis revealed that a high TyG index was significantly associated with metabolic syndrome (MD = 0.83, 95% CI: 0.74–0.93, P < 0.01) (Fig. 13A). These studies demonstrated high heterogeneity (98.18%, P < 0.01), with no significant publication bias detected based on Egger’s and Begg’s tests (P = 0.43 and P = 0.76). Additionally, the trim and fill analysis, which did not include any imputed studies, adjusted the MD to 0.83 (95% CI: 0.74–0.93) (Fig. 13B). The sensitivity analysis indicated that the total effect size remained stable without significant change after the sequential deletion of each study (Fig. 13C). Moreover, the prediction interval for the TyG and metabolic syndrome relationship ranged from 0.47 to 1.20 (Fig. 13D). The power analysis indicated high statistical power (1–β = 1), suggesting adequate sample size (Fig. 10E). The epidemiological strength of the relationship between TyG index and metabolic syndrome was accompanied by low evidence (Table 3).
Fig. 13.
The association between TyG index and metabolic syndrome. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
The correlation between TyG index and NAFLD
A total of 17 studies, comprising a combined sample size of 121,975 individuals, investigated the relationship between the TyG index and NAFLD (Table 2). The included meta-analyses assessed the TyG as both a continuous and categorical variable.
The meta-analysis demonstrated that the TyG index was significantly associated with NAFLD as a continuous variable (OR = 2.36, 95% CI: 1.88–2.97, P < 0.01) (Fig. 14A). The Egger’s regression test showed a significant publication bias (P < 0.01), while the Begg’s regression test did not indicate a significant publication bias (P = 0.24). Additionally, the trim and fill analysis, which did not include any imputed studies, adjusted the OR to 2.36 (95% CI: 1.88–2.97) (Fig. 14B). The sensitivity analysis indicated that the total effect size remained stable without a significant change after the sequential deletion of each study (Fig. 14C). Moreover, the prediction interval for the TyG and metabolic syndrome relationship ranged from 0.99 to 5.68 (Fig. 14D). The power analysis indicated high statistical power (1–β = 1), suggesting an adequate sample size (Fig. 14E).
Fig. 14.
The association between TyG index and NAFLD as a continuous variable. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
The meta-analysis results showed a significant association between the TyG index and NAFLD when TyG was considered as a categorical variable (OR = 6.00, 95% CI: 4.12–8.73, P < 0.01) (Fig. 15A). However, the analysis also revealed significant heterogeneity (91.80%, P < 0.01) in the included studies. The Egger’s and Begg’s regression tests did not show any significant publication bias (P = 0.89 and P = 0.75, respectively). Trim and Fill analysis, without imputing any additional studies, resulted in an odds ratio of 6.00 (95% CI: 4.12–8.73) (Fig. 15B). The sensitivity analysis demonstrated the stability of the overall effect size, as it remained consistent after the sequential removal of each individual study (Fig. 15C). The prediction interval for the relationship between TyG and metabolic syndrome ranged from 1.64 to 21.98, indicating the potential variation in the true effect size across different settings (Fig. 15D). Finally, the power analysis revealed a high statistical power (1–β = 1), suggesting that the sample size used in the meta-analysis was adequate to detect the observed effect (Fig. 15E). The GRADE criteria showed very low evidence of the association between TyG and NAFLD (Table 3).
Fig. 15.
The association between TyG index and NAFLD as a categorical variable. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
TyG index and cerebrovascular diseases
The correlation between TyG index and dementia
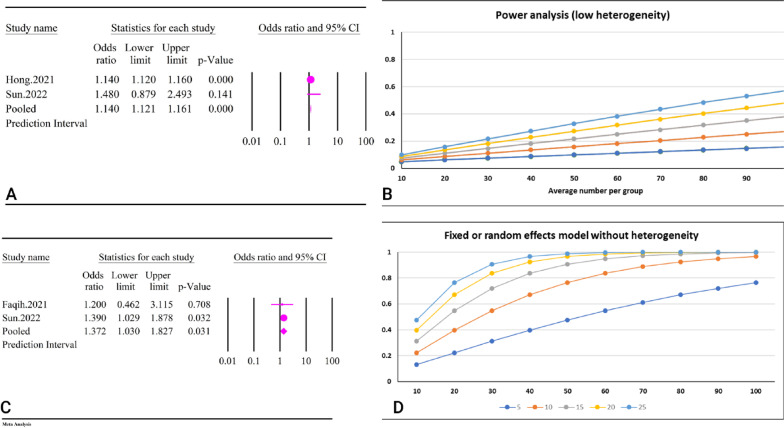
The relationship between TyG index and dementia was assessed considering TyG as both a continuous and categorical variable (Table 2). Two studies with a total sample size of 8,435,216 participants assessed TyG as a categorical variable. The results of the meta-analysis showed that a higher TyG index was significantly associated with an increased risk of dementia (OR = 1.14, 95% CI: 1.12–1.16, P < 0.01) (Fig. 16A). No significant heterogeneity was found across the studies (I2 = 0.00%, P = 0.32). Due to the low number of studies, it was not possible to assess publication bias, prediction interval, and epidemiological robustness. The results of the power analysis showed high statistical power, indicating that the sample size was adequate (Fig. 16B).
Fig. 16.
The association between TyG index and dementia. A Forest plot of TyG as a categorical index B Power analysis of TyG as a categorical index. C Forest plot of TyG as a continuous index. D Power analysis of TyG as a continuous index
TyG was also significantly associated with dementia as a continuous variable based on the results of two studies with a total sample size of 2,524 participants (OR = 1.37, 95% CI: 1.03–1.82, P = 0.03) (Fig. 16C). No significant heterogeneity was observed (I2 = 0.00%, P = 0.77). However, due to the low number of studies, it was not possible to assess publication bias, prediction interval, and epidemiological robustness. The results of the power analysis showed high statistical power, indicating that the sample size was sufficient (Fig. 16D).
The correlation between TyG index and cognitive impairment
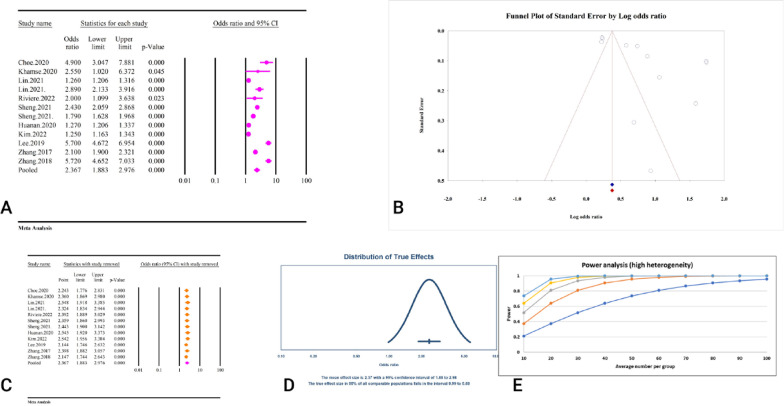
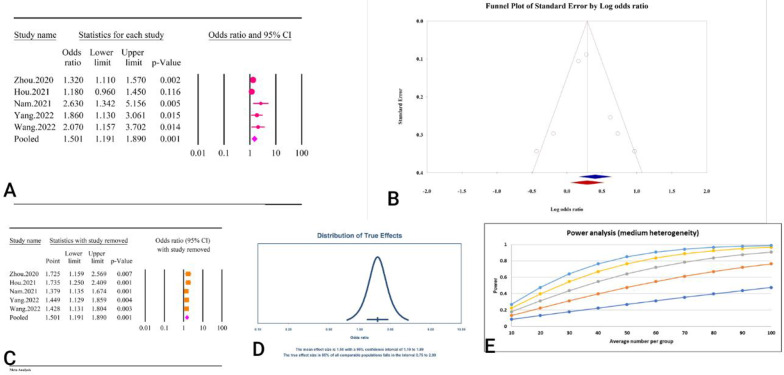
Based on the meta-analysis of five studies including 7,057 participants, TyG index was significantly associated with cognitive impairment as a categorical variable (OR = 2.31, 95% CI: 1.38–3.86, P < 0.01) (Fig. 17A) (Table 2). Significant heterogeneity (I2 = 84.75%, P < 0.01) accompanied the results. While there was no obvious publication bias in the Begg’s regression test (P = 0.46), the Egger’s regression test revealed considerable publication bias (P < 0.01). The OR was modified to 1.67 (95% CI: 1.05–2.64) by the trim and fill analysis results using two imputed trials (Fig. 17B). The removal of each study did not significantly alter the pooled effect, according to the results of the sensitivity analysis (Fig. 17C). The power analysis revealed strong statistical power, indicating that the overall sample size was sufficient, and the prediction interval was 0.37–14.26 (Fig. 17D) (Fig. 17E).
Fig. 17.
The association between TyG index as a categorical variable and cognitive impairment. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
A total of four studies comprising 1,235 individuals assessed the relationship between cognitive impairment and TyG index and as a continuous variable. This association was statistically significant (OR = 3.38, 95% CI: 1.67–6.83, P < 0.01) (Fig. 18A), however, it was accompanied by significant heterogeneity (I2 = 81.56%). No significant publication bias was found based on the Egger’s and Begg’s regression tests (P = 0.84 and P = 0.73, respectively). The results of the trim and fill analysis adjusted the OR to 3.38 (95% CI: 1.67–6.83) without imputing any additional studies (Fig. 18B). The results of the sensitivity analysis indicated that the removal of each individual study did not significantly affect the total estimate (Fig. 18C). Prediction interval ranged from 0.16 to 72.58 (Fig. 18D). The power analysis showed high statistical power, reflecting the adequacy of the sample size (Fig. 18E). The epidemiological strength of the association between TyG index and cognitive impairment was very low based on GRADE criteria (Table 3).
Fig. 18.
The association between TyG index as a continuous variable and cognitive impairment. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
The correlation between TyG index and ischemic stroke
Among the included studies, the correlation between the TyG index and the following outcomes was examined: ischemic stroke, ischemic stroke recurrence, ischemic stroke mortality, ischemic stroke poor functional outcomes, and ischemic stroke neurological worsening (Table 2).
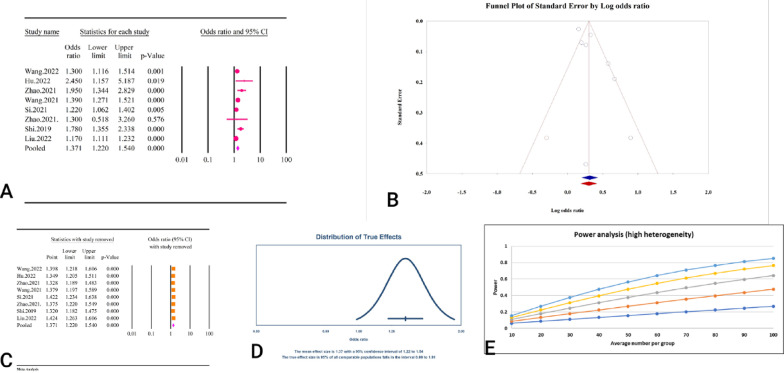
Based on the results of 8 studies including 554,334 individuals, the TyG index was significantly associated with an increased risk of stroke (OR = 1.37, 95% CI: 1.22–1.54, P < 0.01) (Fig. 19A). The results were accompanied by significant heterogeneity (I2 = 74.18%, P < 0.01). The results of the publication bias assessment showed significant bias based on the Egger’s regression test (P = 0.04), however, the Begg’s regression test was not significant (P = 0.90). The results of the trim and fill analysis with one imputed study adjusted the OR to 1.35 (95% CI: 1.20–1.52) (Fig. 19B). The results of the sensitivity analysis showed no significant change in the pooled estimate after the removal of each individual study (Fig. 19C). The prediction interval was calculated as 0.99–1.91, and the power analysis indicated high statistical power (Fig. 19D and E).
Fig. 19.
The association between TyG index and ischemic stroke. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
The results of five studies including 84,774 individuals showed that the TyG index was significantly associated with an increased risk of ischemic stroke recurrence (OR = 1.50, 95% CI: 1.19–1.89, P < 0.01) (Fig. 20A). The analysis exhibited moderate heterogeneity among the included studies (I2 = 56.22%, P = 0.05). The assessment of publication bias suggested the presence of bias based on Egger’s regression test (P = 0.02), but Begg’s regression test was not significant (P = 0.08). The trim and fill analysis, which adjusts for publication bias, estimated the adjusted OR as 1.33 (95% CI: 1.04–1.70) (Fig. 20B). The sensitivity analysis indicated that the pooled estimate was stable (Fig. 20C). The prediction interval calculated was 0.75–2.99, indicating the range of true effect sizes (Fig. 20E). The power analysis showed high statistical power (Fig. 20D).
Fig. 20.
The association between TyG index and ischemic stroke recurrence. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
The results of the analysis including six studies with total sample size of 31,465 showed that the higher TyG index was significantly associated with an increased risk of poor functional outcomes following an ischemic stroke (OR = 1.36, 95% CI: 1.10–1.69, P < 0.01) (Fig. 21A). The analysis exhibited high heterogeneity among the included studies (I2 = 71.40%, P < 0.01). The assessment of publication bias suggested the presence of bias based on both Egger’s regression test (P < 0.01) and Begg’s regression test (P = 0.02). The trim and fill analysis, which adjusts for publication bias, estimated the adjusted OR as 1.25 (95% CI: 1.01–1.54) with two studies imputed (Fig. 21B). The sensitivity analysis indicated that the pooled estimate was stable (Fig. 21C). The prediction interval for the true effect sizes was calculated to be between 0.74 and 2.54, indicating variability in the potential outcomes (Fig. 21D). The power analysis demonstrated high statistical power (Fig. 21E).
Fig. 21.
The association between TyG index and ischemic stroke poor functional outcomes. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
The results of the analysis showed that the TyG index was significantly associated with an increased risk of ischemic stroke mortality (OR = 1.40, 95% CI: 1.14–1.71, P < 0.01) (Fig. 22A). The analysis exhibited high heterogeneity among the included studies (I2 = 70.70%, P < 0.01). The assessment of publication bias using Egger’s regression test (P = 0.19) and Begg’s regression test (P = 0.70) was not significant. The trim and fill analysis did not impute any studies, and thus the adjusted OR remained the same (OR = 1.40, 95% CI: 1.14–1.71) (Fig. 22B). The sensitivity analysis indicated that the pooled estimate was stable (Fig. 22C). The prediction interval for the true effect sizes was calculated to be between 0.76 and 2.57, indicating variability in the potential outcomes (Fig. 22D). The power analysis demonstrated high statistical power (Fig. 22E). The epidemiological strength of the association between TyG index and ischemic stroke mortality was very low based on GRADE criteria (Table 3).
Fig. 22.
The association between TyG index and ischemic stroke mortality. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
TyG index and cardiovascular outcomes
The correlation between TyG index and HF
Among the included studies, the relationship between TyG index and HF was evaluated as both a continuous and categorical variable (Table 2).
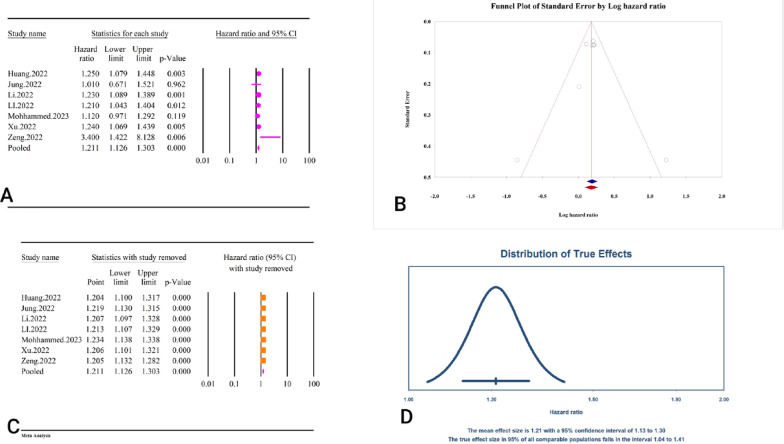
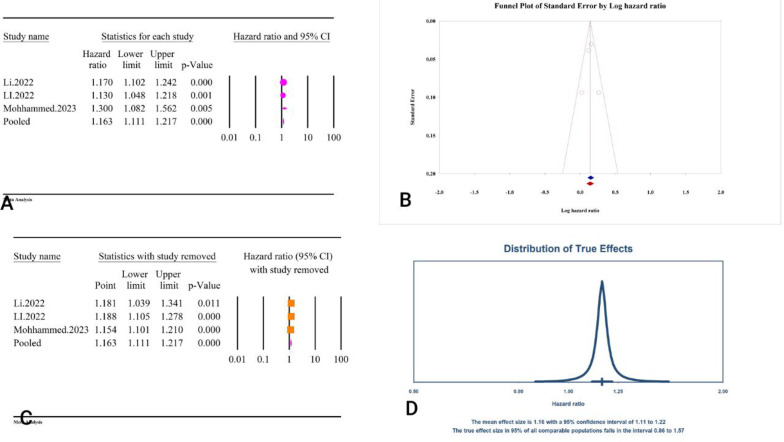
The results of the analysis showed that the TyG index as a categorical variable was significantly associated with an increased risk of heart failure (HR = 1.21, 95% CI: 1.12–1.30, P < 0.01) (Fig. 23A). The analysis exhibited low heterogeneity among the included studies (I2 = 21.43%, P = 0.26). The assessment of publication bias using Egger’s regression test (P = 0.29) and Begg’s regression test (P = 0.76) was not significant. The trim and fill analysis estimated the adjusted HR as 1.19 (95% CI: 1.08–1.31) with one study imputed (Fig. 23B). The sensitivity analysis indicated that the pooled estimate was stable (Fig. 23C). The prediction interval for the true effect sizes was calculated to be between 1.04 and 1.41, indicating variability in the potential outcomes (Fig. 23D). Because the reported results were presented as HR, the assessment of power analysis was not possible.
Fig. 23.
The association between TyG index and heart failure as a categorical variable. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval
The results of the analysis showed that the TyG index was significantly associated with an increased risk of HF as a continuous variable (HR = 1.16, 95% CI: 1.11–1.21, P < 0.01) (Fig. 24A). The analysis exhibited very low heterogeneity among the included studies (I² = 0.64%, P = 0.36). The assessment of publication bias using Egger’s regression test (P = 0.40) and Begg’s regression test (P = 1) was not significant. The trim and fill analysis, which adjusts for publication bias, estimated the adjusted HR as 1.15 (95% CI: 1.09–1.21) with one study imputed (Fig. 24B). The sensitivity analysis indicated that the pooled estimate was stable (Fig. 24C). The prediction interval for the true effect sizes was calculated to be between 0.86 and 1.57, indicating variability in the potential outcomes (Fig. 24D). The epidemiological strength of the association between TyG index and HF was low based on GRADE criteria (Table 3).
Fig. 24.
The association between TyG index and heart failure as a continues variable. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval
The correlation between TyG index and AF
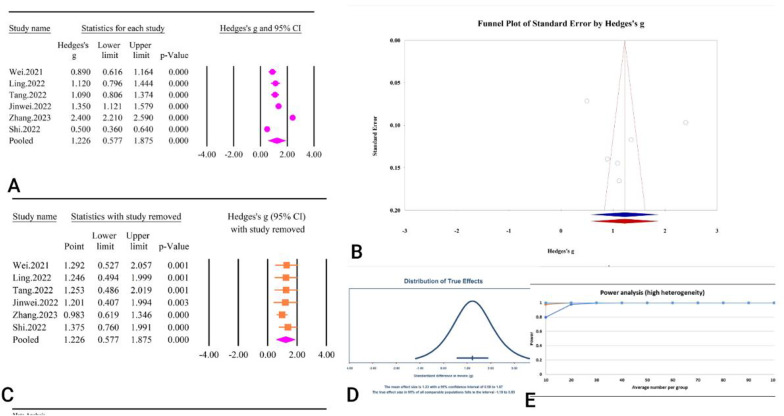
The analysis of six studies comprising 5813 participants revealed a significant association between the TyG index and an increased risk of AF, with an SMD of 1.22 (95% CI: 0.57–1.87, P < 0.01) (Fig. 25A) (Table 2). The heterogeneity among the included studies was extremely high (I2 = 98.04%, P < 0.01). Evaluations for publication bias indicated no significant bias, as evidenced by Egger’s regression test (P = 0.64) and Begg’s regression test (P = 1). The trim and fill method did not identify any missing studies, thus the adjusted SMD remained unchanged (SMD = 1.22, 95% CI: 0.57–1.87) (Fig. 25B). Sensitivity analysis confirmed the robustness of the pooled estimate (Fig. 25C). The prediction interval, ranging from − 1.18 to 3.63, highlighted substantial variability in the possible effect sizes. The power analysis confirmed a high level of statistical power (Fig. 25D). The epidemiological strength of the association between TyG index and AF was low based on GRADE criteria (Table 3).
Fig. 25.
The association between TyG index and atrial fibrillation. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
The correlation between TyG index and HTN
The analysis of 6 studies and 200,044 individuals demonstrated that the TyG index was significantly associated with an increased risk of HTN (RR = 1.52, 95% CI: 1.25–1.85, P < 0.01) (Fig. 26A) (Table 2). The included studies exhibited considerable heterogeneity (I2 = 85.67%, P < 0.01). The evaluation of publication bias showed mixed results: Egger’s regression test indicated the presence of bias (P < 0.01), whereas Begg’s regression test did not show significant bias (P = 0.28). The trim and fill analysis, which corrects for potential publication bias, adjusted the risk ratio to 1.44 (95% CI: 1.20–1.74) with one study imputed (Fig. 26B). The sensitivity analysis confirmed the stability of the pooled estimate (Fig. 26C). The prediction interval for the true effect sizes ranged from 0.78 to 2.98, indicating variability in the potential outcomes (Fig. 26D). The epidemiological strength of the association between TyG index and HTN was very low based on GRADE criteria (Table 3). Because the reported results were presented as RR, the assessment of power analysis was not possible.
Fig. 26.
The association between TyG index and hypertension. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval
The correlation between TyG index and arterial stiffness
Among the 16 included studies with 59,851 individuals, the relationship between TyG index and arterial stiffness was evaluated as both a continuous and categorical variable (Table 2).
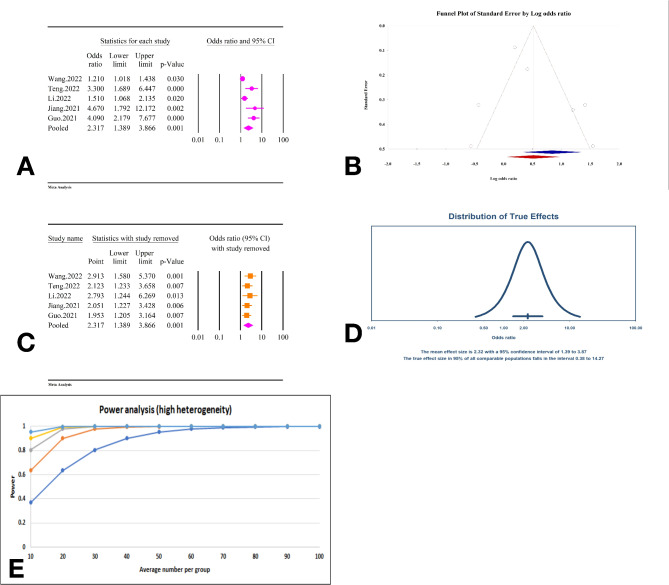
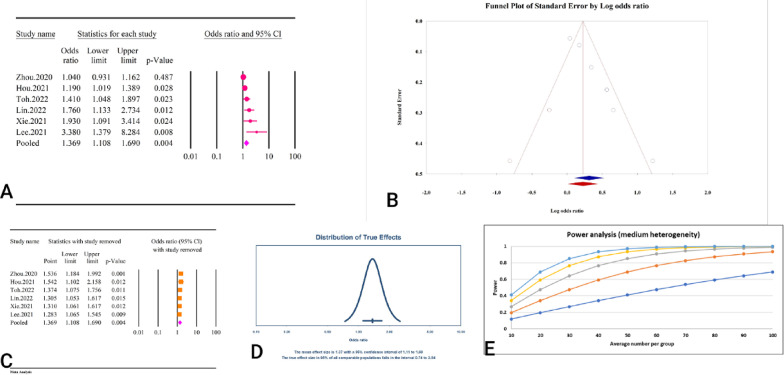
The analysis demonstrated a significant association between the TyG index as a categorical variable and an increased risk of arterial stiffness, with an OR of 1.80 (95% CI: 1.53–2.10, P < 0.01) (Fig. 27A). The studies included exhibited moderate heterogeneity (I2 = 66.26%, P < 0.01). Assessments for publication bias showed no significant bias according to Egger’s regression test (P = 0.35) and Begg’s regression test (P = 0.20). The trim and fill method did not identify any missing studies, thus the adjusted OR remained the same (OR = 1.80, 95% CI: 1.53–2.10) (Fig. 27B). Sensitivity analysis confirmed the robustness of the pooled estimate (Fig. 27C). The prediction interval for the true effect sizes ranged from 1.07 to 3.03, indicating some variability in the potential outcomes (Fig. 27D). The power analysis demonstrated a high level of statistical power (Fig. 27E).
Fig. 27.
The association between TyG index as a categorical variable and atrial stiffness. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval E Power analysis
The findings revealed a significant association between the TyG index as a continuous variable and increased risk of arterial stiffness, with an OR of 1.50 (95% CI: 1.34–1.69, P < 0.01) (Fig. 28A). The studies included in the analysis exhibited substantial heterogeneity (I2 = 81.66%, P < 0.01). Tests for publication bias were not significant, as shown by Egger’s regression test (P = 0.10) and Begg’s regression test (P = 0.16). The trim and fill method suggested the addition of four missing studies, leaving the adjusted OR unchanged (OR = 1.39, 95% CI: 1.23–1.56) (Fig. 28B). Sensitivity analysis indicated the robustness of the combined estimate (Fig. 28C). The prediction interval for the true effect sizes was between 1.03 and 2.21(Fig. 27D). The results demonstrated high statistical power (Fig. 28E). According to the GRADE criteria, the epidemiological strength of the association between the TyG index and arterial stiffness was found to be very low (Table 3).
Fig. 28.
The association between TyG index as a continuous variable and atrial stiffness. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval E Power analysis
The correlation between TyG index and coronary artery calcification
In the 10 included studies, encompassing 27,456 individuals, the relationship between the TyG index and coronary artery calcification was assessed both as a continuous and a categorical variable (Table 2).
Employing the TyG index as a continuous variable, the analysis showed a significant correlation (OR of 1.73 (95% CI: 1.36–2.20, P < 0.01) with an increased risk of coronary artery calcification (Fig. 29A).The included studies demonstrated moderate heterogeneity (I2 = 50.81%, P = 0.08). Tests for publication bias were not significant, as indicated by Egger’s regression test (P = 0.08) and Begg’s regression test (P = 0.22). The trim and fill analysis did not identify any missing studies, thus the adjusted OR remained unchanged (OR = 1.73, 95% CI: 1.36–2.20) (Fig. 29B). Sensitivity analysis confirmed the robustness of the pooled estimate. The prediction interval for the true effect sizes ranged from 0.84 to 3.59, indicating some variability in outcomes (Fig. 29C). The power analysis demonstrated high statistical power (Fig. 29D).
Fig. 29.
The association between TyG index as a continuous variable and coronary artery calcification. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
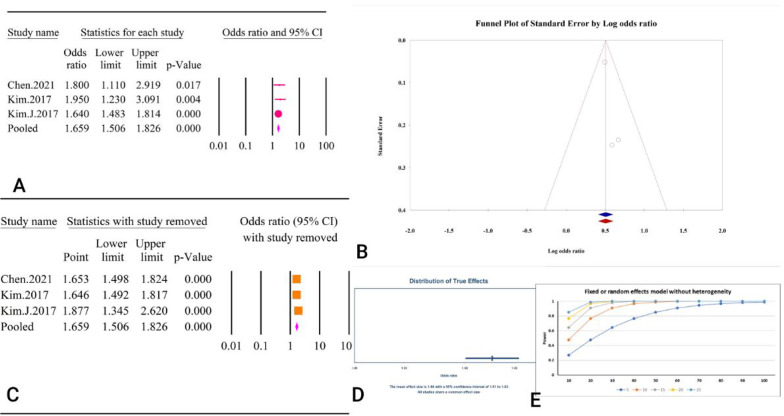
With an OR of 1.65 (95% CI: 1.50–1.82, P < 0.01), our findings demonstrated a strong correlation between the TyG index as a categorical variable and an elevated risk of coronary artery calcification (Fig. 30A).There was no heterogeneity detected among the studies (I2 = 0.00%, P = 0.72). Evaluation for publication bias showed no significant issues according to Egger’s regression test (P = 0.21) and Begg’s regression test (P = 1). The trim and fill method did not indicate any missing studies, so the adjusted OR remained unchanged (OR = 1.65, 95% CI: 1.50–1.82) (Fig. 30B). Sensitivity analysis confirmed the robustness of the pooled estimate (Fig. 30C). The prediction interval, matching the confidence interval from 1.51 to 1.83, suggested consistency in the effect sizes (Fig. 30D). The power analysis indicated high statistical power (Fig. 30E). According to the GRADE criteria, the epidemiological strength of the association between the TyG index and coronary artery calcification was found to be very low (Table 3).
Fig. 30.
The association between TyG index as a categorical variable and coronary artery calcification. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval. E Power analysis
The correlation between TyG index and post PCI events
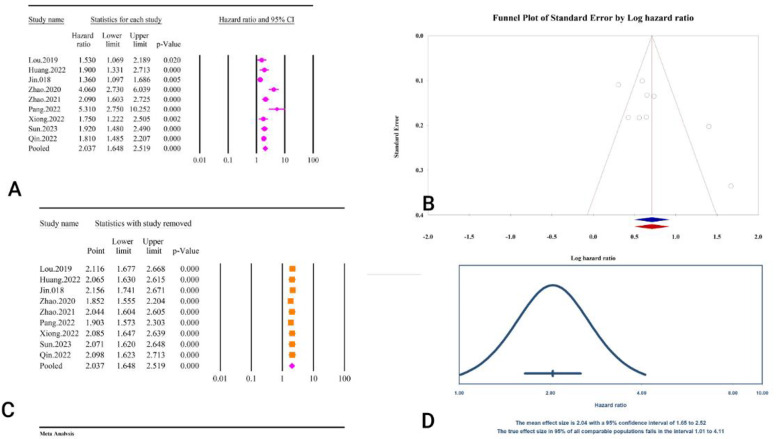
A total of nine studies including 10, 164 participants assessed the correlation between TyG index and post PCI events (Table 2).
The analysis revealed a significant association between the TyG index as a categorical variable and an increased risk of major cardiovascular outcomes following PCI (HR = 2.03,95% CI: 1.64–2.51, P < 0.01) (Fig. 31A). The included studies showed considerable heterogeneity (I² = 77.09%, P < 0.01). Publication bias assessments indicated no significant issues according to Egger’s regression test (P = 0.051) and Begg’s regression test (P = 0.25). The trim and fill method did not suggest any missing studies, leaving the adjusted HR unchanged (HR = 2.03, 95% CI: 1.64–2.51) (Fig. 31B). Sensitivity analysis confirmed the stability of the pooled estimate (Fig. 31C). The prediction interval for the true effect sizes ranged from 1.01 to 4.11, indicating variability in the potential outcomes (Fig. 31D). The results were reported as HR and calculating power analysis was not possible.
Fig. 31.
The association between TyG index as a categorical variable and major cardiovascular outcomes following PCI. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval
The analysis demonstrated a significant association between the TyG index as a continuous variable and an increased risk of major cardiovascular outcomes following PCI, with HR of 1.81 (95% CI: 1.34–2.46, P < 0.01) (Fig. 32A). The included studies exhibited very high heterogeneity (I² = 91.72%, P < 0.01). Tests for publication bias indicated significant bias according to both Egger’s regression test (P = 0.02) and Begg’s regression test (P = 0.02). The trim and fill method did not impute any missing studies, so the adjusted HR remained unchanged (HR = 1.81, 95% CI: 1.34–2.46) (Fig. 32B). Sensitivity analysis confirmed the robustness of the pooled estimate (Fig. 32C). The prediction interval for the true effect sizes ranged from 0.58 to 5.75, reflecting a wide variability in potential outcomes (Fig. 32D). The results were reported as HR and calculating power analysis was not possible.
Fig. 32.
The association between TyG index as a continuous variable and major cardiovascular outcomes following PCI. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval
The epidemiological strength of the association between TyG and post-PCI major cardiovascular outcomes was low based on the GRADE criteria (Table 3).
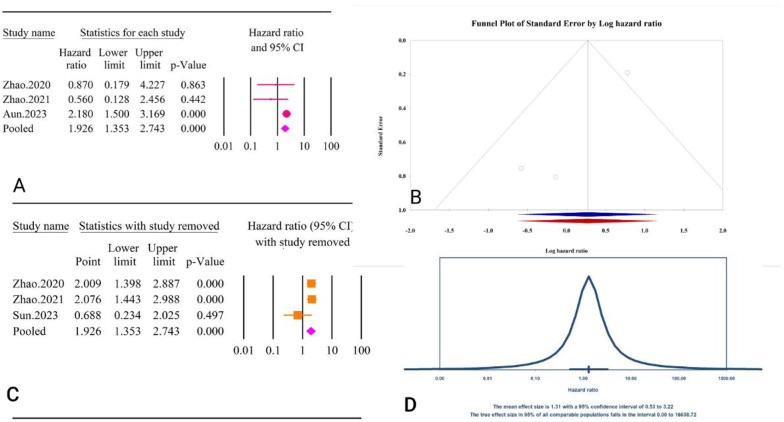
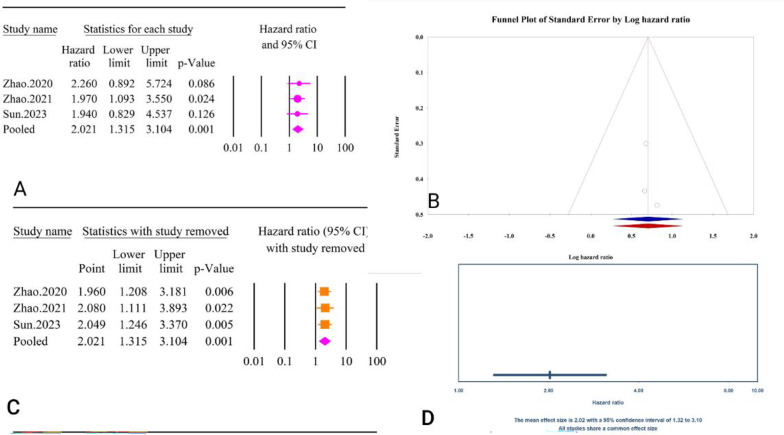
The analysis revealed a significant association between the TyG index as a categorical variable and post-PCI all-cause mortality (HR = 1.92, 95% CI: 1.35–2.74, P < 0.01) (Fig. 33A). The included studies showed moderate heterogeneity (I² = 50.91%, P = 0.13). Publication bias assessments indicated minimal effects, with Egger’s regression test (P = 0.15) and Begg’s regression test (P = 1.00) showing no significant bias. The trim and fill method did not suggest any missing studies, leaving the adjusted HR unchanged (HR = 2.18, 95% CI: 0.97–4.87) (Fig. 33B). Sensitivity analysis showed significant change in pooled result after removal of Sun, 2023 [62] (HR = 0.68, 95% CI: 0.23–2.02, P = 0.49) (Fig. 33C). The prediction interval for the true effect sizes ranged from 0.01 to 16638.72, indicating considerable variability in potential outcomes (Fig. 33D). The results were reported as HR and calculating power analysis was not possible.
Fig. 33.
The association between TyG index as a categorical variable and all-cause mortality following PCI. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval
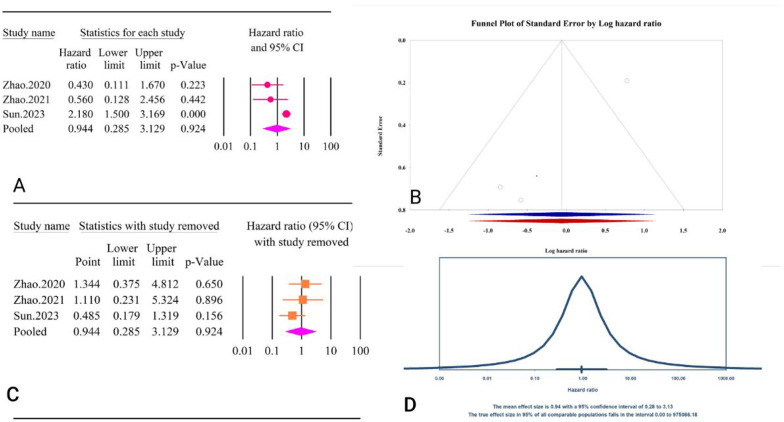
For the TyG index as a continuous variable, the analysis did not show a significant association with post-PCI all-cause mortality (HR = 0.94, 95% CI: 0.28–3.12, P = 0.92) (Fig. 34A). The included studies exhibited high heterogeneity (I2 = 73.95%, P = 0.02). Tests for publication bias indicated no significant bias according to both Egger’s regression test (P = 0.09) and Begg’s regression test (P = 1.00). The trim and fill method did not impute any missing studies, so the adjusted HR remained unchanged (HR = 0.94, 95% CI: 0.28–3.12) (Fig. 34B). The sensitivity analysis showed a significant change in the pooled results after the removal of Zhao.2020 [63] (HR = 1.34, 95% CI: 0.37–4.18, P = 0.65), Zhao,2021 [64] (HR = 1.11, 95% CI: 0.23–5.32, P = 0.89), and Sun, 2023 [62] (HR = 0.48, 95% CI: 0.17–1.31, P = 0.15) (Fig. 34C). The prediction interval for the true effect sizes ranged from 0.01 to 975086.18, indicating wide variability in potential outcomes (Fig. 34D). The results were reported as HR and calculating power analysis was not possible.
Fig. 34.
The association between TyG index as a continuous variable and all-cause mortality outcomes following PCI. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval
The epidemiological strength of the association between TyG and post-PCI all cause mortality was low based on the GRADE criteria (Table 3).
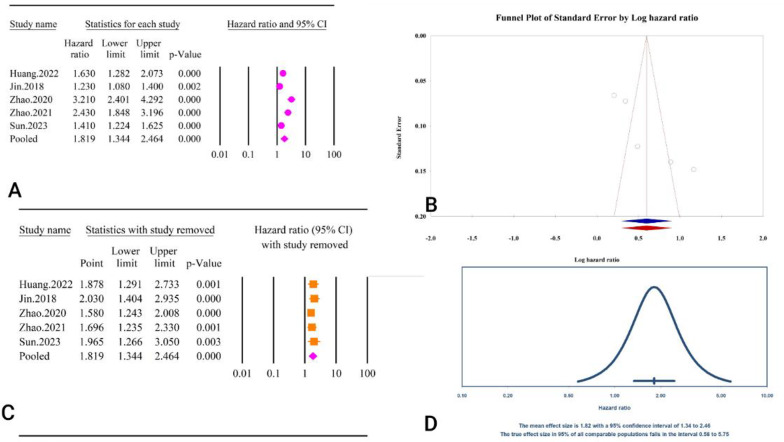
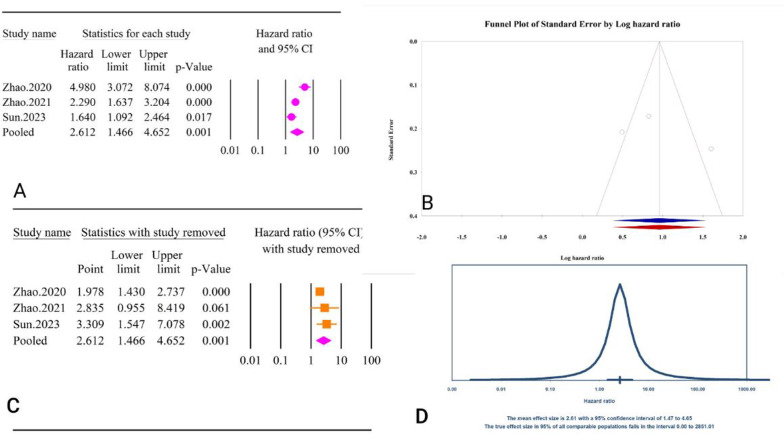
The analysis revealed a significant association between the TyG index as a categorical variable and post-PCI revascularization (HR = 2.61, 95% CI: 1.46–4.65, P < 0.01) (Fig. 35A). The included studies exhibited high heterogeneity (I2 = 83.56%, P < 0.01). Tests for publication bias indicated minimal effects, with Egger’s regression test (P = 0.58) and Begg’s regression test (P = 1.00) showing no significant bias. The trim and fill method did not suggest any missing studies, leaving the adjusted HR unchanged (HR = 2.61, 95% CI: 1.46–4.65) (Fig. 35B). Sensitivity analysis showed that after the elimination of the Zhao.2021 study, the association became insignificant (HR = 2.83, 95% CI: 0.05–8.41, P = 0.06) [64] (Fig. 35C). The prediction interval for the true effect sizes ranged from 0.01 to 2851.01, indicating considerable variability in potential outcomes (Fig. 35D). Calculating power analysis was not possible due to reported results in HR.
Fig. 35.
The association between TyG index as a categorical variable and post-PCI revascularization. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval
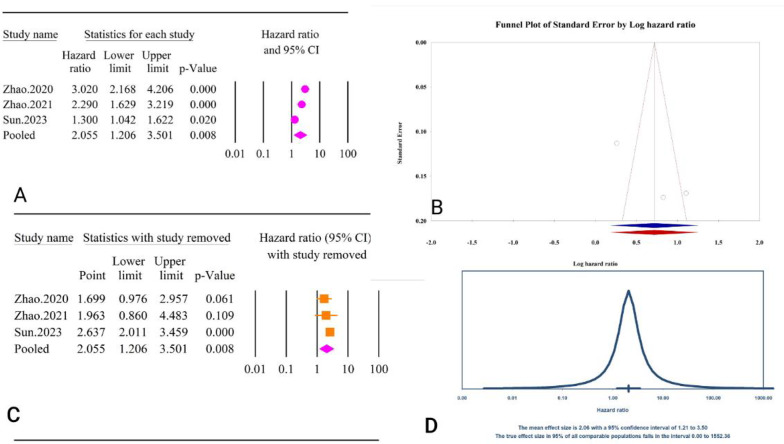
For post-PCI revascularization and TyG index as a continuous variable, the analysis revealed a significant association (HR = 2.05, 95% CI: 1.20–3.50, P < 0.01) (Fig. 36A). The included studies exhibited high heterogeneity (I2 = 89.78%, P < 0.01). Tests for publication bias indicated minimal effects, with Egger’s regression test (P = 0.20) and Begg’s regression test (P = 1.00) showing no significant bias. The trim and fill method did not suggest any missing studies, leaving the adjusted HR unchanged (HR = 2.05, 95% CI: 1.20–3.50) (Fig. 36B). Sensitivity analysis showed changes in the overall summary after elimination Zhao, 2021 (HR = 1.96, 95% CI: 0.86–4.48, P = 0.10) [64] and Zhao, 2020 (HR = 1.69, 95% CI: 0.97–2.95, P = 0.06) (Fig. 36C) [63]. The prediction interval for the true effect sizes ranged from 0.01 to 1552.36, indicating considerable variability in potential outcomes (Fig. 36D).
Fig. 36.
The association between TyG index as a continuous variable and post-PCI revascularization. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval
The epidemiological strength of the association between TyG and post-PCI revascularization was very low based on the GRADE criteria (Table 3).
The analysis revealed a significant association between the TyG index as a categorical variable and post-PCI non-fatal MI (HR = 2.02, 95% CI: 1.31–3.10, P < 0.01) (Fig. 37A). The included studies exhibited no heterogeneity (I2 = 0.00%, P = 0.96). Tests for publication bias indicated minimal effects, with Egger’s regression test (P = 0.57) and Begg’s regression test (P = 0.29) showing no significant bias. The trim and fill method did not suggest any missing studies, leaving the adjusted HR unchanged (HR = 2.02, 95% CI: 1.31–3.10) (Fig. 37B). Sensitivity analysis showed no significant effect of any single study on the overall sample size (Fig. 37C). The prediction interval for the true effect sizes ranged from 1.32 to 3.10, indicating limited variability in potential outcomes (Fig. 37D).
Fig. 37.
The association between TyG index as a categorical variable and post-PCI myocardial infarction. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval
When examining post-PCI non-fatal MI as a continuous variable, the analysis identified a significant association (HR = 2.56, 95% CI: 1.49–4.40, P < 0.01) (Fig. 38A). The included studies displayed moderate heterogeneity (I2 = 63.41%, P = 0.06). Assessment for publication bias revealed minimal influence, with Egger’s regression test (P = 0.12) and Begg’s regression test (P = 1.00) both showing no significant bias. The trim and fill method did not indicate any missing studies, and the adjusted HR remained unchanged (HR = 2.56, 95% CI: 1.49–4.40) (Fig. 38B). Sensitivity analysis demonstrated that the exclusion of any single study did not significantly impact the overall sample size (Fig. 38C). The prediction interval for true effect sizes spanned from 0.01 to 1013.95, reflecting substantial variability in possible outcomes (Fig. 38D).
Fig. 38.
The association between TyG index as a continuous variable and post-PCI myocardial infarction. A Forest plot. B Publication bias. C Sensitivity analysis. D Prediction interval
The epidemiological strength of the association between TyG and post-PCI MI was moderate based on the GRADE criteria (Table 3).
Discussion
It has been proposed that the triglyceride-glucose (TyG) index can be used as an accessible, practical, and affordable substitute marker for the hyperglycemic-hyperinsulinemic clamp test in order to measure insulin resistance (IR) [65]. The TyG index has been manifested to better predict and identify the presence of insulin resistance than the homeostasis model assessment of insulin resistance (HOMA-IR) method, as shown in several studies [66–69]. As the TyG index is highly relevant in predicting IR, it can be used to identify the risk level of various metabolic conditions, such as metabolic syndrome, type 2 diabetes mellitus, non-alcoholic fatty liver disease, cardiovascular disease, and atherosclerosis [70, 71]. A large volume of original and meta-analysis studies has been conducted to evaluate the prognostic and diagnostic value of TyG across various medical conditions.
Our analysis showed a significant relationship between the TyG index and T2DM. Elevated fasting plasma glucose and triglyceride levels are two components of the metabolic syndrome linked to IR and the development of chronic diseases [72]. It is important to note that IR usually appears about 1–2 decades before T2DM is diagnosed [73]. Consequently, early detection of IR is crucial for devising effective health interventions to prevent the onset of T2DM [74]. Multiple studies conducted with populations from America, Europe, and Asia have found that a higher TyG index is significantly associated with IR, despite some baseline variables being influenced by ethnicity [75–81].
Research has found that over 60% of individuals diagnosed with T2DM will go on to develop DR within the first 10 years of their disease onset [82]. Recent studies have shown a strong association between IR and DR [83]. In the early stages of DR, there are typically no obvious symptoms [84]. As a result, many patients only seek medical attention when they start experiencing blurred vision and vision loss, which indicates a serious stage. This late presentation is not favorable for subsequent recovery and prognosis. Accordingly, early detection of DR is crucial. Therefore, a noninvasive and convenient index should be identified for screening and detecting early-stage patients [38]. Our final analysis found a link between a higher TyG index and an increased risk of developing DR. Therefore, the TyG index, as an easily accessible and routinely available indicator, may represent a potentially valuable clinical option for the early diagnosis of DR.
Our research discovered that a high TyG index could indicate or predict GDM. The available evidence suggests that the main cause or pathogenesis of GDM is associated with IR and decreased insulin secretion during pregnancy [85]. Previous studies have found a relationship between a high TyG index and the development of GDM [86–88]. However, other studies failed to find such a correlation [87]. Previous research has shown that triglyceride (TG) levels fluctuate during pregnancy to meet fetal needs [89]. TG levels decrease in the first 2 months, then gradually increase over the next 7 months. Therefore, comparing the TyG index before or around 2 months of pregnancy may be more sensitive. In addition, individual differences among the study participants may contribute to the inconsistency and heterogeneity observed in TyG index levels. For example, it’s important to consider potential racial differences in physiology that could impact the TyG index [41].
Our study showed that individuals with a higher TyG index are more susceptible to developing coronary artery disease. The TyG index is a highly accurate and precise indicator for detecting IR, which increases the risk of developing cardiovascular disease [90]. Normally, insulin promotes vasodilation by stimulating nitric oxide production. However, when insulin signaling is dysregulated, it can reduce the availability of nitric oxide, leading to increased vascular stiffness [91]. The nonenzymatic glycosylation of lipids is a factor that contributes to atherosclerosis, along with hypertriglyceridemia and hyperglycemia. Hypertriglyceridemia can result in the production of low-density lipoproteins, promoting atherosclerosis and weakening the protective impact of high-density lipoproteins [92]. Additionally, hyperglycemia can accelerate atherosclerosis directly or indirectly [93]. Our study demonstrated that a higher TyG index is associated with the development and progression of heart failure. Indeed, IR is widely recognized for its harmful effects, such as oxidative stress, endothelial dysfunction, and myocardial remodeling. These contribute to defective cardiac function and the development of heart failure [23, 94].
Various biomarkers have been identified and evaluated for the risk assessment of AF, such as high-sensitivity troponin I [95], C-reactive protein (CRP) [96], fibroblast growth factor-23 (FGF-23) [97], the N-terminal pro-B-type natriuretic peptide (NT-proBNP) [96, 98], Galectin-3 [99], and several micro-RNAs [100]. Other biomarkers related to baseline risk factors, such as diabetes [101] and IR [87], have also been examined for their dual diagnostic and prognostic purposes. Our final analysis found an essential relationship between a higher TyG index and the development of AF. Therefore, the TyG index, as an established, cost-effective, and easily measurable indicator of IR, may assist clinicians in identifying patients with a higher risk of atrial fibrillation (AF). This could help in the early detection of atrial fibrillation, allowing for timely intervention and management [56].
Our final analysis also found a positive relationship between the TyG index and the development of HTN. Previous research has indicated that IR is associated with low-level systemic inflammation, which may contribute to endothelial dysfunction [102–104]. Additionally, IR may also affect the metabolism of sodium in the kidneys, stimulate the sympathetic nervous system’s activity [105], and modulate the secretion of vasoactive substances [106]. All of these factors have been linked to the development of hypertension.
In the context of OSA, research has shown a positive correlation between the TYG index and the presence of the condition. Chronic intermittent hypoxia and sleep deprivation have been extensively linked to the development of IR through multiple pathways. [107] The activation ofHypoxia-Inducible Factor 1 (HIF-1) triggers a cascade of inflammatory signaling and impairs insulin signaling, contributing to the onset of IR [108, 109]. Additionally, hypoxia disrupts sympathetic nervous system function, leading to increased oxidative stress and altered adipokine secretion, which can further exacerbate IR [110]. Although confounding factors like obesity and other metabolic conditions play a role in this regard, Bikov’s research has shown that even after adjusting for factors like body mass index (BMI), there remains a positive correlation between the presence of OSA and the TYG index [111].
Clinical studies have established a clear association between the presence of IR and CKD, and these IR levels have been shown to rise progressively alongside the worsening stages of CKD [112, 113]. TYG, as a promising marker of IR, has been closely linked to an increased risk and prevalence of CKD in this study and previous ones [114, 115]. Multiple mechanisms underlie the association between IR and CKD. IR disrupts the delicate balance between vasodilation and vasoconstriction within the kidney, ultimately leading to glomerular hyperfiltration [116]. It can interfere with mitochondrial function and energy metabolism in proximal tubular cells and disrupt the cytoskeletal architecture of podocytes, ultimately leading to dysfunction in the filtration barrier and proteinuria [117–120]. By addressing the important role of IR in the pathogenesis of CKD, TYG as a reliable surrogate marker of IR can play a crucial role first in the identification of the risk of developing CKD, second in predicting its progression to end-stage levels (ESRD), and third in identifying the risk of all-cause mortality in patients with CKD [121, 16].
CIN, a form of acute kidney injury following contrast exposure, is typically seen in patients undergoing invasive treatments for non-ST-segment elevation myocardial infarction (NSTEMI) [122, 123]. It can occur even in patients with normal baseline kidney function and is associated with higher mortality rates and hospital stays [124]. Therefore, a proper risk assessment and finding high-risk patients for timely prophylactic management are crucial. As reported in previous studies, diabetes is a well-established risk factor for the development of CIN [125, 126]. Thus, TyG index was evaluated in non-diabetic NSTEMI patients after percutaneous coronary intervention, and several original investigations discovered a high association between the TyG index and the risk of CIN. These findings were consistent with our results [127, 128].
NAFLD and MAFLD are two terms used to describe metabolic diseases related to steatosis in the liver, which are closely associated with the underlying condition of IR [129]. In this meta-umbrella study, after considering all original articles within various meta-analyses, there was a close relationship between metabolic syndrome and NAFLD with the TyG index. IR can manifest in both the liver and peripheral tissues. Hepatic IR impairs the liver’s ability to suppress glucose production, leading to hyperglycemia [130]. Peripheral IR, on the other hand, reduces the inhibitory effect of insulin on lipolysis, resulting in an increased flow of free fatty acids to the liver [131]. This excessive influx of fatty acids, combined with impaired fat metabolism in the liver, ultimately contributes to the development of hepatic steatosis, the hallmark of NAFLD and MAFLD [132]. Given the central role of IR in the pathogenesis of these metabolic liver diseases, it is crucial to identify a reliable marker that can accurately assess IR. Recent evidence suggests that the TyG index may be a more sensitive and reliable indicator of IR compared to the traditional HOMA-IR, particularly in the context of NAFLD and metabolic syndrome [67, 133].
There aren’t so many studies assessing the relationship between TyG and the risk of cognitive impairment and dementia. In the last meta-analysis assessing this correlation, there were only 10 studies included [43]. However, in our study, it was demonstrated that there is a significant association between TyG and both cognitive impairment and dementia. Drugs such as statins and hypoglycemic agents like metformin are important to consider as they are known to decrease the risk of cognitive impairment as well as affect the TyG index measurement [134, 135]. In addition, other factors like ethnicity, APOE genotype, and underlying diseases such as diabetes and hypertension are important factors that should be taken into consideration in future studies to make further rational analyses [136].
Stroke, as an acute neurological condition, greatly reduces the quality of life of patients and causes significant social and economic burdens in societies [137]. In our study, after assuming all the data, the results revealed that the risk of ischemic stroke and its recurrence was significantly related to the value of the TyG index. In a study on 10,132 participants of American adults without a history of stroke at baseline with a median follow-up of 26.6 years and after considering potential confounders, they reported that for each unit increase in the TyG index, there is an increase of 32.1% in the risk of stroke incidence [138]. Also, in our results, it was demonstrated that many complications of stroke, such as poor functional outcome, worsening of neurological state, and risk of mortality, have a significant correlation with the TyG index. However, data on stroke adverse events is controversial in different studies [139]. Yang et al. revealed that although the relationship between mortality and TyG is significant, they didn’t find a significant association in terms of poor functional outcomes and neurological worsening [15]. This could be explained by some sources of heterogeneity in these studies, such as the severity of the stroke itself, the quality of rehabilitation, secondary prevention methods, the existence of CYP2C19 polymorphisms, family support, and so many other factors that should be taken into consideration to better understand these relationships [15, 140, 141]. Ultimately, TyG could be considered to optimize risk stratification for stroke in the general population and could be a valuable marker for screening and management of high-risk patients.
Strengths, limitations, and future suggestions
This umbrella review has several strengths. It integrates findings from 32 meta-analyses, offering a comprehensive and detailed synthesis of the evidence on the diagnostic utility of the TyG Index across various medical conditions. The review follows rigorous methodology by adhering to established guidelines from the Cochrane Handbook and PRISMA standards, ensuring a systematic and transparent approach to study selection, data extraction, and analysis. Furthermore, the use of the AMSTAR2 checklist for quality assessment and GRADE criteria for evaluating the epidemiological strength of the associations adds robustness to the review process.
However, there are several limitations to consider. The included meta-analyses exhibited substantial heterogeneity, which could affect the reliability of the pooled estimates. The heterogeneity might stem from differences in study populations, diagnostic criteria, and analytical methods across the original studies. Moreover, the definitions of the TyG index and the cut-off values varied among the included studies in the meta-analyses, which is another source of heterogeneity. Additionally, while publication bias was generally low, it cannot be entirely ruled out, particularly in areas with fewer studies. Another limitation was that 22 of the 32 included studies were from a single nationality (China), which may impact the robustness and comprehensiveness of the results. In addition, the overall quality of the included meta-analyses varied, with some being assessed as low or critically low quality, which may influence the robustness of the conclusions drawn.
Future research should focus on addressing the sources of heterogeneity identified in this review. High-quality, large-scale prospective studies are needed to validate the associations between the TyG Index and various medical conditions. Standardizing the diagnostic criteria and analytical methods used in studies involving the TyG Index could help reduce variability and improve comparability. Moreover, there are metabolic diseases such as polycystic ovarian syndrome, obesity, and dyslipidemia for which meta-analyses should be conducted to evaluate the prognostic value of the TyG index. Additionally, further research should explore the potential mechanisms underlying the associations between the TyG Index and different diseases, which could provide deeper insights into its clinical utility. Investigating the TyG Index in diverse populations and settings would also enhance the generalizability of the findings, supporting its broader application in clinical practice.
Conclusion
The TyG Index shows significant promise as a screening marker for a wide range of medical conditions, including chronic kidney disease, type 2 diabetes mellitus, ischemic stroke, non-alcoholic fatty liver disease, cognitive impairment, coronary artery calcification, obstructive sleep apnea, gestational diabetes mellitus, metabolic syndrome, dementia, heart failure, atrial fibrillation, hypertension, and post-percutaneous coronary intervention events. Despite the encouraging associations, the substantial heterogeneity and varying quality of the included studies indicate the necessity for further rigorous research to confirm these findings. This comprehensive umbrella review provides valuable insights into the current evidence, supporting the potential clinical utility of the TyG Index and guiding future research efforts.
Electronic supplementary material
Acknowledgements
Not applicable.
Author contributions
The concept development for this research was carried out by E.AS, S.S.N, and L.D.P. The design and planning of the methodology to generate the results were handled by E.AS and S.S.N. Supervision, including oversight, organization, implementation, and manuscript writing, was provided by E.A.S, S.N, P.B, and M.T.U. Data collection and processing, which involved conducting experiments, organizing data, and reporting findings, were managed by E.AS, MH.K, N.L, and A.H. The analysis and interpretation of the data, including statistical analysis, evaluation, and presentation of the results, were the responsibility of A.A and MH.K. The literature search was performed by E.A.S and D.A. All authors contributed substantively to writing the manuscript.
Funding
None.
Data availability
No datasets were generated or analysed during the current study. The datasets used and/or analyzed during the current study can be provided from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bodaghi A, Fattahi N, Ramazani A. Biomarkers: Promising and valuable tools towards diagnosis, prognosis and treatment of Covid-19 and other diseases. Heliyon. 2023;9(2):e13323. 10.1016/j.heliyon.2023.e13323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drugan T, Leucuța D. Evaluating Novel Biomarkers for Personalized Medicine. Diagnostics (Basel). 2024. 10.3390/diagnostics14060587. 10.3390/diagnostics14060587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho YK, Han KD, Kim HS, Jung CH, Park JY, Lee WJ. Triglyceride-glucose index is a useful marker for Predicting Future Cardiovascular Disease and Mortality in young Korean adults: a Nationwide Population-based Cohort Study. J Lipid Atheroscler. 2022;11(2):178–86. 10.12997/jla.2022.11.2.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massimino M, Monea G, Marinaro G, Rubino M, Mancuso E, Mannino GC, Andreozzi F. The Triglycerides and Glucose (TyG) Index Is Associated with 1-Hour Glucose Levels during an OGTT. Int J Environ Res Public Health. 2022. 10.3390/ijerph20010787. 10.3390/ijerph20010787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Primo D, Izaola O, de Luis DA. Triglyceride-glucose index cutoff point is an accurate marker for Predicting the prevalence of metabolic syndrome in obese caucasian subjects. Ann Nutr Metab. 2023;79(2):238–45. 10.1159/000526988 [DOI] [PubMed] [Google Scholar]
- 6.Ramdas Nayak VK, Satheesh P, Shenoy MT, Kalra S. Triglyceride glucose (TyG) index: a surrogate biomarker of insulin resistance. J Pak Med Assoc. 2022;72(5):986–8. 10.47391/JPMA.22-63 [DOI] [PubMed] [Google Scholar]
- 7.Jiang M, Li X, Wu H, Su F, Cao L, Ren X, Hu J, Tatenda G, Cheng M, Wen Y. Triglyceride-glucose index for the diagnosis of metabolic syndrome: a cross-sectional study of 298,652 individuals receiving a Health Check-Up in China. Int J Endocrinol. 2022;2022:3583603. 10.1155/2022/3583603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alizargar J, Hsieh NC, Wu SV. The correct formula to calculate triglyceride-glucose index (TyG). J Pediatr Endocrinol Metab. 2020;33(7):945–6. 10.1515/jpem-2019-0579 [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Zhang T, He S, Jia S, Zhang Z, Ye R, Yang X, Chen X. RETRACTED ARTICLE: Association of metabolic syndrome with TyG index and TyG-related parameters in an urban Chinese population: a 15-year prospective study. Diabetol Metab Syndr. 2022;14(1):84. 10.1186/s13098-022-00855-4 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Zhang R, Guan Q, Zhang M, Ding Y, Tang Z, Wang H, Zhang W, Chen Y, Jiang R, Cui Y, et al. Association between triglyceride-glucose Index and Risk of Metabolic Dysfunction-Associated fatty liver disease: a Cohort Study. Diabetes Metab Syndr Obes. 2022;15:3167–79. 10.2147/DMSO.S383907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araújo SP, Juvanhol LL, Bressan J, Hermsdorff HHM. Triglyceride glucose index: a new biomarker in predicting cardiovascular risk. Prev Med Rep. 2022;29:101941. 10.1016/j.pmedr.2022.101941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. 10.1186/s12933-022-01511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang S, Wang C, Zhang J, Liu Z, Bai Y, Chen Z, Huang H, He Y. Triglyceride-glucose index and coronary artery disease: a systematic review and meta-analysis of risk, severity, and prognosis. Cardiovasc Diabetol. 2023;22(1):170. 10.1186/s12933-023-01906-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Zhu L, Li Y. Association between the triglyceride glucose index and diabetic retinopathy in type 2 diabetes: a meta-analysis. Front Endocrinol. 2023;14:1302127. 10.3389/fendo.2023.1302127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Huang X, Wang Y, Leng L, Xu J, Feng L, Jiang S, Wang J, Yang Y, Pan G, et al. The impact of triglyceride-glucose index on ischemic stroke: a systematic review and meta-analysis. Cardiovasc Diabetol. 2023;22(1):2. 10.1186/s12933-022-01732-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren X, Jiang M, Han L, Zheng X. Association between triglyceride-glucose index and chronic kidney disease: a cohort study and meta-analysis. Nutr Metab Cardiovasc Dis. 2023;33(6):1121–8. 10.1016/j.numecd.2023.03.026 [DOI] [PubMed] [Google Scholar]
- 17.Shuster JJ. Cochrane handbook for systematic reviews for interventions, Version 5.1. 0, published 3/2011. Julian PT Higgins and Sally Green, Editors. In. Wiley Online Library; 2011. [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. 10.1136/bmj.n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017. 10.1136/bmj.j4008. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011. 10.1136/bmj.d4002. 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The triglyceride-glucose index, an insulin resistance marker, was non-linear Associated with all-cause and Cardiovascular Mortality in the General Population. Front Cardiovasc Med. 2020;7:628109. 10.3389/fcvm.2020.628109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Chan JSK, Guan B, Peng S, Wu X, Lu X, Zhou J, Hui JMH, Lee YHA, Satti DI, et al. Triglyceride-glucose index and the risk of heart failure: evidence from two large cohorts and a mendelian randomization analysis. Cardiovasc Diabetol. 2022;21(1):229. 10.1186/s12933-022-01658-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Zhong H, Shao Y, Hua Y, Zhou X, Luo D. Association between the homeostasis model assessment of insulin resistance and coronary artery calcification: a meta-analysis of observational studies. Front Endocrinol (Lausanne). 2023;14:1271857. 10.3389/fendo.2023.1271857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DelBello MP, Kadakia A, Heller V, Singh R, Hagi K, Nosaka T, Loebel A. Systematic review and network Meta-analysis: Efficacy and Safety of Second-Generation antipsychotics in Youths with Bipolar Depression. J Am Acad Child Adolesc Psychiatry. 2022;61(2):243–54. 10.1016/j.jaac.2021.03.021 [DOI] [PubMed] [Google Scholar]
- 26.Beydoun HA, Ng TKS, Beydoun MA, Shadyab AH, Jung SY, Costanian C, Saquib N, Ikramuddin FS, Pan K, Zonderman AB, et al. Biomarkers of glucose homeostasis as mediators of the relationship of body mass index and waist circumference with COVID-19 outcomes among postmenopausal women: the women’s Health Initiative. Clin Nutr. 2023;42(9):1690–700. 10.1016/j.clnu.2023.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pranata R, Huang I, Irvan, Lim MA, Vania R. The association between triglyceride-glucose index and the incidence of type 2 diabetes mellitus-a systematic review and dose-response meta-analysis of cohort studies. Endocrine. 2021;74(2):254–62. 10.1007/s12020-021-02780-4 [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A, Solis RC. Diagnostic Accuracy of the Triglyceride and Glucose Index for Insulin Resistance: A Systematic Review. Int J Endocrinol. 2020. 10.1155/2020/4678526. 10.1155/2020/4678526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brito ADM, Hermsdorff HHM, Filgueiras MS, Suhett LG, Vieira-Ribeiro SA, Franceschini S, Novaes JF. Predictive capacity of triglyceride-glucose (TyG) index for insulin resistance and cardiometabolic risk in children and adolescents: a systematic review. Crit Rev Food Sci Nutr. 2021;61(16):2783–92. 10.1080/10408398.2020.1788501 [DOI] [PubMed] [Google Scholar]
- 30.Behnoush AH, Mousavi A, Ghondaghsaz E, Shojaei S, Cannavo A, Khalaji A. The importance of assessing the triglyceride-glucose index (TyG) in patients with depression: a systematic review. Neurosci Biobehav Rev. 2024;159:105582. 10.1016/j.neubiorev.2024.105582 [DOI] [PubMed] [Google Scholar]
- 31.Ibrahim Abougalambou SS, AbaAlkhail H, Abougalambou AS. The knowledge, attitude and practice among diabetic patient in central region of Saudi Arabia. Diabetes Metab Syndr. 2019;13(5):2975–81. 10.1016/j.dsx.2019.07.049 [DOI] [PubMed] [Google Scholar]
- 32.Ahmad A, Lim LL, Morieri ML, Tam CH, Cheng F, Chikowore T, Dudenhöffer-Pfeifer M, Fitipaldi H, Huang C, Kanbour S, et al. Precision prognostics for cardiovascular disease in type 2 diabetes: a systematic review and meta-analysis. Commun Med (Lond). 2024;4(1):11. 10.1038/s43856-023-00429-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stelmach-Mardas M, Brajer-Luftmann B, Kuśnierczak M, Batura-Gabryel H, Piorunek T, Mardas M. Body Mass Index Reduction and Selected Cardiometabolic Risk Factors in Obstructive Sleep Apnea: Meta-Analysis. J Clin Med. 2021. 10.3390/jcm10071485. 10.3390/jcm10071485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, Ma J, Zhao Y, Zhu W, Wang J. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):124. 10.1186/s12933-022-01546-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo JW, Duan WH, Yu YQ, Song L, Shi DZ. Prognostic significance of triglyceride-glucose index for adverse Cardiovascular events in patients with coronary artery disease: a systematic review and Meta-analysis. Front Cardiovasc Med. 2021;8:774781. 10.3389/fcvm.2021.774781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu A-r, Jin Q, Shen Z, Zhang J, Fu Q. Association between the risk of hypertension and triglyceride glucose index in Chinese regions: a systematic review and dose-response meta-analysis of a regional update. Front Cardiovasc Med. 2023;10:1242035. 10.3389/fcvm.2023.1242035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan F, Yan S, Wang J, Cui Y, Chen F, Fang F, Cui W. Association between triglyceride glucose index and risk of cerebrovascular disease: systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):226. 10.1186/s12933-022-01664-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou J, Zhu L, Li Y. Association between the triglyceride glucose index and diabetic retinopathy in type 2 diabetes: a meta-analysis. Front Endocrinol (Lausanne). 2023;14:1302127. 10.3389/fendo.2023.1302127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling Q, Chen J, Liu X, Xu Y, Ma J, Yu P, Zheng K, Liu F, Luo J. The triglyceride and glucose index and risk of nonalcoholic fatty liver disease: a dose-response meta-analysis. Front Endocrinol (Lausanne). 2022;13:1043169. 10.3389/fendo.2022.1043169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song T, Su G, Chi Y, Wu T, Xu Y, Chen C. Triglyceride-glucose index predicts the risk of gestational diabetes mellitus: a systematic review and meta-analysis. Gynecol Endocrinol. 2022;38(1):10–5. 10.1080/09513590.2021.1940932 [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Chi R, Jiang Y, Chen B, Chen Y, Chen Z. Triglyceride glycemic index as a biomarker for gestational diabetes mellitus: a systemic review and meta-analysis. Endocr Connect. 2021;10(11):1420–7. 10.1530/EC-21-0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun C, Hu L, Li X, Zhang X, Chen J, Li D, Zhang J, Liu L, Wu M. Triglyceride-glucose index’s link to cardiovascular outcomes post-percutaneous coronary intervention in China: a meta-analysis. ESC Heart Fail. 2024. 10.1002/ehf2.14679. 10.1002/ehf2.14679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, Ling Q, Wu Y, Zhang M. Association between the triglyceride glucose index and cognitive impairment and dementia: a meta-analysis. Front Aging Neurosci. 2023;15:1278730. 10.3389/fnagi.2023.1278730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Yang W, Jiang X. Association between triglyceride-glucose index and hypertension: a Meta-analysis. Front Cardiovasc Med. 2021;8:644035. 10.3389/fcvm.2021.644035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu F, Ling Q, Xie S, Xu Y, Liu M, Hu Q, Ma J, Yan Z, Gao Y, Zhao Y, et al. Association between triglyceride glucose index and arterial stiffness and coronary artery calcification: a systematic review and exposure-effect meta-analysis. Cardiovasc Diabetol. 2023;22(1):111. 10.1186/s12933-023-01819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong H, Shao Y, Guo G, Zhan Y, Liu B, Shao M, Li L. Association between the triglyceride-glucose index and arterial stiffness: a meta-analysis. Med (Baltim). 2023;102(10):e33194. 10.1097/MD.0000000000033194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao C, Xu H, Jin T, Xu K, Xu Z, Zhu L, Liu M. Triglyceride-glucose index and the incidence of stroke: a meta-analysis of cohort studies. Front Neurol. 2022;13:1033385. 10.3389/fneur.2022.1033385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng X, Yao Y, Wu L, Cheng C, Tang Q, Xu S. Triglyceride-glucose index and the risk of stroke: a systematic review and dose-response Meta-analysis. Horm Metab Res. 2022;54(3):175–86. 10.1055/a-1766-0202 [DOI] [PubMed] [Google Scholar]
- 49.Ma X, Han Y, Jiang L, Li M. Triglyceride-glucose index and the prognosis of patients with Acute ischemic stroke: a Meta-analysis. Horm Metab Res. 2022;54(6):361–70. 10.1055/a-1853-9889 [DOI] [PubMed] [Google Scholar]
- 50.Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride–glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20(1):76. 10.1186/s12933-021-01268-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Yan S, Cui Y, Chen F, Piao M, Cui W. The Diagnostic and Prognostic Value of the Triglyceride-Glucose Index in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD): A Systematic Review and Meta-Analysis. Nutrients. 2022. 10.3390/nu14234969. 10.3390/nu14234969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Ren L, Chang C, Luo L. Triglyceride-glukose index predicts adverse events in patients with Acute Coronary Syndrome: a Meta-analysis of Cohort studies. Horm Metab Res. 2021;53(9):594–601. 10.1055/a-1518-7503 [DOI] [PubMed] [Google Scholar]
- 53.Khalaji A, Behnoush AH, Khanmohammadi S, Ghanbari Mardasi K, Sharifkashani S, Sahebkar A, Vinciguerra C, Cannavo A. Triglyceride-glucose index and heart failure: a systematic review and meta-analysis. Cardiovasc Diabetol. 2023;22(1):244. 10.1186/s12933-023-01973-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Behnoush AH, Khalaji A, Ghondaghsaz E, Masrour M, Shokri Varniab Z, Khalaji S, Cannavo A. Triglyceride-glucose index and obstructive sleep apnea: a systematic review and meta-analysis. Lipids Health Dis. 2024;23(1):4. 10.1186/s12944-024-02005-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nabipoorashrafi SA, Seyedi SA, Rabizadeh S, Ebrahimi M, Ranjbar SA, Reyhan SK, Meysamie A, Nakhjavani M, Esteghamati A. The accuracy of triglyceride-glucose (TyG) index for the screening of metabolic syndrome in adults: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2022;32(12):2677–88. 10.1016/j.numecd.2022.07.024 [DOI] [PubMed] [Google Scholar]
- 56.Azarboo A, Behnoush AH, Vaziri Z, Daneshvar MS, Taghvaei A, Jalali A, Cannavo A, Khalaji A. Assessing the association between triglyceride-glucose index and atrial fibrillation: a systematic review and meta-analysis. Eur J Med Res. 2024;29(1):118. 10.1186/s40001-024-01716-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sajdeya O, Beran A, Mhanna M, Alharbi A, Burmeister C, Abuhelwa Z, Malhas SE, Khader Y, Sayeh W, Assaly R, et al. Triglyceride glucose index for the prediction of subclinical atherosclerosis and arterial stiffness: a Meta-analysis of 37,780 individuals. Curr Probl Cardiol. 2022;47(12):101390. 10.1016/j.cpcardiol.2022.101390 [DOI] [PubMed] [Google Scholar]
- 58.Beran A, Ayesh H, Mhanna M, Wahood W, Ghazaleh S, Abuhelwa Z, Sayeh W, Aladamat N, Musallam R, Matar R, et al. Triglyceride-Glucose Index for Early Prediction of Nonalcoholic Fatty Liver Disease: A Meta-Analysis of 121,975 Individuals. J Clin Med. 2022. 10.3390/jcm11092666. 10.3390/jcm11092666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akbar MR, Pranata R, Wibowo A, Irvan, Sihite TA, Martha JW. The association between triglyceride-glucose index and major adverse cardiovascular events in patients with acute coronary syndrome - dose-response meta-analysis. Nutr Metab Cardiovasc Dis. 2021;31(11):3024–30. 10.1016/j.numecd.2021.08.026 [DOI] [PubMed] [Google Scholar]
- 60.da Silva A, Caldas APS, Rocha D, Bressan J. Triglyceride-glucose index predicts independently type 2 diabetes mellitus risk: a systematic review and meta-analysis of cohort studies. Prim Care Diabetes. 2020;14(6):584–93. 10.1016/j.pcd.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 61.Chang WT, Liu CC, Huang YT, Wu JY, Tsai WW, Hung KC, Chen IW, Feng PH. Diagnostic efficacy of the triglyceride-glucose index in the prediction of contrast-induced nephropathy following percutaneous coronary intervention. Front Endocrinol (Lausanne). 2023;14:1282675. 10.3389/fendo.2023.1282675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun T, Huang X, Zhang B, Ma M, Chen Z, Zhao Z, Zhou Y. Prognostic significance of the triglyceride-glucose index for patients with ischemic heart failure after percutaneous coronary intervention. Front Endocrinol (Lausanne). 2023;14:1100399. 10.3389/fendo.2023.1100399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, Zhou YJ. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc Diabetol. 2020;19(1):108. 10.1186/s12933-020-01086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, Zhou YJ. Triglyceride-glucose index as a surrogate marker of insulin resistance for Predicting Cardiovascular outcomes in nondiabetic patients with Non-ST-Segment elevation Acute Coronary Syndrome undergoing percutaneous coronary intervention. J Atheroscler Thromb. 2021;28(11):1175–94. 10.5551/jat.59840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51. 10.1210/jc.2010-0288 [DOI] [PubMed] [Google Scholar]
- 66.Kim B, Choi HY, Kim W, Ahn C, Lee J, Kim JG, Kim J, Shin H, Yu JM, Moon S. The cut-off values of surrogate measures for insulin resistance in the Korean population according to the Korean Genome and Epidemiology Study (KOGES). PLoS ONE. 2018;13(11):e0206994. 10.1371/journal.pone.0206994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee SB, Kim MK, Kang S, Park K, Kim JH, Baik SJ, Nam JS, Ahn CW, Park JS. Triglyceride glucose index is Superior to the Homeostasis Model Assessment of Insulin Resistance for Predicting nonalcoholic fatty liver disease in Korean adults. Endocrinol Metab (Seoul). 2019;34(2):179–86. 10.3803/EnM.2019.34.2.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo P, Cao Y, Li P, Li W, Song Z, Fu Z, Zhou H, Yi X, Zhu L, Zhu S. TyG Index Performs Better Than HOMA-IR in Chinese Type 2 Diabetes Mellitus with a BMI < 35 kg/m2: A Hyperglycemic Clamp Validated Study. Med (Kaunas). 2022. 10.3390/medicina58070876. 10.3390/medicina58070876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJ, Geloneze B. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–100. 10.1016/j.diabres.2011.05.030 [DOI] [PubMed] [Google Scholar]
- 70.Li S, Guo B, Chen H, Shi Z, Li Y, Tian Q, Shi S. The role of the triglyceride (triacylglycerol) glucose index in the development of cardiovascular events: a retrospective cohort analysis. Sci Rep. 2019;9(1):7320. 10.1038/s41598-019-43776-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, Kim JH, Park JS. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–73. 10.2337/dc18-1920 [DOI] [PubMed] [Google Scholar]
- 72.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. 10.1186/1741-7015-9-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5(4):444–70. 10.4239/wjd.v5.i4.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y, Guo L, Li L, Yang C, Guang P, Huang F, Chen Z, Wang L, Hu J. Early diagnosis of type 2 diabetes based on Near-Infrared Spectroscopy Combined with Machine Learning and Aquaphotomics. Front Chem. 2020;8:580489. 10.3389/fchem.2020.580489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Calcaterra V, Montalbano C, de Silvestri A, Pelizzo G, Regalbuto C, Paganelli V, Albertini R, Cave FD, Larizza D, Cena H. Triglyceride Glucose Index as a Surrogate Measure of Insulin Sensitivity in a Caucasian Pediatric Population. J Clin Res Pediatr Endocrinol. 2019. 10.4274/jcrpe.galenos.2019.2019.0024. 10.4274/jcrpe.galenos.2019.2019.0024 [DOI] [PubMed] [Google Scholar]
- 76.Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W. The triglyceride-glucose index, a predictor of type 2 diabetes development: a retrospective cohort study. Prim Care Diabetes. 2020;14(2):161–7. 10.1016/j.pcd.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 77.Dikaiakou E, Vlachopapadopoulou EA, Paschou SA, Athanasouli F, Panagiotopoulos Ι, Kafetzi M, Fotinou A, Michalacos S. Τriglycerides-glucose (TyG) index is a sensitive marker of insulin resistance in Greek children and adolescents. Endocrine. 2020;70(1):58–64. 10.1007/s12020-020-02374-6 [DOI] [PubMed] [Google Scholar]
- 78.Liu EQ, Weng YP, Zhou AM, Zeng CL. Association between Triglyceride-Glucose Index and Type 2 Diabetes Mellitus in the Japanese Population: A Secondary Analysis of a Retrospective Cohort Study. Biomed Res Int. 2020. 10.1155/2020/2947067. 10.1155/2020/2947067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Locateli JC, Lopes WA, Simões CF, de Oliveira GH, Oltramari K, Bim RH, de Souza Mendes VH, Remor JM, Lopera CA. Nardo Junior N: Triglyceride/glucose index is a reliable alternative marker for insulin resistance in south American overweight and obese children and adolescents. J Pediatr Endocrinol Metab. 2019;32(10):1163–70. 10.1515/jpem-2019-0037 [DOI] [PubMed] [Google Scholar]
- 80.Minh HV, Tien HA, Sinh CT, Thang DC, Chen CH, Tay JC, Siddique S, Wang TD, Sogunuru GP, Chia YC, et al. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J Clin Hypertens (Greenwich). 2021;23(3):529–37. 10.1111/jch.14155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park B, Lee HS, Lee YJ. Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: a 12-year longitudinal study of the Korean genome and epidemiology study cohort. Transl Res. 2021;228:42–51. 10.1016/j.trsl.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 82.Begum T, Rahman A, Nomani D, Mamun A, Adams A, Islam S, Khair Z, Khair Z, Anwar I. Diagnostic accuracy of detecting Diabetic Retinopathy by using Digital Fundus photographs in the Peripheral Health facilities of Bangladesh: Validation Study. JMIR Public Health Surveill. 2021;7(3):e23538. 10.2196/23538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arıkan S, Erşan İ, Eroğlu M, Yılmaz M, Tufan HA, Gencer B, Kara S, Aşık M. Does retinal neurodegeneration seen in Diabetic patients begin in the insulin resistance stage? Turk J Ophthalmol. 2016;46(6):264–9. 10.4274/tjo.68888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Platania CBM, Maisto R, Trotta MC, D’Amico M, Rossi S, Gesualdo C, D’Amico G, Balta C, Herman H, Hermenean A, et al. Retinal and circulating miRNA expression patterns in diabetic retinopathy: an in silico and in vivo approach. Br J Pharmacol. 2019;176(13):2179–94. 10.1111/bph.14665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bowers K, Yeung E, Williams MA, Qi L, Tobias DK, Hu FB, Zhang C. A prospective study of prepregnancy dietary iron intake and risk for gestational diabetes mellitus. Diabetes Care. 2011;34(7):1557–63. 10.2337/dc11-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pazhohan A, Rezaee Moradali M, Pazhohan N. Association of first-trimester maternal lipid profiles and triglyceride-glucose index with the risk of gestational diabetes mellitus and large for gestational age newborn. J Matern Fetal Neonatal Med. 2019;32(7):1167–75. 10.1080/14767058.2017.1402876 [DOI] [PubMed] [Google Scholar]
- 87.Sánchez-García A, Rodríguez-Gutiérrez R, Saldívar-Rodríguez D, Guzmán-López A, Castillo-Castro C, Mancillas-Adame L, Santos-Santillana K, González-Nava V, González-González JG. Diagnostic accuracy of the triglyceride-glucose index for gestational diabetes screening: a practical approach. Gynecol Endocrinol. 2020;36(12):1112–5. 10.1080/09513590.2020.1742687 [DOI] [PubMed] [Google Scholar]
- 88.Liu M, Chen Y, Chen D. Association between transthyretin concentrations and gestational diabetes mellitus in Chinese women. Arch Gynecol Obstet. 2020;302(2):329–35. 10.1007/s00404-020-05599-y [DOI] [PubMed] [Google Scholar]
- 89.Wiznitzer A, Mayer A, Novack V, Sheiner E, Gilutz H, Malhotra A, Novack L. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population-based study. Am J Obstet Gynecol. 2009;201(5):e482481–488. 10.1016/j.ajog.2009.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–97. 10.1111/eci.12583 [DOI] [PubMed] [Google Scholar]
- 91.Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, Sowers JR. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766. 10.1016/j.metabol.2021.154766 [DOI] [PubMed] [Google Scholar]
- 92.Ginsberg HN, Packard CJ, Chapman MJ, Borén J, Aguilar-Salinas CA, Averna M, Ference BA, Gaudet D, Hegele RA, Kersten S, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42(47):4791–806. 10.1093/eurheartj/ehab551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nahmias A, Stahel P, Xiao C, Lewis GF. Glycemia and atherosclerotic Cardiovascular Disease: exploring the gap between risk marker and risk factor. Front Cardiovasc Med. 2020;7:100. 10.3389/fcvm.2020.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res. 2017;113(4):389–98. 10.1093/cvr/cvx012 [DOI] [PubMed] [Google Scholar]
- 95.Rienstra M, Yin X, Larson MG, Fontes JD, Magnani JW, McManus DD, McCabe EL, Coglianese EE, Amponsah M, Ho JE, et al. Relation between soluble ST2, growth differentiation factor-15, and high-sensitivity troponin I and incident atrial fibrillation. Am Heart J. 2014;167(1):109–e115102. 10.1016/j.ahj.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, Tofler GH, Selhub J, Jacques PF, Wolf PA, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121(2):200–7. 10.1161/CIRCULATIONAHA.109.882241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chua W, Purmah Y, Cardoso VR, Gkoutos GV, Tull SP, Neculau G, Thomas MR, Kotecha D, Lip GYH, Kirchhof P, et al. Data-driven discovery and validation of circulating blood-based biomarkers associated with prevalent atrial fibrillation. Eur Heart J. 2019;40(16):1268–76. 10.1093/eurheartj/ehy815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sinner MF, Stepas KA, Moser CB, Krijthe BP, Aspelund T, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, et al. B-type natriuretic peptide and C-reactive protein in the prediction of atrial fibrillation risk: the CHARGE-AF Consortium of community-based cohort studies. Europace. 2014;16(10):1426–33. 10.1093/europace/euu175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mohtasham Kia Y, Cannavo A, Bahiraie P, Alilou S, Saeedian B, Babajani N, Ghondaghsaz E, Khalaji A, Behnoush AH. Insights into the Role of Galectin-3 as a Diagnostic and Prognostic Biomarker of Atrial Fibrillation. Dis Markers. 2023. 10.1155/2023/2097012. 10.1155/2023/2097012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rizal A, Waranugraha Y, Wikananda AP, Yuniadi Y. Identification of microRNAs as diagnostic biomarkers for atrial fibrillation: a systematic review and meta-analysis. Front Cardiovasc Med. 2023;10:1128708. 10.3389/fcvm.2023.1128708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Selvi NMK, Nandhini S, Sakthivadivel V, Lokesh S, Srinivasan AR, Sumathi S. Association of triglyceride-glucose index (TyG index) with HbA1c and Insulin Resistance in type 2 diabetes Mellitus. Maedica (Bucur). 2021;16(3):375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.da Silva AA, do Carmo JM, Li X, Wang Z, Mouton AJ, Hall JE. Role of Hyperinsulinemia and Insulin Resistance in hypertension: metabolic syndrome revisited. Can J Cardiol. 2020;36(5):671–82. 10.1016/j.cjca.2020.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mancusi C, Izzo R, di Gioia G, Losi MA, Barbato E, Morisco C. Insulin resistance the Hinge between Hypertension and Type 2 diabetes. High Blood Press Cardiovasc Prev. 2020;27(6):515–26. 10.1007/s40292-020-00408-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006. 10.1161/CIRCRESAHA.116.305697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why). J Hypertens. 2001;19(3 Pt 2):523–8. 10.1097/00004872-200103001-00001 [DOI] [PubMed] [Google Scholar]
- 106.Wheatcroft SB, Williams IL, Shah AM, Kearney MT. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med. 2003;20(4):255–68. 10.1046/j.1464-5491.2003.00869.x [DOI] [PubMed] [Google Scholar]
- 107.Ryan S. Adipose tissue inflammation by intermittent hypoxia: mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J Physiol. 2017;595(8):2423–30. 10.1113/JP273312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luo Z, Tian M, Yang G, Tan Q, Chen Y, Li G, Zhang Q, Li Y, Wan P, Wu J. Hypoxia signaling in human health and diseases: implications and prospects for therapeutics. Signal Transduct Target Ther. 2022;7(1):218. 10.1038/s41392-022-01080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gabryelska A, Szmyd B, Panek M, Szemraj J, Kuna P, Białasiewicz P. Serum hypoxia-inducible factor-1α protein level as a diagnostic marker of obstructive sleep apnea. Pol Arch Intern Med. 2020;130(2):158–60. 10.20452/pamw.15220 [DOI] [PubMed] [Google Scholar]
- 110.Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab. 2010;24(5):843–51. 10.1016/j.beem.2010.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bikov A, Frent SM, Meszaros M, Kunos L, Mathioudakis AG, Negru AG, Gaita L, Mihaicuta S. Triglyceride-Glucose Index in Non-Diabetic, Non-Obese Patients with Obstructive Sleep Apnoea. J Clin Med. 2021. 10.3390/jcm10091932. 10.3390/jcm10091932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vladu M, Clenciu D, Efrem IC, Forțofoiu MC, Amzolini A, Micu ST, Moţa M, Forțofoiu M. Insulin resistance and chronic kidney disease in patients with type 1 diabetes Mellitus. J Nutr Metab. 2017;2017:6425359. 10.1155/2017/6425359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liao MT, Sung CC, Hung KC, Wu CC, Lo L, Lu KC. Insulin resistance in patients with chronic kidney disease. J Biomed Biotechnol. 2012;2012:691369. 10.1155/2012/691369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao YM, Chen WJ, Deng ZL, Shang Z, Wang Y. Association between triglyceride-glucose index and risk of end-stage renal disease in patients with type 2 diabetes mellitus and chronic kidney disease. Front Endocrinol (Lausanne). 2023;14:1150980. 10.3389/fendo.2023.1150980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cui C, Liu L, Zhang T, Fang L, Mo Z, Qi Y, Zheng J, Wang Z, Xu H, Yan H, et al. Triglyceride-glucose index, renal function and cardiovascular disease: a national cohort study. Cardiovasc Diabetol. 2023;22(1):325. 10.1186/s12933-023-02055-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tuttle KR. Back to the future: glomerular hyperfiltration and the Diabetic kidney. Diabetes. 2017;66(1):14–6. 10.2337/dbi16-0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hallan S, Sharma K. The role of Mitochondria in Diabetic kidney disease. Curr Diab Rep. 2016;16(7):61. 10.1007/s11892-016-0748-0 [DOI] [PubMed] [Google Scholar]
- 118.Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, Pu M, Sharma S, You YH, Wang L, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013;24(11):1901–12. 10.1681/ASN.2013020126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12(4):329–40. 10.1016/j.cmet.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ma S, Qiu Y, Zhang C. Cytoskeleton Rearrangement in Podocytopathies: An Update. Int J Mol Sci. 2024. 10.3390/ijms25010647. 10.3390/ijms25010647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duan S, Zhou M, Lu F, Chen C, Chen S, Geng L, Zhang C, Guo H, Zeng M, Sun B, et al. Triglyceride-glucose index is associated with the risk of chronic kidney disease progression in type 2 diabetes. Endocrine. 2023;81(1):77–89. 10.1007/s12020-023-03357-z [DOI] [PubMed] [Google Scholar]
- 122.Mandurino-Mirizzi A, Munafò A, Crimi G. Contrast-Associated Acute Kidney Injury. J Clin Med. 2022. 10.3390/jcm11082167. 10.3390/jcm11082167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li M, Li L, Qin Y, Luo E, Wang D, Qiao Y, Tang C, Yan G. Elevated TyG index predicts incidence of contrast-Induced Nephropathy: a retrospective cohort study in NSTE-ACS patients implanted with DESs. Front Endocrinol (Lausanne). 2022;13:817176. 10.3389/fendo.2022.817176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103(5):368–75. 10.1016/S0002-9343(97)00150-2 [DOI] [PubMed] [Google Scholar]
- 125.Khan FR, Nawaz T, Sajjad W, Hussain S, Amin M, Ali H. Evaluating the Differential risk of contrast-Induced Nephropathy among Diabetic and non-diabetic patients following percutaneous coronary intervention. Cureus. 2024;16(2):e53493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shoukat S, Gowani SA, Jafferani A, Dhakam SH. Contrast-induced nephropathy in patients undergoing percutaneous coronary intervention. Cardiol Res Pract. 2010. 10.4061/2010/649164. 10.4061/2010/649164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gursoy E, Baydar O. The triglyceride-glucose index and contrast-induced nephropathy in non-ST elevation myocardial infarction patients undergoing percutaneous coronary intervention. Med (Baltim). 2023;102(2):e32629. 10.1097/MD.0000000000032629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aktas H, Inci S, Gul M, Gencer S, Yildirim O. Increased triglyceride-glucose index predicts contrast-induced nephropathy in non-diabetic NSTEMI patients: a prospective study. J Investig Med. 2023;71(8):838–44. 10.1177/10815589231182317 [DOI] [PubMed] [Google Scholar]
- 129.Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13(1):9–19. [PMC free article] [PubMed] [Google Scholar]
- 130.Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42(13–14):1331–46. 10.1016/j.clinbiochem.2009.05.018 [DOI] [PubMed] [Google Scholar]
- 131.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–89. 10.1002/hep.23280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015;14:121. 10.1186/s12944-015-0123-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu J, Guan L, Zhao M, Li Q, Song A, Gao L, Lin H, Zhao J. Association between the triglyceride-glucose index and outcomes of nonalcoholic fatty liver disease: a large-Scale Health Management Cohort Study. Diabetes Metab Syndr Obes. 2021;14:2829–39. 10.2147/DMSO.S316864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chu CS, Tseng PT, Stubbs B, Chen TY, Tang CH, Li DJ, Yang WC, Chen YW, Wu CK, Veronese N, et al. Use of statins and the risk of dementia and mild cognitive impairment: a systematic review and meta-analysis. Sci Rep. 2018;8(1):5804. 10.1038/s41598-018-24248-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Samaras K, Makkar S, Crawford JD, Kochan NA, Wen W, Draper B, Trollor JN, Brodaty H, Sachdev PS. Metformin Use is Associated with slowed cognitive decline and reduced Incident Dementia in older adults with type 2 diabetes: the Sydney Memory and Ageing Study. Diabetes Care. 2020;43(11):2691–701. 10.2337/dc20-0892 [DOI] [PubMed] [Google Scholar]
- 136.Schiepers OJ, Harris SE, Gow AJ, Pattie A, Brett CE, Starr JM, Deary IJ. APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Mol Psychiatry. 2012;17(3):315–24. 10.1038/mp.2010.137 [DOI] [PubMed] [Google Scholar]
- 137.Lopez-Bastida J, Oliva Moreno J, Worbes Cerezo M, Perestelo Perez L, Serrano-Aguilar P, Montón-Álvarez F. Social and economic costs and health-related quality of life in stroke survivors in the Canary Islands, Spain. BMC Health Serv Res. 2012;12:315. 10.1186/1472-6963-12-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang X, Liu Q, Wang T, Tian W, Chen X, Zhang J, Li Q, Ma D, Zhao L, Chen Z, et al. Triglyceride-glucose index and the risk of stroke in American adults: findings from the atherosclerosis risk in communities study. Diabetol Metab Syndr. 2023;15(1):187. 10.1186/s13098-023-01161-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nam KW, Kang MK, Jeong HY, Kim TJ, Lee EJ, Bae J, Jeon K, Jung KH, Ko SB, Yoon BW. Triglyceride-glucose index is associated with early neurological deterioration in single subcortical infarction: early prognosis in single subcortical infarctions. Int J Stroke. 2021;16(8):944–52. 10.1177/1747493020984069 [DOI] [PubMed] [Google Scholar]
- 140.Wang Y, Meng X, Wang A, Xie X, Pan Y, Johnston SC, Li H, Bath PM, Dong Q, Xu A, et al. Ticagrelor versus Clopidogrel in CYP2C19 loss-of-function carriers with stroke or TIA. N Engl J Med. 2021;385(27):2520–30. 10.1056/NEJMoa2111749 [DOI] [PubMed] [Google Scholar]
- 141.Gittler M, Davis AM. Guidelines for adult Stroke Rehabilitation and Recovery. JAMA. 2018;319(8):820–1. 10.1001/jama.2017.22036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study. The datasets used and/or analyzed during the current study can be provided from the corresponding author on reasonable request.