Abstract
Background
In the past two decades, the impacts of Helium-Neon (He–Ne) laser on stress resistance and secondary metabolism in plants have been studied, but the signaling pathway which by laser regulates this process remains unclear. Therefore, the current study sought to explore the role of RBOH-dependent signaling in He–Ne laser-induced salt tolerance and elicitation of secondary metabolism in Salvia officinalis. Seeds were primed with He–Ne laser (6 J cm− 2) and peroxide hydrogen (H2O2, 5 mM) and 15-old-day plants were exposed to two salinity levels (0, 75 mM NaCl).
Results
Salt stress reduced growth parameters, chlorophyll content and relative water content (RWC) and increased malodialdehyde (MDA) and H2O2 contents in leaves of 45-old-day plants. After 48 h of salt exposure, higher transcription levels of RBOH (encoding NADPH oxidase), PAL (phenylalanine ammonia-lyase), and RAS (rosmarinic acid synthase) were recorded in leaves of plants grown from seeds primed with He–Ne laser and/or H2O2. Despite laser up-regulated RBOH gene in the early hours of exposing to salinity, H2O2 and MDA contents were lower in leaves of these plants after 30 days. Seed pretreatment with He–Ne laser and/or H2O2 augmented the accumulation of anthocyanins, total phenol, carnasol, and rosmarinic acid and increased total antioxidant capacity under non-saline and more extensively at saline conditions. Indeed, these treatments improved RWC, and K+/Na+ ratio, enhanced the activities of superoxide dismutase and ascorbate peroxidase and proline accumulation, and significantly decreased membrane injury and H2O2 content in leaves of 45-old-day plants under salt stress. However, applying diphenylene iodonium (DPI as an inhibitor of NADPH oxidase) and N, N-dimethyl thiourea (DMTU as a H2O2 scavenger) after laser priming reversed the aforementioned effects which in turn resulted in the loss of laser-induced salt tolerance and secondary metabolism.
Conclusions
These findings for the first time deciphered that laser can induce a transient RBOH-dependent H2O2 burst, which might act as a downstream signal to promote secondary metabolism and salt stress alleviation in S. officinalis plants.
Keywords: Anthocyanin, Antioxidant enzyme, NADPH oxidase, Proline, K+/Na+ ratio, Phenylalanine ammonia-lyase
Introduction
Salt stress is one of the major environmental challenges that restricts the growth and development of plants due to disturbing water relations and nutrient balances and triggers oxidative damage in plants [1]. Plants attenuate these detrimental effects, through osmotic adjustment, Na+ removal, and the activation of the antioxidative system and the biosynthesis of phenolic compounds and anthocyanins [1–6]. The phenolic compounds directly attenuate oxidative stress in cells due to their ability to donate electrons to reactive oxygen species (ROS), and chelate metal ions (Cu+ 2 and Fe+ 2) involved in the Fenton reaction. In addition, polyphenols can be used as reducing agents for the action of antioxidant enzymes and thus indirectly also enhance ROS scavenging [7].
In recent years, modern technologies such as seed irradiation with Helium-Neon (He–Ne) laser have attained to enhance stress tolerance in plants [8]. The growing experimental evidence has exhibited that the appropriate dosage of He–Ne laser can withstand plants against abiotic stress such as salinity [9], drought stress [10], cadmium toxicity [11], and ultraviolet-B radiation [12]. The effect of laser on plants might be related to its electromagnetic, optical, and thermal impacts on biomolecules [13]. A study using transcriptomic and physiological analysis exhibited that He-Ne laser pretreatment up-regulated transcription of genes implicated in modulating nutrient uptake and transport, photosynthesis, ROS homeostasis, and osmotic adjustment and consequently led to higher drought acclimation in wheat seedlings [14]. Several studies depicted that the laser elicited the production of anthocyanins in Arabidopsis [15] and apple [16], increased phenolic compounds in buckwheat sprouts [17], and changed phenolic profile in sunflower under drought stress [18]. However, the signaling network which by laser regulates stress tolerance and secondary metabolism has been rarely studied.
Under exposure to various stresses, overproduction of ROS causes oxidative damage in cells; whereas at low concentrations ROS can serve as signal molecules to promote developmental processes and protective strategies in plants. Among ROS, hydrogen peroxide (H2O2) is more stable and its role as a signal molecule in stress tolerance acquisition has been defined in plants [19]. The experimental evidence has demonstrated a distinct role of respiratory burst oxidase homolog (RBOH) in the production of an endogenous H2O2 burst during salt tolerance [2, 20]. RBOH genes are encoding plasma membrane-localized NADPH oxidase enzymes that catalyze the transfer of electrons from NADPH to O2 and the formation of O2˙¯, and then superoxide immediately is converted to H2O2 by SOD activity [21]. Under stress conditions, RBOH–generated H2O2 diffuses into the cytosol and regulates adaptive responses [19]. A study exhibited that the rbohD gene is essential for salt acclimation and rbohD mutants of Arabidopsis were more susceptible to hypoxia and salt stress and showed lesser ability for K+ retention and uptake Na+ and Cl− more than wild plants [22]. Other studies also showed that the inhibition of NADPH- oxidase activity by diphenylene iodonium (DPI) or ROS scavenging by N, N-dimethyl thiourea (DMTU) reduced the expression level of Δ1-pyrroline-5-carboxylate synthetase and proline content [23] and the gene expression and activities of APX, GR, and CAT enzymes [24] in Arabidopsis under salt stress. Sun et al. [25] also reported that DPI or DMTU enhanced K+ efflux and decreased Na+/H+ antiporter activity in NaCl-stressed calluses of Populus euphratica. Wu et al. [26] argued that ROS acts as signal molecules to upregulate genes involved in anthocyanin biosynthesis and increasing anthocyanins led to reducing ROS and maintaining photosynthetic capacity in radish plants under stressful conditions. The content of phenolics and flavonoids, as well as antioxidant properties (DPPH and ABTS capacities) in chia (Salvia hispanica), increased in response to H2O2 seed pretreatment [27]. A study also exhibited that H2O2 burst generated by NADPH-oxidase enzyme is involved in triggering salvianolic acid biosynthesis induced by salicylic acid in Salvia miltiorrhiza [28]. The above literature, imply to the role of H2O2 as a key player in the acquisition of salt tolerance and elicitation of secondary metabolism in plants.
Recently, the role of RBOH also has been elucidated in the tolerance of excess light stress [29, 30]. A study also showed that light exposure upregulates the expression of genes related to H2O2 signaling in plants and both light radiation and H2O2 treatment share many same genes including genes responsive to growth and development and stress tolerance [31]. Therefore, the motivation of RBOH-dependent signaling in plants by laser photons also is very possible. However, the role of H2O2 in He–Ne laser-induced secondary metabolism and salt tolerance has been not investigated in any plant species to date. Herein, we investigated this context in Salvia officinalis L. plants.
Common sage (S. officinalis L.) is a medicinal plant whose most effective antioxidant constituents are rosmarinic acid, carnosic acid, and carnosol. The superoxide scavenging capacity of the rosmarinic acid is higher than trolox and the radical scavenging activity of carnosol is similar to α-tocopherol [32]. The biosynthesis of phenolics is promoted by the phenylalanine ammonia-lyase (PAL) enzyme in the phenylpropanoid pathway. In one branch of this pathway after several stages, finally rosmarinic acid is synthesized by rosmarinic acid synthase (RAS) [33]. Carnosic acid is a labdane-type phenolic diterpene specific to some species of the Lamiaceae family such as common sage and rosemary [34]. Carnosic acid and its major oxidized derivative, carnosol, possess antioxidative properties and can protect linolenic acid and monogalactosyl diacylglycerol against hydroxyl radicals and singlet oxygen. This compound exhibits an antimicrobial effect and is used as a preservative in the food industry [35]. Previous studies showed that various elicitors such as salinity, heavy metal stress, nitric oxide, salicylic acid, H2O2, and laser influence the production of rosmarinic acid, carnosic acid, and carnosol in Salvia species [34, 36–38].Therefore, S. officinalis was selected for this study and hypothesized that H2O2 signaling is likely involved in the acquisition of laser-induced salt tolerance and elicitation of secondary metabolism in this medicinal herb. In the current study, we compared the impact of seed pretreatment with He–Ne laser and H2O2 on adaptive responses under salt stress. Furthermore, we explored the role of RBOH-dependent signaling in laser-induced salt tolerance and rosmarinic acid and carnosol biosynthesis in S. officinalis plants through inhibiting NADPH-oxidase activity by DPI and H2O2 scavenging by DMTU and analysis of expression of RBOH gene.
Materials and methods
Seed pre-treatments and plant cultivation
The results of a previous experiment [38] showed that seed pretreatment with 5 mM H2O2 for 6 h or with an energy dose of 6 J cm− 2 He-Ne laser and 75 mM salinity were the best levels to stimulate secondary metabolism in S. officinalis seedlings. However, the underlying mechanism of how laser and H2O2 function together for eliciting proline, carnosol, and anthocyanin and enhancing SOD and APX activities and the role of RBOH-dependent signaling in laser-induced responses was not assessed in mentioned study. Therefore, these optimal levels of treatments were used in the current experiment to assess the role of H2O2 in laser-elicited secondary metabolism and salt tolerance, through scavenging endogenous H2O2 by N-dimethyl thiourea (DMTU) and inhibition of NADPH- oxidase activity by diphenylene iodonium (DPI) in laser-treated plants.
Seeds of S. officinalis were provided from the Medicinal Plant Research Center of Isfahan, Iran, and surfaced-sterilized (by 2% (v/v) sodium hypochlorite) seeds were treated by the following methods.
Before priming seeds to He–Ne laser, uniform seeds were rinsed for 6 h in distilled water and then were surface-dried using filter paper. A portable He–Ne laser (Mahfanavar sn: 9812210039-A29 made in Iran) with a wavelength of 632.8 nm, beam diameter 12 mm was applied for seed irradiation, and a power⁄energy meter was used for setting the laser output power. Seeds were irradiated with output power 10 mWcm− 2 for 10 min which was equal energy dose of 6 J cm− 2 He–Ne laser. It is worth noting that energy dose of laser is defined as the power (W) × time (sec) as described by Muthusamy et al. [39]. The laser irradiation was carried out after 15 min of the laser warm-up time to avoid any possible error in the laser power stability. The laser power was monitored before and after each exposure using a laser power meter to ensure proper energy delivery to the target site. Another group of seeds were primed by soaking in 5 mM H2O2 solution for 6 h. For lowering endogenous H2O2 levels, laser-treated seeds were rinsed in solutions of 1 mM diphenylene iodonium (200 µM DPI as an inhibitor of NADPH- oxidase) or N, N-dimethyl thiourea (1 mM DMTU as a H2O2 scavenger). Pre-treatments included:
(1) Control (distilled water); (2) H2O2; (3) laser; (4) laser + H2O2; (5) laser + DPI; (6) laser + DMTU.
The number of 10 seeds from each of the above treatments was sown in plastic pots containing 2 kg cocopeat and pearlite in a 1:1 ratio and one week after seed germination, plants were thinned to 5 per pot. The plants were grown in the greenhouse under a relative humidity of 65% and a photoperiod of 14 h day /10 h night. At first, pots were irrigated daily with distilled water, and after 15 days, plants were irrigated with quarter-strength Hoagland’s solution containing 75 mM NaCl or nutrient solution without salt 3 times per week. Furthermore, once every two weeks, non-saline irrigation water was used to avoid salt accumulation in the medium.
Previous studies showed that the peak of RBOH-dependent H2O2 burst occurs in the early hours of stress exposure and then it drops [40, 41]. Therefore, leaf samples were collected 48 h after implementing the salt stress and quickly used for gene analysis. After 4 weeks of exposure to salinity stress, the fresh samples transferred to a − 80 °C freezer for measuring biochemical attributes. Then, 45-old-day plants were harvested, and fresh and dry weights (were dried for 4 days at room temperature) of shoots, were determined and Na+ and K+ concentrations were measured in dried shoots.
Measurement of chlorophyll content
To measure total chlorophyll content, leaves were extracted in 80% acetone and the optical absorbance of filtrates was recorded at 645 and 663 nm. Total chlorophyll content was calculated using the formula suggested by Arnon [42].
Quantification of proline content and relative water content
The relative water content (RWC) in fresh leaves was determined by the following formula:
RWC (%) = [(FW − DW) ∕ (TW − DW)] × 100
Where FW, DW, and TW respectively are fresh weight, dry weight (after being oven-dried at 75 ◦C), and turgor weight (after floating the leaves in water for 14 h) of leaf samples [43].
To quantify the proline contents in leaves, ninhydrin reagent was applied according to Bates et al. [44] method. Briefly, leaf samples were homogenized in 3% sulfosalicylic acid and after centrifugation at 4 °C, the supernatant was mixed with ninhydrin reagent and glacial acid. Tubes were placed in a boiling water bath for 40 min and after cooling, toluene was added and incubated at room temperature. The absorbance was read at 520 nm and the proline content was calculated using the standard curve.
Quantification of Na+ and K+ concentrations
Dried leaf samples (0.1 g) were converted to ashes at 560 °C and then digested using HCl acid. After removal of excess HCl by heating, residual sediment was dissolved in distilled water and the absorbance was read by a flame photometer. The Na+ and K+ contents of each leaf sample were determined using the standard curve [3].
Evaluation of oxidative biomarkers
The malondialdehyde (MDA) was measured in the supernatant of leaves homogenized in trichloroacetic acid based on the thiobarbituric acid (TBA) reaction using the method adopted by Heath and Packer [45]. The optical absorbance was read at 532 and 600 nm, and the MDA content was calculated using an extinction coefficient of 155 mM− 1 cm− 1.
Hydrogen peroxide content was quantified by the method adopted by Velikova et al. [46] using a KI reagent and recording of optical absorbance at 390 nm. H2O2 content in the fresh weight (FW) of leaves was determined by referring to the standard curve.
Histochemical localization of H2O2 and O2˙¯ was detected by the method described by Vafadar et al. [3]. Leaf samples were incubated in either 3,3′-diaminobenzidine (DAB) for 8 h in darkness or nitroblue tetrazolium (NBT) for 30 min. After the decolorization of leaves, by 20 min boiling in 95% ethanol solution, dark yellow spots in DAB staining and dark blue spots in NBT staining were considered as H2O2 and O2˙¯production in leaf tissues.
Determination of antioxidant enzyme activities
First, leaf samples were macerated using an extraction buffer in a mortar on the ice bath. After centrifugation, the supernatant was applied for enzyme assay.
To assay the ascorbate peroxidase (APX) activity, declining absorbance at 290 nm was followed for 1 min and, APX activity was calculated using an extinction coefficient 2.8 mM–1 cm–1 as previously explained by Valivand et al. [47].
Superoxide dismutase (SOD) activity was assayed based on recording the decrease in absorbance of nitro-blue tetrazolium (NBT) dye caused by the enzyme at 560 nm as previously described by Pirooz et al. [37]. One unit of SOD activity was defined as the amount of enzyme that inhibited the 50% reduction of NBT.
Quantitative RT‑PCR
To further decipher the link between endogenous H2O2 burst and He–Ne laser-induced responses, the expression of PAL, RBOH, and RAS genes was analyzed in samples. The LiCl-Phenol chloroform method was applied for the extraction of total RNA and cDNA synthesis was done using a kit (Smobio Co., South Korea). 18 S rRNA gene was applied as an internal control. The list of the primers used in this survey has been indicated in Table 1. For each reaction, 2X SYBR-Green Real-time PCR Master Mix (Bio fact, Soth Korea) was applied. The PCR program was promoted in hot start at 95 °C for 15 min, followed by 40 cycles at 95 °C for 20 s, 55 °C for 30 s and 72 °C for 30 s. The melting curve was assessed between 65 °C and 95 °C 0.3 °C ramping rate per 1 min. Finally, fold changes were computed using the 2−ΔΔCT method.
Table 1.
Characterization of the primers used for amplification of genes in this study
| Name | Sequence | Amplicon length | Target gene | Accession Number |
|---|---|---|---|---|
| FBRasrr | 5’- GTATGGTCGCAAGGCTGAAAC-3’ | 135 bp | 18 S rRNA | KX709367.1 |
| RBRasrr | 5’- GAGCTCTCAGTCTGTCAATCC-3’ | |||
| FRBOHsa | 5’- GGGCTACAAATACAAAAGTGGG-3’ | 113 | RBOH | |
| RRBOHsa | 5’- CACTGAGATAGTCATCCCCTG-3’ | |||
| PAL1-F | 5’-ACCTACCTCGTCGCCCTATGC-3’ | 169 bp | PAL | DQ408636.1 |
| PAL1-R | 5’-CCACGCGGATCAAGTCCTTCT-3’ | |||
| FPirRAS | 5’- CAAGTGTGGCGCTGCGTCTG-3’ | 167 bp | RAS | KF220570.1 |
| RPirRAS | 5’-ACCAGTTCGCCGCACAGAGC-3’ |
Quantification of anthocyanin concentration and total phenol content
To quantify the total anthocyanin concentration, ground leaves (0.5 g) were soaked and shaken in acidified methanol in the dark at room temperature for 24 h. The mixture was centrifuged and the optical density of the supernatant was read at 657 and 530 nm using acidified methanol as blank. The formula A = (A530 − 0.25 A657) was used to modify absorption relative to chlorophyll and the anthocyanidin content was computed using the molar extinction coefficient 29,600 by the method described by Ereifej et al. [48]. The anthocyanin content was represented as mg of cyanidin-3-glucoside equivalent per g of the dry weight of leaves.
Total phenol content (TFC) was quantified by adding Folin–Ciocalteu reagent and Na2CO3 to plant extract and reading at 760 nm as previously applied by Amooaghaie et al. [38].
Quantification of carnosol and rosmarinic acid by HPLC analysis
The amounts of carnosol and rosmarinic acid were quantified by high-performance liquid chromatography using a 515 Waters HPLC pump, with 2487 dual wavelength absorbance detector (Waters, Milford, MA, USA) on a Nova-Pak C18, 3.9 × 150 mm (Waters, Milford, MA, USA). At first, the dried leaves (2 g) was soaked in 15 mL of methanol (15 mL) and shaken for 24 h. Then, extracts were dried and remaining was dissolved in 10% sodium bicarbonate (5 mL) and chlorophylls and fats washed were removed using ethyl acetate (1 mL). After acidifying the aqueous phase using 2 N hydrochloric acid (to a pH of 2.0, the phenolic compounds were extracted using diethyl and were dried. Then, the organic part was dissolved in 1 mL HPLC solvent A: B (1:1), filtered with a 0.22 μm syringe filter, and injected into the HPLC system for quantification. The solvents, the columns, and times were adjusted as previously adopted by Amooaghaie et al. [38]. Injection and quantification of standards (Sigma-Aldrich, USA) also was done in the same way, Millennium software was applied [49].
Evaluation of total antioxidant capacity
The total antioxidant capacity was estimated by the phosphomolybdenum assay as described by Jan et al. [50]. The leaf extract of each sample was mixed with reagent solution (4 mM ammonium molybdate, 0.6 M sulfuric acid, and 28 mM sodium phosphate) and tubes laid in a water bath at 95 °C for 90 min. After cooling, the optical absorbance of the resultant solution was read at 765 nm. Total antioxidant capacity was calculated using the following equation:
Total antioxidant capacity (%) = [(Abs of control − Abs of sample)/ Abs of control] × 100.
Statistical analysis
A factorial experiment with a randomized complete design was conducted with 3 replicates. Data were subjected to ANOVA analysis using SAS version 9, and the significance of means differences was assessed by Duncan’s multiple range tests at the P < 0.05 level.
Results
The role of H2O2 in laser-induced improvement of growth parameters
Seed pretreatment with laser and/or H2O2 significantly improved the fresh and dry weight of shoots and total Chl. in leaves under non-saline conditions (Fig. 1A-C).
Fig. 1.
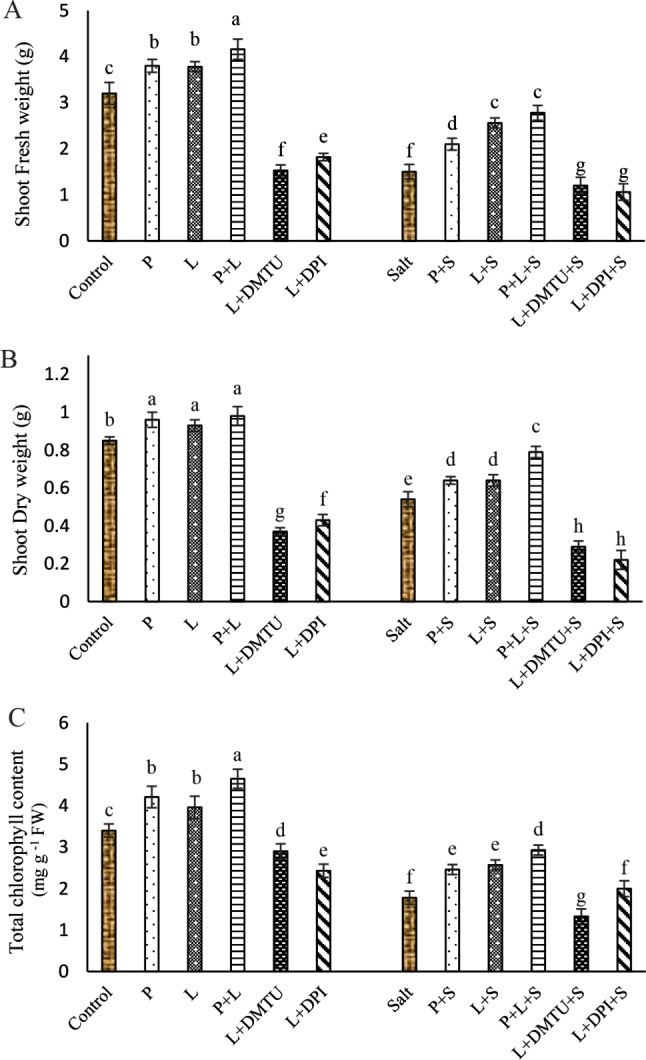
. Impact of seed pretreatment with H2O2 and He–Ne laser irradiation alone or together to DPI and DMTU on fresh weight (A) and dry weight (B) of shoots and total Chl. (C) in leaves of Salvia officinalis plants under salt stress
Mean ± SE (n = 3) followed by different letters represent a significant difference between treatments at p < 0.05, based on Duncan’s multiple range tests. P = H2O2 L = He–Ne laser S = salt stress.
Salt stress significantly reduced shoot fresh and dry weights of shoots and total Chl. and seed pretreatment with laser and/or H2O2 significantly improved these attributes (Fig. 1A-C). Seed irradiation with He-Ne laser increased fresh and dry weight of shoots and total Chl. respectively by 20.4, 70.66, and 18.5% compared to salt treatment alone. However, under both saline and non-saline conditions, the laser-induced improvement of the fresh and dry weight of shoots and total Chl. was reversed when NADPH oxidase activity was inhibited by DPI or endogenous H2O2 was scavenged by DMTU (Fig. 1A-C).
The role of H2O2 in laser-induced effects on proline content and relative water content
Seed pretreatment with laser and/or H2O2 significantly did not affect proline and RWC under non-saline conditions (Fig. 2A, B). Salinity increased proline content by 1.68 fold and decreased RWC by 40.63% in the leaves of sage seedlings in comparison to control (Fig. 2A, B). Seed pretreatment with laser and/or H2O2 significantly improved proline content and RWC in leaves. Seed irradiation with laser increased proline content by 2.03 fold and RWC 29.79% in leaves compared to salt treatment alone. The increase of proline content and RWC induced by laser was reduced by applying DPI, and DMTU (Fig. 2A, B) which meant that ROS signaling likely is involved in laser-induced proline production.
Fig. 2.
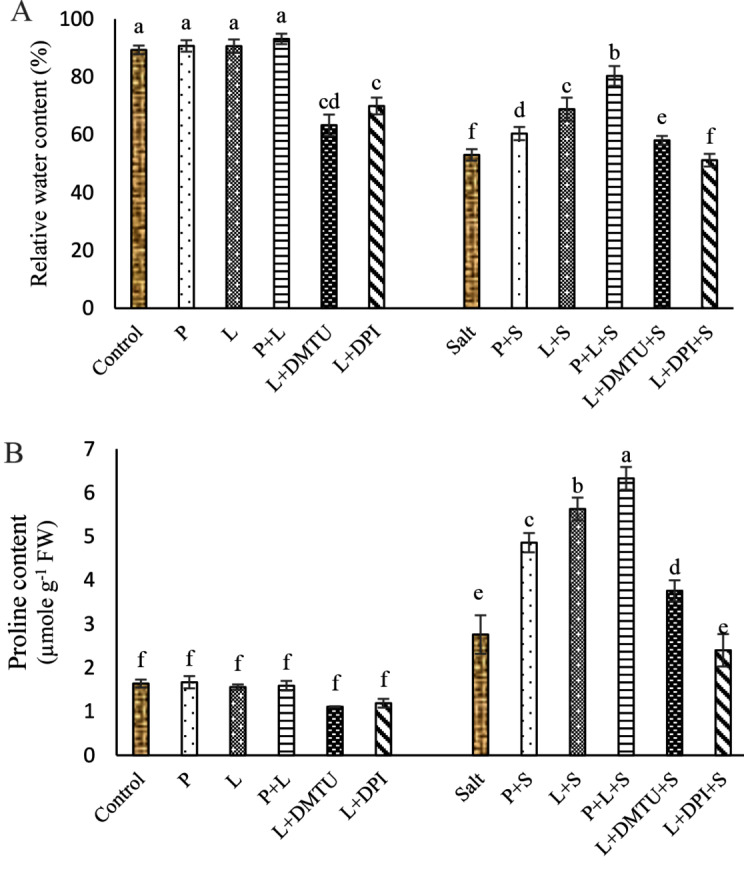
Impact of seed pretreatment with H2O2 and He–Ne laser irradiation alone or together to DPI and DMTU on relative water content (A) and proline content (B) in Salvia officinalis plants under salt stress
Mean ± SE (n = 3) followed by different letters represent a significant difference between treatments at p < 0.05, based on Duncan’s multiple range tests. P = H2O2 L = He–Ne laser S = salt stress.
The role of H2O2 in laser-induced effects on Na+ and K+ contents
Seed pretreatment with laser and/or H2O2 significantly did not affect the Na+ content but improved the K+ content under non-saline conditions (Fig. 3A, B).
Fig. 3.
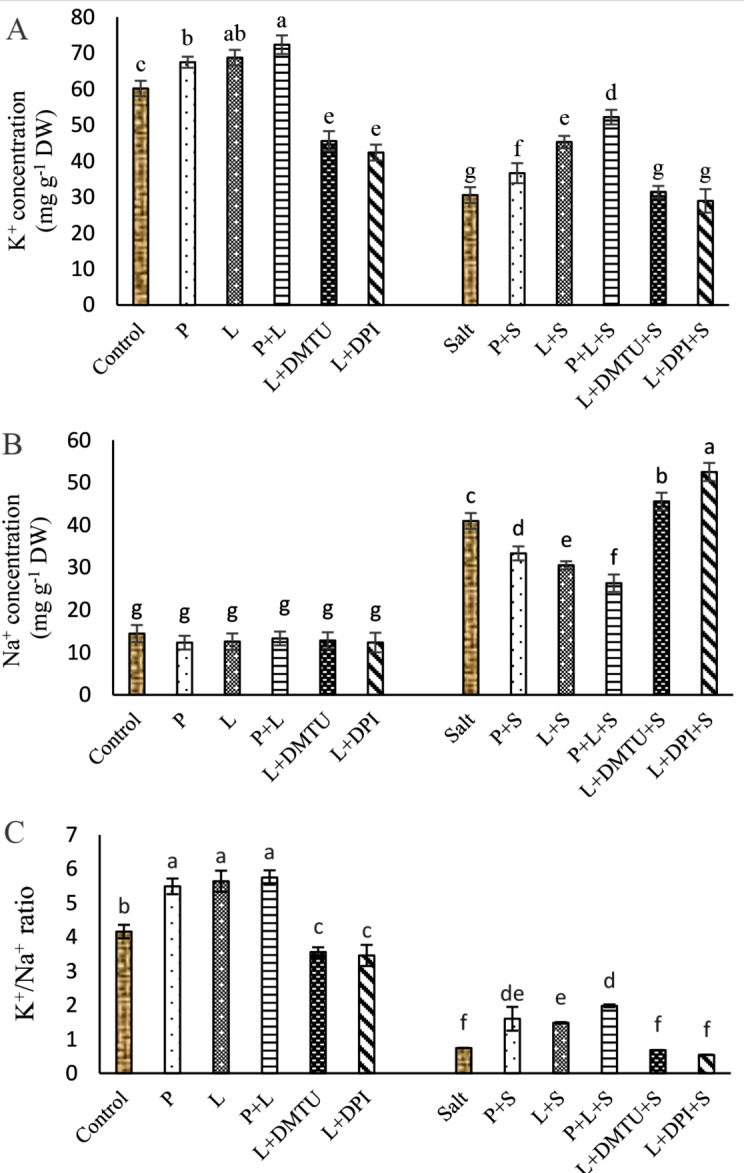
. Impact of seed pretreatment with H2O2 and He–Ne laser irradiation alone or together to DPI and DMTU on Na+ (A) and K+ (B) contents and K+/Na+ ratio (C) in Salvia officinalis plants under salt stress
Mean ± SE (n = 3) followed by different letters represent a significant difference between treatments at p < 0.05, based on Duncan’s multiple range tests. P = H2O2 L = He–Ne laser S = salt stress.
Salt stress decreased the K+ content by 50% and increased the Na+ content by 2.83 folds (Fig. 3A, B) in leaves. Therefore, salinity decreased the K+/Na+ ratio in comparison to the control plants (Fig. 3C). Seed pretreatment with laser and/or H2O2 significantly improved the K+/Na+ ratio in salt-stressed plants. Pretreatment with laser, increased K+ content by 48.37% and reduced Na+ content in leaves by 25.46% compared to salt treatment alone (Fig. 3A, B). However, applying DPI, and DMTU on laser-primed seeds, significantly increased Na+ content, but diminished the contents of K+ and consequently lowered K+/Na+ ratio in salt-stressed plants (Fig. 3A-C).
The role of H2O2 in laser-induced effects on oxidative stress biomarkers
Seed pretreatment with laser and/or H2O2 significantly did not affect the MDA content, but slightly increased the H2O2 content in leaves under non-saline conditions (Fig. 4A, B).
Fig. 4.
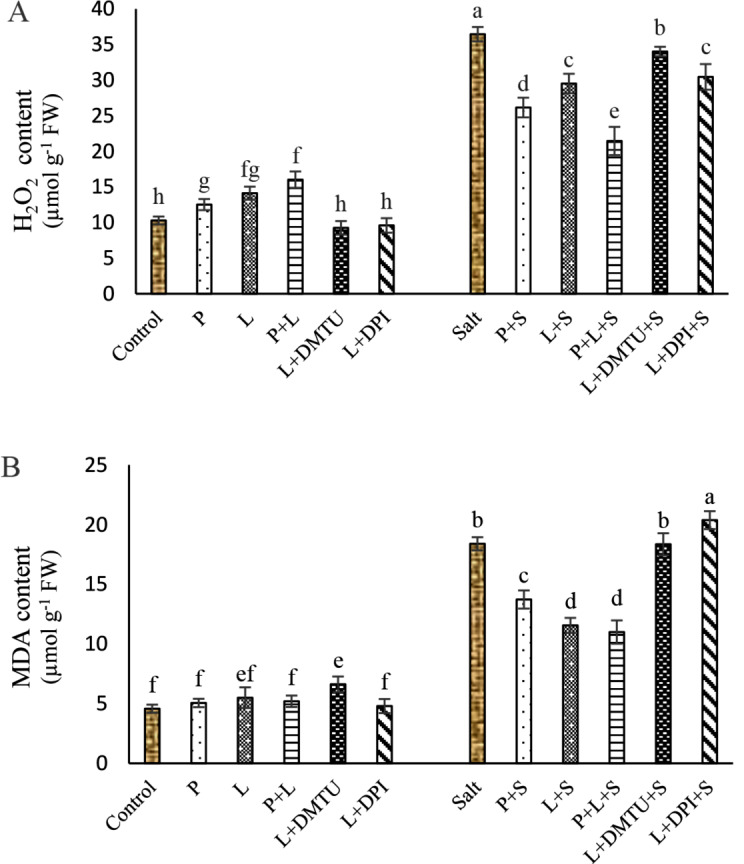
Impact of seed pretreatment with H2O2 and He–Ne laser irradiation alone or together to DPI and DMTU on H2O2 (A) and MDA (B) contents in Salvia officinalis plants under salt stress
Mean ± SE (n = 3) followed by different letters represent a significant difference between treatments at p < 0.05, based on Duncan’s multiple range tests. P = H2O2 L = He–Ne laser S = salt stress.
The role of H2O2 in laser-induced effects on antioxidant enzymes
Seed pretreatment with laser and/or H2O2 significantly did not affect APX activity but increased the SOD activity under non-saline conditions (Fig. 5A, B).
Fig. 5.
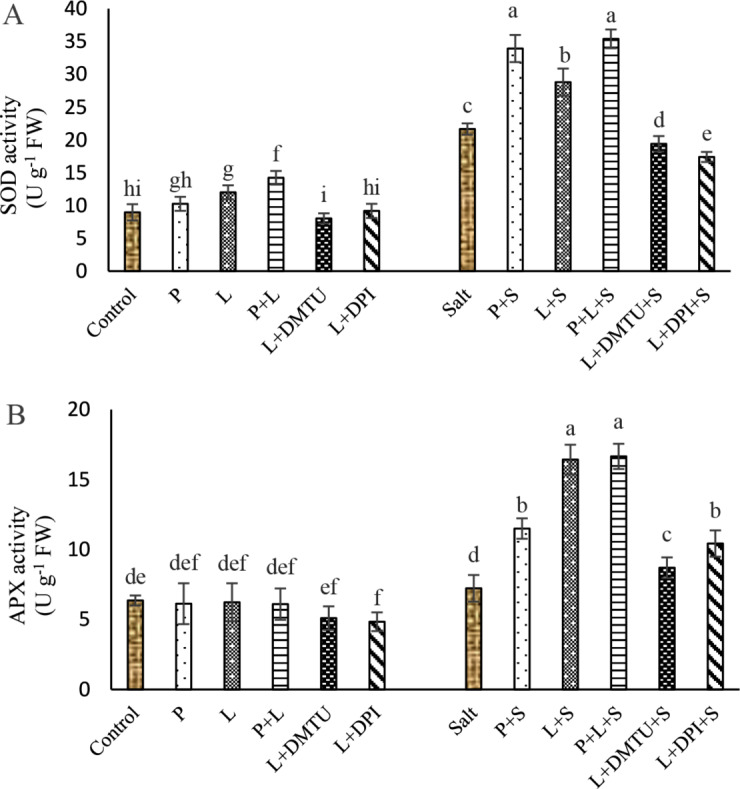
Impact of seed pretreatment with H2O2 and He–Ne laser irradiation alone or together to DPI and DMTU on the activities of SOD (A) and APX (B) in Salvia officinalis plants under salt stress
Mean ± SE (n = 3) followed by different letters represent a significant difference between treatments at p < 0.05, based on Duncan’s multiple range tests P = H2O2 L = He–Ne laser S = salt stress.
Salt stress significantly increased SOD activity, but had no significant effect on APX activity. Seed pretreatment with laser and/or H2O2 significantly enhanced SOD and APX activity in salt-stressed plants. Seed irradiation with laser enhanced SOD activity by 32.96% and APX activity by 2.27 fold in the leaves compared to salt stress alone (Fig. 5). The stimulatory effects of laser on SOD and APX activity were nullified by applying DPI, and DMTU.
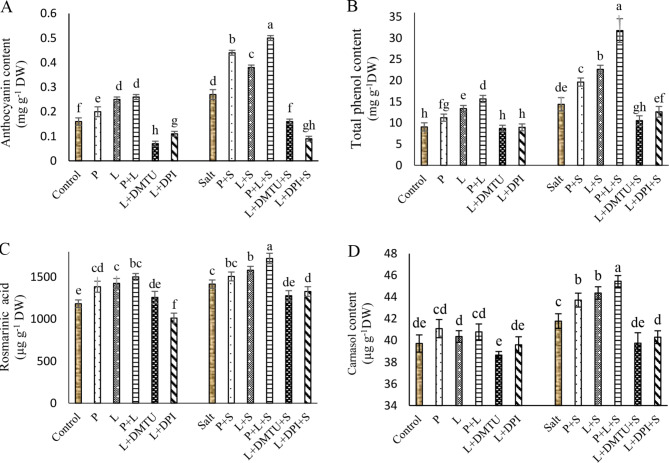
The role of H2O2 in laser-induced effects on the content of anthocyanin total phenol rosmarinic acid and carnosol
Seed pretreatment with laser and/or H2O2 significantly increased the content of anthocyanin, total phenol and rosmarinic acid but did not affect carnosol content under non-saline conditions (Fig. 6A-D).
Fig. 6.
Impact of seed pretreatment with H2O2 and He–Ne laser irradiation alone or together to DPI and DMTU on the content of anthocyanin (A), total phenol content (B), rosmarinic acid (C) and carnosol (A) in Salvia officinalis plants under non-stress and salt stress
Mean ± SE (n = 3) followed by different letters represent a significant difference between treatments at p < 0.05, based on Duncan’s multiple range tests. P = H2O2 L = He–Ne laser S = salt stress.
Salt stress significantly increased the content of anthocyanin, total phenol, carnosol and rosmarinic acid. Seed pretreatment with He–Ne laser and/or H2O2 significantly increased the content of these compounds under both non-saline and saline conditions. Seed irradiation with laser enhanced the amount of anthocyanin, total phenol, carnosol and rosmarinic acid respectively by 40.74, 64.37, 11/66, 6.19% in salt-stressed plants. However, additive treatment with DPI, and DMTU after He–Ne laser irradiation caused a significant decrease in the amount of these compounds under both saline and non-saline conditions (Fig. 6A-D).
The role of H2O2 in laser-induced effects on total antioxidant capacity
Salt stress significantly increased total antioxidant capacity (TAC) and seed pretreatment with He–Ne laser and H2O2 significantly enhanced it under non-saline and saline conditions. However, treatment with DPI, and DMTU after He–Ne laser irradiation caused a significant decrease in TAC (Fig. 7).
Fig. 7.
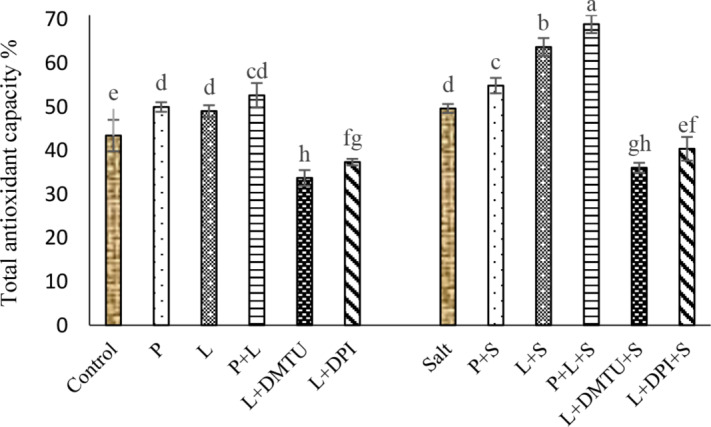
Impact of seed pretreatment with H2O2 and He–Ne laser irradiation alone or together to DPI and DMTU on total antioxidant capacity in Salvia officinalis plants under non-stress and salt stress
Mean ± SE (n = 3) followed by different letters represent a significant difference between treatments at p < 0.05, based on Duncan’s multiple range tests. P = H2O2 L = He–Ne laser S = salt stress.
The role of H2 The role of HO2 in laser-induced effects on the expression of RBOH PAL and RAS genes
Seed pretreatment with laser and/or H2O2 significantly increased the expression of RBOH, PAL, and RAS genes under non-saline conditions (Fig. 8A-C). After 48 h imposing to salt stress higher expression of RBOH, PAL, and RAS genes were recorded, and He–Ne laser illumination and/or H2O2 pretreatment intensified transcription of these genes. However, applying DPI, and DMTU on laser-primed seeds remarkably reduced the expression of RBOH, PAL, and RAS genes in plants exposed to salt stress (Fig. 8A-C).
Fig. 8.
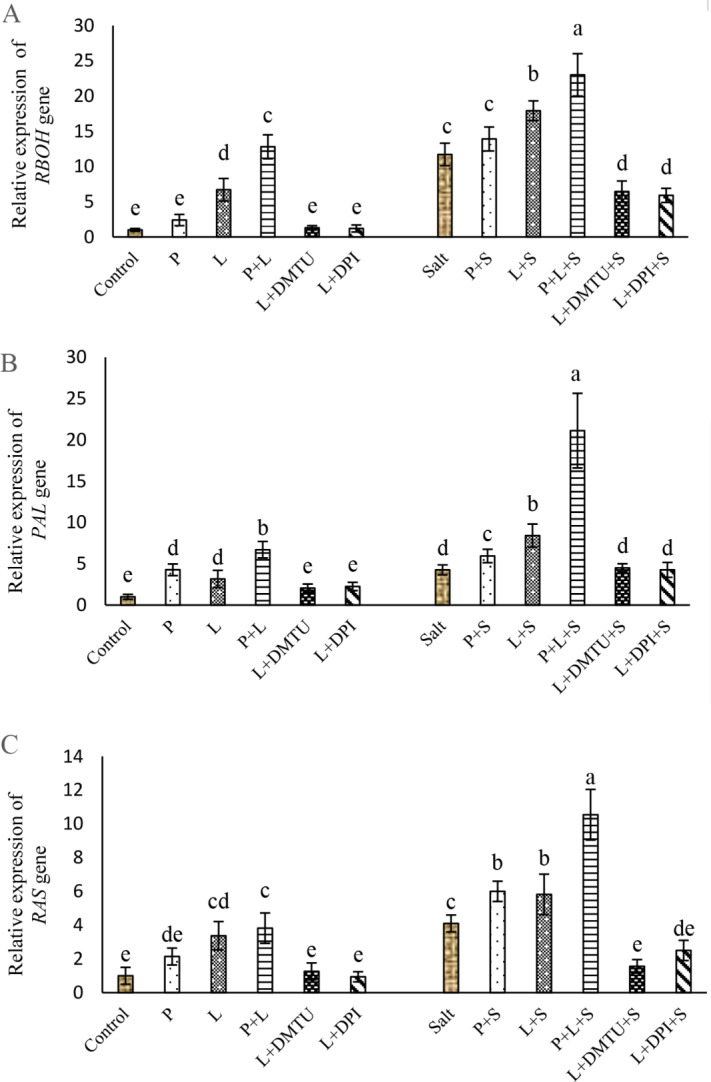
Impact of seed pretreatment with H2O2 and He–Ne laser irradiation alone or together to DPI and DMTU on the expression of RBOH (A), PAL (B), and RAS (C) genes in Salvia officinalis plants under non-stress and salt stress
Mean ± SE (n = 3) followed by different letters represent a significant difference between treatments at p < 0.05, based on Duncan’s multiple range tests. P = H2O2 L = He–Ne laser S = salt stress.
Discussion
Numerous studies have shown that the He-Ne laser can improve the growth, metabolism, and stress tolerance in plants [8–18]. Hernandez et al. [13] believe that absorbed energy during seed irradiation with the laser may be transformed into chemical energy and at a later time can improve seed germination and growth. However, there is not enough empirical evidence regarding the mechanism by which He-Ne laser effects are translated as biochemical responses. The current study provided evidence that suggests laser through the motivation of an RBOH-dependent H2O2 burst may mediate salt tolerance and secondary metabolism in S. officinalis.
As expected, salinity (75 mM NaCl) reduced shoot growth of S. officinalis plants (Fig. 1A, B) which agreed with the findings of Tounekti et al. [34]. Seed laser irradiation similar to H2O2 pretreatment increased growth parameters and Chl content under non-saline and saline conditions (Fig. 1A-C). Likewise, the improvement of growth and Chl content in water-stressed sunflower in response to He-Ne laser radiation [18] and in salt-stressed Silybum marianum [51] by seed pretreatment with H2O2 has been reported earlier. It has been suggested that red photons of the He–Ne laser (632.8 nm) may be absorbed by the phytochromes and these photoreceptors can regulate the growth, Chl biosynthesis, and stress tolerance in plants [52]. However, in the current study reversing laser effects on growth and Chl content after H2O2 scavenging by DMTU or inhibiting of RBOH-mediated H2O2 generation by DPI, suggests laser mediates these responses through H2O2 signaling. It has been well defined that the lower dose of H2O2 can act as a signal to regulate hormonal situations, Chl biosynthesis, photosynthesis, and defensive responses, resulting in improved growth and stress tolerance in plants [19, 21]. It is also noticeable that salinity likely increased Chl degradation due to the high generation of ROS in leaves. Seed pretreatment with H2O2 or/and laser reduced ROS content (Fig. 4) and oxidative damage of Chl in leaves (Fig. 1C) which likely enhanced photosynthesis and improved growth parameters.
Indeed, it is likely that the improvement of RWC levels by H2O2 and/or laser (Fig. 2A) retained safeguarding turgor and consequently improved the growth of S.officinalis plants under salinity stress. Likewise, recovery of growth and water content by H2O2 in water-deficit-exposed quinoa [53] and by laser irradiation in drought-stressed wheat [10] has been published earlier. The impact of H2O2 and laser on proline accumulation (Fig. 2B) was responsible for the maintenance of RWC (Fig. 2A) in S.officinalis leaves under salt stress. These results were in agreement with previous reports, where proline accumulation was increased in water deficit-exposed sunflower [18] by seed laser irradiation and in salt-accrued wheat [54] by seed H2O2 pretreatment. Proline not only as an osmoticum contributes to the osmotic adjustment but also displays an important role in the stability of membranes and enzymes due to participating in ROS scavenging under saline conditions [55]. Our results depicted that laser-induced proline accumulation in leaves of S. officinalis was regulated by RBOH-dependent signaling; because H2O2 scavenging by DMTU or the inhibition of RBOH-mediated H2O2 generation by DPI in early hours of salt exposure, abolished stimulatory impacts of laser on proline accumulation in 45-old-day plants (Fig. 2B). A study depicted that RBOH-mediated H2O2 was required for enhancing the activity of Δ1-pyrroline-5-carboxylate synthetase (the enzyme underlying proline biosynthesis), reducing the activity of proline dehydrogenase (an enzyme involved in proline degradation) and consequently increasing proline accumulation in wheat roots under salt stress and these impacts were reversed by DMTU and DPI [56]. Therefore, it is likely that laser also evokes RBOH-dependent H2O2 generation, and this H2O2 influences the gene expression or activity of enzymes underlying biosynthesis or degradation of proline.
Disruption of Na+ and K+ homeostasis is another reason for decreasing plant growth under salt stress [4, 57]. Increasing Na+ content and conversely decreasing K+ in salt-stressed S. officinalis plants (Fig. 3A, B) agreed with the findings of Es‑sbihi et al. [58]. However, H2O2 and laser pretreatments reduced Na+ accumulation and increased K+ contents in leaves (Fig. 3A, B). These findings validate previous reports, where Na+ and K+ homeostasis was modified by seed pretreatment with H2O2 in wheat [57] and sunflower [59] under salt stress. To the best of our knowledge, the effect of laser irradiation on Na+ and K+ homeostasis in salt-stressed plants has never been published. The reversion of the laser effect on the re-establishment of the K+/Na+ ratio in S. officinalis leaves by DMTU and DPI (Fig. 3C) points to the role of RBOH-dependent signaling in this phenomenon. The role of H2O2 in triggering Ca2+ cascades and ultimately regulating Na+ efflux from the cytosol of cells through Ca2+-dependent activation of SOS1 (as a plasma membrane Na+/H+ antiporter) has been well demonstrated in Arabidopsis [4, 20]. Wang et al. [60] found that RBOH-dependent signaling is required for K+ homeostasis in saline conditions; because Knockout of OsRbohA down-regulated the expression of low or high-affinity K+ transporters and relative-channel genes (OsAKT1, OsHAK5, OsHAK1, and OsGORK), while overexpression of OsRbohA, enhanced the transcripts of these genes and lowered the loss of K+ ions in roots of salt-stressed rice. Therefore, it is likely that the laser through triggering RBOH-signaling, regulated the gene expression or the action of transporters involved in the removal of excess Na+ and K+ influx and consequently improved the K+/Na+ ratio.
Salinity stress increased H2O2 content and led to membrane peroxidation in leaves (Fig. 4). The decline of these oxidative biomarkers by H2O2 and/or laser treatment likely was related to enhancing the function of SOD, and APX enzymes by these treatments (Fig. 5). Similarly, former studies also have depicted that increasing the gene expression and/or the activities of antioxidant enzymes in tall fescue plants [9] by laser radiation, and in wheat [54] by H2O2 seed priming, improved salt-induced oxidative stress in these plants. However, the nullification of the stimulatory effects of laser on SOD and APX activity by DMTU and DPI (Fig. 5) in the current study; suggests that laser enhanced the activities of these enzymes through RBOH-dependent signaling. Similarly, applying DMTU and the inhibition of RBOH-dependent signaling with DPI or imidazole reduced the activities of APX, GR, and CAT in salt-stressed Arabidopsis thaliana seedlings [23]. A study also demonstrated that salt tolerance induced by seed priming with H2O2 or osmo-priming in alfalfa depended on RBOH-generated H2O2 to induce the gene expression and activity of antioxidant enzymes and proline accumulation [2]. Therefore, it can be proposed that up-regulating the gene expression and activities of antioxidant enzymes through RBOH-dependent H2O2 might be involved in the progress of effects of He–Ne laser on the management of salt-induced oxidative stress in S. officinalis plants.
Impact of H2O2 and/or laser pretreatment on lowering H2O2 content and lipid peroxidation in salt-stressed plants might be also related to the elicitation of secondary metabolites such as anthocyanins and other phenolic compounds (Fig. 6) by these treatments. Anthocyanins are water-soluble pigments, and antioxidant compounds that have the ability to scavenge ROS as well as prevent their production [5]. Thus, it can be supposed that increasing anthocyanin content in leaves by He-Ne laser or H2O2 (Fig. 6A), contributed to salt tolerance in S. officinalis plants. Similarly, it has been reported that anthocyanin content increased in leaves of salt-stressed basil [60] by H2O2 treatment and in water deficit-exposed sunflower [18] in response to He-Ne laser irradiation. Dudareva et al. [15] believe that likely red photon of laser trigger anthocyanin biosynthesis through phytochrome activation. In the current study reversing effect of the He-Ne laser on anthocyanin content by DMTU and DPI (Fig. 6A), indicates that this impact mediates through RBOH-dependent H2O2 generation. In support of our hypothesis, Wu et al. [23] reported that increasing anthocyanin accumulation in the hypocotyls of radish sprouts under stressful conditions depends on light and H2O2. They observed that exogenous H2O2 enhanced expressions of anthocyanin biosynthesis-related transcription factors and applying DMTU severely inhibited anthocyanin biosynthesis in radish.
Phenolics are antioxidant compounds that owing to their ROS scavenging capacity can reduce degradation and excess excitation of chlorophyll in leaves, especially under stressful conditions [7]. He-Ne laser irradiation or H2O2 pretreatment increased the accumulation of total phenolics, rosmarinic acid, and carnosol (Fig. 6B-C) in S. officinalis leaves even under non-saline conditions, and upregulating PAL and RAS genes was responsible for this result. Increment of rosmarinic acid accumulation in Salvia miltiorrhiza cell cultures by H2O2 treatment [36] and in Cymbopogon proximus sprouts in response to laser irradiation [61] under non-stress conditions have been also reported earlier. However, there is no report concerning the effect of laser and H2O2 on carnosol accumulation in plants. Likely, the impact of laser on Chl content (Fig. 1C), improved photosynthesis and consequently increased the carbon availability for the biosynthesis of these compounds. Besides, our results for the first time depicted that laser as well as H2O2 treatment intensified the expression of PAL and RAS genes (Fig. 8B, C), and this response was reversed by applying DPI and DMTU. These novel findings suggest that laser pretreatment induced an RBOH-dependent H2O2 generation and H2O2 as a downstream signal stimulated de novo synthesis of phenolics such as rosmarinic acid via activating respective gene expression. In line with our hypothesis, Hao et al. [36] argued that triggering an endogenous H2O2 burst is required in rosmarinic acid production elicited by salicylic acid in Salvia miltiorrhiza cell cultures; because the inhibition of NADPH- oxidase by imidazole or quenching H2O2 burst by DMTU blocked rosmarinic acid accumulation in this herb.
Our results showed that laser and H2O2 enhanced the production of phenolics with stronger antioxidant activity in salt-stressed plants, as was verified by results of total antioxidant capacity (Fig. 7). Previous studies revealed that carnosic acid and its major oxidized derivative, carnosol, protect lipids from oxidation [35]. Both rosmarinic acid and carnosol have strong antioxidative properties and their accumulation can contribute to ROS scavenging in leaves [62]. It has been formerly reported that increasing carnosol in S. officinalis [63] and rosmarinic acid in Dracocephalum kotschyi Boiss. [3] improved salt-induced oxidative stress in these plants. Taken together, the above data support this assumption that RBOH-dependent H2O2 generation is involved in He–Ne laser-elicited secondary metabolism in plants, especially under salt stress. The analysis of RBOH transcripts further supported this assumption. Our results for the first time exhibited that seed laser irradiation upregulated RBOH gene expression in leaves under non-stress conditions (Fig. 8A). Importantly, this laser-induced upregulation of the RBOH gene did not increase lipid peroxidation (Fig. 4B) and even corresponded with the enhanced growth (Fig. 1) and the increased contents of secondary metabolites (Fig. 6) in 45-old-day plants. This suggests that laser motivated a transit and controlled RBOH-dependent H2O2 burst and induced a mode of like to stressful conditions which in turn stimulated the growth and secondary metabolism under non-stress conditions. The accordance of higher levels of PAL and RAS transcripts with higher expression of the RBOH gene in laser-primed plants (Fig. 8) also further endorses this assumption. There is no report concerning laser-induced upregulating RBOH transcripts and the acquisition of H2O2 burst in the literature. However, a transient increase of lipid peroxidation in wheat callus after He-Ne laser irradiation was reported by Salyaev et al. [64] that indirectly points to the potential ability of the laser to induce a transient ROS burst.
Interestingly, higher levels of RBOH transcripts in laser-primed plants after 48 h imposing to salinity (Fig. 8A), not only did not aggravate oxidative stress but also was accompanied by lower contents of H2O2 and MDA in leaves of 45-old-day plants (Fig. 4). In addition, the higher levels of RBOH transcripts in laser-treated plants in early hours of salt exposure (Fig. 8A) were closely linked with positive effects conferred by He-Ne laser on proline content (Fig. 2), the activity of antioxidant enzymes (Fig. 5), the accumulation of anthocyanin, total phenolics, rosmarinic acid, and carnosol (Fig. 6) and Na+/K+ homeostasis (Fig. 3) in 45-old-day plants and, all these responses were impaired by applying DMTU and DPI. These novel findings again suggest that laser irradiation likely is motivating a transient RBOH-dependent H2O2 burst in the early hours after salt exposure that might act as a downstream signal for activating the antioxidant system and consequently reducing oxidative stress in 45-old-day plants. However, further evidence using rboh mutants should validate this opinion.
It is known that seed priming can initiate a ‘‘transcriptional memory’’ that can be shared later and mediate metabolism and stress tolerance in plants [65]. The positive effects of laser radiation on seeds are ascribed to its optical, electromagnetic, and thermal properties [13]. Previous studies showed that priming with mild heat stress [66], magnetopriming of seeds [67] and light exposure [31, 68] can induce RBOH-dependent H2O2 and stress memory in plants which can enhance plant tolerance under subsequent exposures to stress. The above literature and our data suggest this proposal that the optical, thermal, and magnetic effects of the laser may act as mild stressors that activate RBOH-mediated H2O2 generation. This H2O2 burst or other signals elicited by H2O2 might induce epigenetic modifications and establish a laser-induced ‘transcriptional memory’ in seeds. This memory is likely decoded in mature plants and is accountable for inducing secondary metabolism and alleviating salt-induced oxidative, osmotic, and ionic stresses in laser-primed plants. Our data showed that laser energy indirectly and via RBOH-dependent signaling was directed to the metabolic pathways and manifested as the upregulation of RAS and PAL genes, the stimulation of proline and secondary metabolites biosynthesis, and the activation of the antioxidant enzymes. Therefore, we suggest hypothesis of Hernandez et al. [13] be modified as that optical, electromagnetic, and thermal effects of laser can motivate signaling cascades and initiate a ‘transcriptional memory’ that is subsequently shared and regulates the growth, metabolism, and stress tolerance in plants.
Conclusions
In conclusion, the outcomes of this study show that the laser-induced upregulating RBOH gene was concomitant with increasing antioxidant enzyme activities, proline accumulation, and improving Na+/K+ homeostasis in salt-stressed plants. Furthermore, laser-induced adaptive responses under salt stress were diminished by DPI and DMTU. These findings highlight the role of RBOH-mediated H2O2 generation in the progress of impacts of the He–Ne laser on salt tolerance. However, our results are preliminary and more evidence is required to prove the above hypothesis. In addition, the role of crosstalk of H2O2 generated by RBOHs with transcription factors and other signaling components such as Ca2+, NO, phytohormones and phytochromes cannot be ruled out. The more detail should be addressed in future studies using RBOH mutants and transcriptomic, proteomic and metabolomic analysis.
Acknowledgements
The authors are very grateful to Dr. Moradi and Mr Mohsen Zamani for contributing to laser irradiation.
Author contributions
R. A supervised and planned the study and A.A and M.Gh advised and set up RT-PCR and HPLC analysis respectively. F.M performed experiments. R.A and F.M analyzed data and wrote the manuscript. All authors reviewed and confirmed the final manuscript.
Funding
The authors would like to thank the Plant Science Department of Shahrekord University, Iran, for the financial support of this research.
Data availability
The data that support the findings of this study are available from the first author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable. This manuscript does not involve researching about humans or animals.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shams M, Khadivi A. Mechanisms of salinity tolerance and their possible application in the breeding of vegetables. BMC Plant Biol. 2023;23:139. 10.1186/s12870-023-04152-8. 10.1186/s12870-023-04152-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amooaghaie R, Tabatabaie F. Osmopriming-induced salt tolerance during seed germination of alfalfa most likely mediates through H2O2 signaling and upregulation of heme oxygenase Protoplasma. 2017; 254(4): 1791–803. [DOI] [PubMed]
- 3.Vafadar F, Amooaghaie R, Ehsanzadeh P, et al. Melatonin improves the photosynthesis in Dracocephalum Kotschyi under salinity stress in a Ca2+/CaM-dependent manner. Funct Plant Biol. 2021;49:89–101. 10.1071/FP21233 [DOI] [PubMed] [Google Scholar]
- 4.Chung JS, Zhu JK, Bressan RA, et al. Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. J Plant. 2008;53:554–65. 10.1111/j.1365-313X.2007.03364.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landi M, Tattini M, Gould KS. Multiple functional roles of anthocyanins in plant-environment interactions. Environ Exp Bot. 2015;119:4–17. 10.1016/j.envexpbot.2015.05.012 [DOI] [Google Scholar]
- 6.Mahajan M, Kuiry R, Pal PK. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J App Res Med Aromat Plants. 2020;18:100255. [Google Scholar]
- 7.Sharma A, Shahzad B, Rehman A, et al. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules. 2019;24. 10.3390/molecules24132452. [DOI] [PMC free article] [PubMed]
- 8.Ali SI, Gaafar AA, Metwally SA, et al. The reactive influences of pre-sowing He-Ne laser seed irradiation and drought stress on growth, fatty acids, phenolic ingredients, and antioxidant properties of Celosia argentea. Sci Hortic. 2020;261:108989. 10.1016/j.scienta.2019.108989 [DOI] [Google Scholar]
- 9.Li Y, Gao L, Han R. Endogenous nitric oxide mediates He-Ne laser-induced adaptive responses in salt stressed-tall fescue leaves. Biosci Biotechnol Biochem. 2016;80(10):1887–97. 10.1080/09168451.2016.1179091 [DOI] [PubMed] [Google Scholar]
- 10.Qiu Z, He Y, Zhang Y, et al. Characterization of miRNAs and their target genes in He-Ne laser pretreated wheat seedlings exposed to drought stress. Ecotox Environ Safe. 2018;164:611–7. 10.1016/j.ecoenv.2018.08.077 [DOI] [PubMed] [Google Scholar]
- 11.Qiu Z, Li J, Zhang M, et al. He–Ne laser pretreatment protects wheat seedlings against cadmium-induced oxidative stress. Ecotox Environ Safe. 2013;88:135–41. 10.1016/j.ecoenv.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Han R, Sun Y. Damage repair effect of He–Ne laser on wheat exposed to enhanced ultraviolet-B radiation. Plant Physiol Biochem. 2021;57:218–21. 10.1016/j.plaphy.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 13.Hernandez AC, Dominguez PA, Cruz OA, et al. Laser in agriculture. Int Agrophys. 2010;24:407–22. [Google Scholar]
- 14.Qiu Z, Yuan M, He Y, et al. Physiological and transcriptome analysis of He-Ne laser pretreated wheat seedlings in response to drought stress. Sci Rep. 2017;7:1–12. 10.1038/s41598-017-06518-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudareva L, Tarasenko V, Rudikovskaya E. Involvement of photoprotective compounds of a phenolic nature in the response of Arabidopsis thaliana leaf tissues to low-intensity laser radiation. Photochem Photobiol. 2020;96:1243–50. 10.1111/php.13289 [DOI] [PubMed] [Google Scholar]
- 16.Kazemzadeh-beneh H, Mahna N, Safari E, et al. Effects of diode and He-Ne laser on in vitro production of anthocyanin in apple cell suspension culture. Inter J Hort Sci Technol. 2015;2:205–12. [Google Scholar]
- 17.Almuhayawi MS, Hassan AH, Abdel-Mawgoud M, et al. Laser light is a promising approach to improve the nutritional value, antioxidant capacity and anti-inflammatory activity of flavonoid-rich buckwheat sprouts. Food Chem. 2021;345:128788. 10.1016/j.foodchem.2020.128788 [DOI] [PubMed] [Google Scholar]
- 18.Mahmood S, Afzal B, Perveen S, et al. He-Ne laser seed treatment improves the nutraceutical metabolic pool of sunflowers and provides better tolerance against water deficit. Front Plant Sci. 2021;12:804. 10.3389/fpls.2021.579429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman JM, Muhlemann JK, Chapman JM, Muhlemann JK, Gayomba SR, Muday GK. RBOH-dependent ROS synthesis and ROS scavenging by plant specialized metabolites to modulate plant development and stress responses. Chem Res Toxicol. 2019;32:370–96. 10.1021/acs.chemrestox.9b00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurusu T, Kuchitsu K, Tada Y. Plant signaling networks involving Ca2+ and Rboh/Nox-mediated ROS production under salinity stress. Front. Plant Sci. 2015; 6: p.427. [DOI] [PMC free article] [PubMed]
- 21.Chen Q, Yang G. Signal function studies of ROS, especially RBOH-dependent ROS, in plant growth, development and environmental stress. J Plant Grow Regul. 2020;39:157–71. 10.1007/s00344-019-09971-4 [DOI] [Google Scholar]
- 22.Wang F, Chen ZH, Liu X, et al. The loss of RBOHD function modulates root adaptive responses to combined hypoxia and salinity stress in Arabidopsis. Environ Exp Bot. 2019;158:125–35. 10.1016/j.envexpbot.2018.11.020 [DOI] [Google Scholar]
- 23.Rejeb KB, Lefebvre-de Vos D, Le Disquet I, Leprince AS, Bordenave M, et al. Hydrogen peroxide produced by NADPH oxidases increases proline accumulation during salt or mannitol stress in Arabidopsis thaliana. New Phytol. 2015;208(4):1138–48. 10.1111/nph.13550. hal-01187624. 10.1111/nph.13550 [DOI] [PubMed] [Google Scholar]
- 24.Rejeb KB, Benzarti M, Debez A, et al. NADPH oxidase-dependent H2O2 production is required for salt-induced antioxidant defense in Arabidopsis thaliana. J Plant Physiol. 2014;174:5–15. 10.1016/j.jplph.2014.08.022 [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Wang MG, Ding MQ et al. H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis. [DOI] [PubMed]
- 26.Wu Q, Su N, Zhang X, et al. Hydrogen peroxide, nitric oxide and UV RESISTANCE LOCUS8 interact to mediate UV-B-induced anthocyanin biosynthesis in radish sprouts. Sci Rep. 2016;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gómez-Velázquez HD, Aparicio-Fernández X, Reynoso-Camacho R. Chia sprouts elicitation with salicylic acid and hydrogen peroxide to improve their phenolic content, antioxidant capacities in vitro and the antioxidant status in obese rats. Plant Foods Hum Nutr. 2021;76:363–70. 10.1007/s11130-021-00912-9 [DOI] [PubMed] [Google Scholar]
- 28.Guo H, Dang X, Dong J. Hydrogen peroxide and nitric oxide are involved in salicylic acid-induced salvianolic acid B production in Salvia miltiorrhiza cell cultures. Molecules. 2014;19:5913–24. 10.3390/molecules19055913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong H, Hua L, Reyna-Llorens I et al. Photosynthesis-independent production of reactive oxygen species in the rice bundle sheath during high light is mediated by NADPH oxidase. Proceedings of the National Academy of Sciences. 2021; 118: p.e202270211. [DOI] [PMC free article] [PubMed]
- 30.Devireddy AR, Liscum E, Mittler R. Phytochrome B is required for systemic stomatal responses and reactive oxygen species signaling during light stress. Plant Physiol. 2020;184:1563–72. 10.1104/pp.20.01084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng H, Zhang Q, Guo D. Genes that respond to H2O2 are also evoked under light in Arabidopsis. Mol Plant. 2013;6(1):226–8. 10.1093/mp/sss108 [DOI] [PubMed] [Google Scholar]
- 32.Ghorbani A, Esmaeilizadeh M. Pharmacological properties of Salvia officinalis and its components. J Tradit Complement Med. 2017;7:433–40. 10.1016/j.jtcme.2016.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trócsányi E, György Z, Zámboriné-Németh É. New insights into rosmarinic acid biosynthesis based on molecular studies. Curr Plant Biol. 2020;23:100162. 10.1016/j.cpb.2020.100162 [DOI] [Google Scholar]
- 34.Tounekti T, Hernández I, Müller M, et al. Kinetin applications alleviate salt stress and improve the antioxidant composition of leaf extracts in Salvia officinalis. Plant Physiol Biochem. 2011;49:1165–76. 10.1016/j.plaphy.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 35.Loussouarn M, Krieger-Liszkay A, Svilar L, et al. Carnosic acid and carnosol, two major antioxidants of rosemary, act through different mechanisms. Plant Physiol. 2017;175:1381–94. 10.1104/pp.17.01183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao W, Guo H, Zhang J et al. Hydrogen peroxide is involved in salicylic acid-elicited rosmarinic acid production in Salvia miltiorrhiza cell cultures. Sci World J Article. 2014; ID 843764. 10.1155/2014/843764 [DOI] [PMC free article] [PubMed]
- 37.Pirooz P, Amooaghaie R, Ahadi A, et al. Silicon-induced nitric oxide burst modulates systemic defensive responses of Salvia officinalis under copper toxicity. Plant Physiol Biochem. 2021;162:752–61. 10.1016/j.plaphy.2021.02.048 [DOI] [PubMed] [Google Scholar]
- 38.Amooaghaie R, Mardani Korrani F, Ghanadian M et al. Hybrid priming with He–Ne laser and hydrogen peroxide advances phenolic composition and antioxidant quality of Salvia officinalis under saline and non–saline condition. J Plant Grow Regul,2023. 10.1007/s00344-023-11156
- 39.Muthusamy A, Kudwa PP, Prabhu V, et al. Influence of Helium-Neon laser irradiation on seed germination in vitro and physico-biochemical characters in seedlings of brinjal (Solanum melongena L.) var. Mattu Gulla J Photochem Photobiol. 2021;88:1227–35. 10.1111/j.1751-1097.2012.01162.x [DOI] [PubMed] [Google Scholar]
- 40.Mei Y, Chen H, Shen W, et al. Hydrogen peroxide is involved in hydrogen sulfide-induced lateral root formation in tomato seedlings. BMC Plant Biol. 2017;17:162. 10.1186/s12870-017-1110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karimi-Baram A, Amooaghaie R, Ghorbanpour M et al. RBOH-dependent H2O2 burst induced by silicon dioxide nanoparticles establishes systemic acquired acclimation in quinoa under lead toxicity. Sci Hort. 2024.
- 42.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1. 10.1104/pp.24.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valivand M, Amooaghaie R. Calcium signaling confers nickel tolerance in Cucurbita pepo. Int J Phytoremed. 2021;23:362–73. 10.1080/15226514.2020.1814992 [DOI] [PubMed] [Google Scholar]
- 44.Bates LS, Waldren RP, Teare I. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–7. 10.1007/BF00018060 [DOI] [Google Scholar]
- 45.Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–98. 10.1016/0003-9861(68)90654-1 [DOI] [PubMed] [Google Scholar]
- 46.Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. 10.1016/S0168-9452(99)00197-1 [DOI] [Google Scholar]
- 47.Valivand M, Amooaghaie R, Ahadi A. Seed priming with H2S and Ca2+ trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiol Biochem. 2019;143:286–98. 10.1016/j.plaphy.2019.09.016 [DOI] [PubMed] [Google Scholar]
- 48.Ereifej KI, Feng H, Rababah T, et al. Chemical composition, phenolics, anthocyanins concentration and antioxidant activity of ten wild edible plants. Food Nut Sci. 2015;6:581. [Google Scholar]
- 49.Ghanadian M, Shamaeezadeh N, Mohammadi P, et al. A new validated high performance liquid chromatography method for standardization of rosmarinic acid in Salvia extracts. J Herb Med Pharma. 2018;7:44–50. 10.15171/jhp.2018.08 [DOI] [Google Scholar]
- 50.Jan S, Khan MR, Rashid U, et al. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of Monotheca Buxifolia fruit. Osong Public Health Res Perspect. 2013;4:246–54. 10.1016/j.phrp.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Migahid M, Elghobashy R, Bidak L, et al. Priming of Silybum marianum (L.) Gaertn seeds with H2O2 and magnetic field ameliorates seawater stress. Heliyon. 2019;5:e01886. 10.1016/j.heliyon.2019.e01886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dudareva L, Phytochrome. (The main agent of action of low-intensity He–Ne laser radiation on seeds of cultivated plants: a review. J Plant Growth Regul. 2024;43:382–401. 10.1007/s00344-023-11118-5. 10.1007/s00344-023-11118-5 [DOI] [Google Scholar]
- 53.Iqbal H, Yaning C, Waqas M, et al. Differential response of quinoa genotypes to drought and foliage-applied H2O2 in relation to oxidative damage, osmotic adjustment and antioxidant capacity. Ecotox Environ Safe. 2018;164:344–54. 10.1016/j.ecoenv.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 54.Habib N, Ali Q, Ali S, et al. Use of nitric oxide and hydrogen peroxide for better yield of wheat (Triticum aestivum L.) under water deficit conditions: growth, osmoregulation, and antioxidative defense mechanism. Plants. 2020;9:285. 10.3390/plants9020285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55..Rejeb KB, Abdelly C, Savouré A. How reactive oxygen species and proline face stress together. Plant Biochem Physiol. 2014;80:278–84. 10.1016/j.plaphy.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 56.Liu L, Huang L, Lin X, et al. Hydrogen peroxide alleviates salinity-induced damage through enhancing proline accumulation in wheat seedlings. Plant Cell Rep. 2020;39:567–75. 10.1007/s00299-020-02513-3 [DOI] [PubMed] [Google Scholar]
- 57.Abdel Latef AAH, Kordrostami M, Zakir A, et al. Eustress with H2O2 facilitates plant growth by improving tolerance to salt stress in two wheat cultivars. Plants. 2019;8:303. 10.3390/plants8090303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Es-sbihi FZ, Hazzoumi Z, Aasfar A, et al. Improving salinity tolerance in Salvia officinalis L. by foliar application of salicylic acid. Chem Biol Technol Agri. 2021;8:1–12. [Google Scholar]
- 59.Silva PCC, Azevedo Neto AD, Gheyi HR, et al. Salt tolerance induced by hydrogen peroxide priming on seed is related to improvement of ion homeostasis and antioxidative defense in sunflower plants. J Plant Nutr. 2020;44:1207–21. 10.1080/01904167.2020.1862202 [DOI] [Google Scholar]
- 60.Wang Q, Ni L, Cui Z, et al. The NADPH oxidase OsRbohA increases salt tolerance by modulating K+ homeostasis in rice. Crop J. 2022. 10.1016/j.cj.2022.03.004. 10.1016/j.cj.2022.03.004 [DOI] [Google Scholar]
- 61.Okla MK, El-Tayeb MA, Qahtan AA, et al. Laser light treatment of seeds for improving the biomass photosynthesis, chemical composition and biological activities of lemongrass sprouts. Agronomy. 2021;11:478. 10.3390/agronomy11030478 [DOI] [Google Scholar]
- 62.Gohari G, Alavi Z, Esfandiari E, et al. Interaction between hydrogen peroxide and sodium nitroprusside following chemical priming of Ocimum basilicum L. against salt stress. Physiol Plant. 2020;168:361–73. 10.1111/ppl.13020 [DOI] [PubMed] [Google Scholar]
- 63.Tounekti T, Khemira H. NaCl stress-induced changes in the essential oil quality and abietane diterpene yield and composition in common sage. J Intercult Ethnopharmacol. 2015;4:208. 10.5455/jice.20150405064135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salyaev RK, Dudareva LV, Lankevich SV. Effect of low-intensity laser radiation on the lipid peroxidation in wheat callus culture. Russ J Plant Physiol. 2003;50:498–500. 10.1023/A:1024720707041 [DOI] [Google Scholar]
- 65.Liu H, Able AJ. Priming crops for the future: rewiring stress memory. Trends Plant Sci. 2022;27. 10.1016/j.tplants.2021.11.015. [DOI] [PubMed]
- 66.Sun M, Jiang F, Zhou R, et al. Respiratory burst oxidase homologue-dependent H2O2 is essential during heat stress memory in heat sensitive tomato. Sci Hort. 2019;258:108777. 10.1016/j.scienta.2019.108777 [DOI] [Google Scholar]
- 67.Anand A, Kumari A, Thakur M et al. Hydrogen peroxide signaling integrates with phytohormones during the germination of magnetoprimed tomato seeds. Scient Rep 201; 9:8814. [DOI] [PMC free article] [PubMed]
- 68.Feng XJ, Li JR, Qi SL, et al. Light affects salt stress-induced transcriptional memory of P5CS1 in Arabidopsis. Proc Natl Acad Sci India Sect B Biol Sci. 2016;113:8335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the first author upon reasonable request.