Abstract
Angiotensin I-converting enzyme (ACE) is a key enzyme in the regulation of systemic blood pressure and plays a major role in the renin-angiotensin and bradykinin-kinin systems, at the luminal surface of the vascular endothelia. To identify the promoter region, the transcription regulatory elements and the cell specificity of the ACE gene, five successive DNA deletions of the 5' upstream region (-1214, -754, -472, -343, -132 bp relative to the start site of transcription) were isolated and fused in sense and antisense orientations to the bacterial chloramphenicol acetyltransferase (CAT) reporter gene in the promoterless plasmid pBLCAT3. Promoter activities were measured in transient transfection assays using three different cell lines from rabbit endothelium (RE), human embryocarcinoma (Tera-1) and hepatocarcinoma cells (HepG2). All five fragments of the ACE promoter region directed expression of the CAT gene when transfected into the endothelial and the embryocarcinoma cells, which contain endogenous ACE mRNA and express ACE activity. In contrast only minimal levels of promoter activity were obtained on transfection into hepatocarcinoma cells in which endogenous ACE mRNA and ACE activity were not detected. Transfection of RE and Tera-1 cells demonstrated that promoter activity was defined by the length of the ACE promoter sequence inserted into the construct. The 132 bases located upstream from the transcription start site were sufficient to confer ACE promoter activity, whereas the sequences upstream from -472 bp and between -343 bp and -132 bp were responsible for a decrease of promoter activity. Furthermore, the minimal 132 bp of the ACE promoter contains elements which direct cell-specific CAT expression. In addition, the DNA transfection study in the presence of dexamethasone suggested that the potential glucocorticoid regulatory elements, located in the sequence of the ACE promoter, are not functional.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruneval P., Hinglais N., Alhenc-Gelas F., Tricottet V., Corvol P., Menard J., Camilleri J. P., Bariety J. Angiotensin I converting enzyme in human intestine and kidney. Ultrastructural immunohistochemical localization. Histochemistry. 1986;85(1):73–80. doi: 10.1007/BF00508656. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen D., Piekarz R. L., Hsu S. I., DePinho R. A., Carrasco N., Horwitz S. B. Structural and functional analysis of the mouse mdr1b gene promoter. J Biol Chem. 1991 Feb 5;266(4):2239–2244. [PubMed] [Google Scholar]
- Costerousse O., Allegrini J., Lopez M., Alhenc-Gelas F. Angiotensin I-converting enzyme in human circulating mononuclear cells: genetic polymorphism of expression in T-lymphocytes. Biochem J. 1993 Feb 15;290(Pt 1):33–40. doi: 10.1042/bj2900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb D. W., Dixon J. E. A method for increasing the sensitivity of chloramphenicol acetyltransferase assays in extracts of transfected cultured cells. Anal Biochem. 1987 May 15;163(1):88–92. doi: 10.1016/0003-2697(87)90096-0. [DOI] [PubMed] [Google Scholar]
- Cushman D. W., Cheung H. S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971 Jul;20(7):1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- Defendini R., Zimmerman E. A., Weare J. A., Alhenc-Gelas F., Erdös E. G. Angiotensin-converting enzyme in epithelial and neuroepithelial cells. Neuroendocrinology. 1983 Jul;37(1):32–40. doi: 10.1159/000123512. [DOI] [PubMed] [Google Scholar]
- Devinoy E., Maliénou-N'Gassa R., Thépot D., Puissant C., Houdebine L. M. Hormone responsive elements within the upstream sequences of the rabbit whey acidic protein (WAP) gene direct chloramphenicol acetyl transferase (CAT) reporter gene expression in transfected rabbit mammary cells. Mol Cell Endocrinol. 1991 Oct;81(1-3):185–193. doi: 10.1016/0303-7207(91)90217-g. [DOI] [PubMed] [Google Scholar]
- Dorfman D. M., Wilson D. B., Bruns G. A., Orkin S. H. Human transcription factor GATA-2. Evidence for regulation of preproendothelin-1 gene expression in endothelial cells. J Biol Chem. 1992 Jan 15;267(2):1279–1285. [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- El-Dorry H. A., Bull H. G., Iwata K., Thornberry N. A., Cordes E. H., Soffer R. L. Molecular and catalytic properties of rabbit testicular dipeptidyl carboxypeptidase. J Biol Chem. 1982 Dec 10;257(23):14128–14133. [PubMed] [Google Scholar]
- Erdös E. G., Skidgel R. A. The angiotensin I-converting enzyme. Lab Invest. 1987 Apr;56(4):345–348. [PubMed] [Google Scholar]
- Fleming R. E., Gitlin J. D. Structural and functional analysis of the 5'-flanking region of the rat ceruloplasmin gene. J Biol Chem. 1992 Jan 5;267(1):479–486. [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gumucio D. L., Rood K. L., Blanchard-McQuate K. L., Gray T. A., Saulino A., Collins F. S. Interaction of Sp1 with the human gamma globin promoter: binding and transactivation of normal and mutant promoters. Blood. 1991 Oct 1;78(7):1853–1863. [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Howard T. E., Shai S. Y., Langford K. G., Martin B. M., Bernstein K. E. Transcription of testicular angiotensin-converting enzyme (ACE) is initiated within the 12th intron of the somatic ACE gene. Mol Cell Biol. 1990 Aug;10(8):4294–4302. doi: 10.1128/mcb.10.8.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. N., Hung H. L., Stanfield-Oakley S. A., High K. A. Characterization of the human blood coagulation factor X promoter. J Biol Chem. 1992 Aug 5;267(22):15440–15446. [PubMed] [Google Scholar]
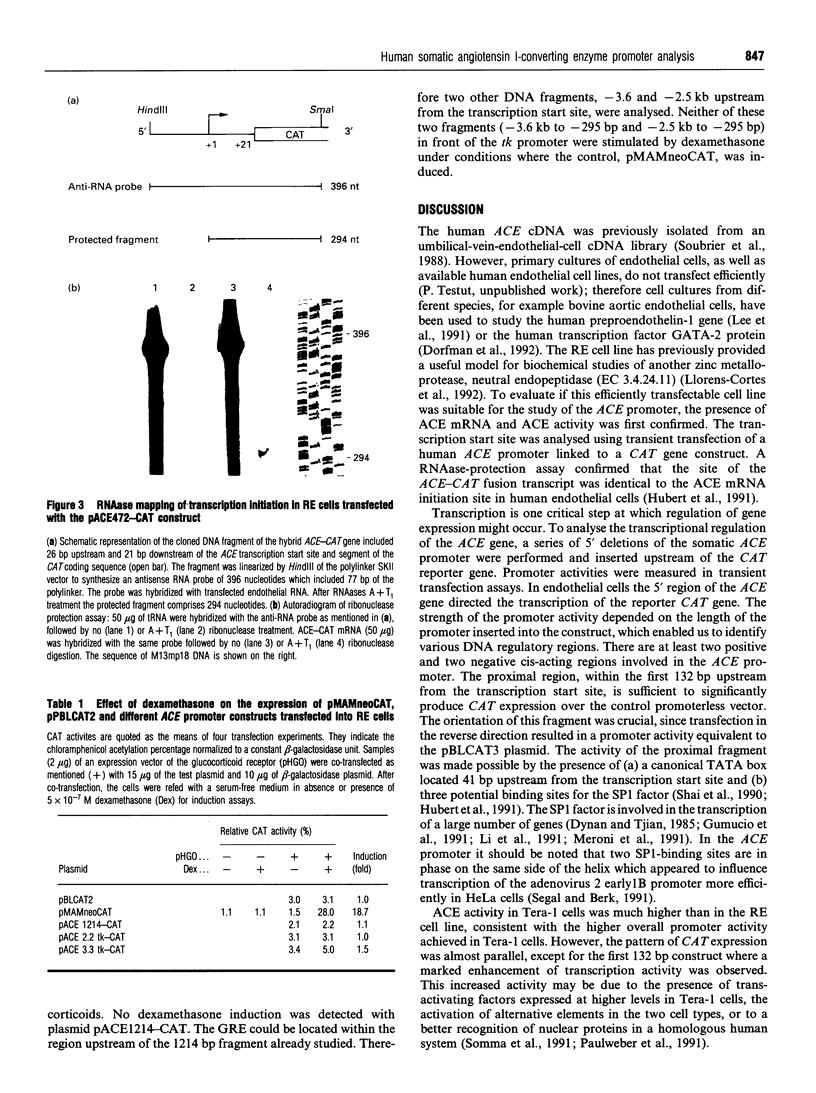
- Hubert C., Houot A. M., Corvol P., Soubrier F. Structure of the angiotensin I-converting enzyme gene. Two alternate promoters correspond to evolutionary steps of a duplicated gene. J Biol Chem. 1991 Aug 15;266(23):15377–15383. [PubMed] [Google Scholar]
- Krulewitz A. H., Baur W. E., Fanburg B. L. Hormonal influence on endothelial cell angiotensin-converting enzyme activity. Am J Physiol. 1984 Sep;247(3 Pt 1):C163–C168. doi: 10.1152/ajpcell.1984.247.3.C163. [DOI] [PubMed] [Google Scholar]
- Kumar R. S., Thekkumkara T. J., Sen G. C. The mRNAs encoding the two angiotensin-converting isozymes are transcribed from the same gene by a tissue-specific choice of alternative transcription initiation sites. J Biol Chem. 1991 Feb 25;266(6):3854–3862. [PubMed] [Google Scholar]
- Lange A. J., Espinet C., Hall R., el-Maghrabi M. R., Vargas A. M., Miksicek R. J., Granner D. K., Pilkis S. J. Regulation of gene expression of rat skeletal muscle/liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Isolation and characterization of a glucocorticoid response element in the first intron of the gene. J Biol Chem. 1992 Aug 5;267(22):15673–15680. [PubMed] [Google Scholar]
- Langford K. G., Shai S. Y., Howard T. E., Kovac M. J., Overbeek P. A., Bernstein K. E. Transgenic mice demonstrate a testis-specific promoter for angiotensin-converting enzyme. J Biol Chem. 1991 Aug 25;266(24):15559–15562. [PubMed] [Google Scholar]
- Lanzillo J. J., Stevens J., Dasarathy Y., Yotsumoto H., Fanburg B. L. Angiotensin-converting enzyme from human tissues. Physicochemical, catalytic, and immunological properties. J Biol Chem. 1985 Dec 5;260(28):14938–14944. [PubMed] [Google Scholar]
- Lee M. E., Dhadly M. S., Temizer D. H., Clifford J. A., Yoshizumi M., Quertermous T. Regulation of endothelin-1 gene expression by Fos and Jun. J Biol Chem. 1991 Oct 5;266(28):19034–19039. [PubMed] [Google Scholar]
- Li R., Knight J. D., Jackson S. P., Tjian R., Botchan M. R. Direct interaction between Sp1 and the BPV enhancer E2 protein mediates synergistic activation of transcription. Cell. 1991 May 3;65(3):493–505. doi: 10.1016/0092-8674(91)90467-d. [DOI] [PubMed] [Google Scholar]
- Lieberman J., Sastre A. Angiotensin-converting enzyme activity in postmortem human tissues. Lab Invest. 1983 Jun;48(6):711–717. [PubMed] [Google Scholar]
- Llorens-Cortes C., Huang H., Vicart P., Gasc J. M., Paulin D., Corvol P. Identification and characterization of neutral endopeptidase in endothelial cells from venous or arterial origins. J Biol Chem. 1992 Jul 15;267(20):14012–14018. [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni G., Malgaretti N., Pontoglio M., Ottolenghi S., Taramelli R. Functional analysis of the human lecithin cholesterol acyl transferase gene promoter. Biochem Biophys Res Commun. 1991 Nov 14;180(3):1469–1475. doi: 10.1016/s0006-291x(05)81361-0. [DOI] [PubMed] [Google Scholar]
- Nadaud S., Houot A. M., Hubert C., Corvol P., Soubrier F. Functional study of the germinal angiotensin I-converting enzyme promoter. Biochem Biophys Res Commun. 1992 Nov 30;189(1):134–140. doi: 10.1016/0006-291x(92)91535-x. [DOI] [PubMed] [Google Scholar]
- Okabe T., Yamagata K., Fujisawa M., Watanabe J., Takaku F., Lanzillo J. J., Fanburg B. L. Increased angiotensin-converting enzyme in peripheral blood monocytes from patients with sarcoidosis. J Clin Invest. 1985 Mar;75(3):911–914. doi: 10.1172/JCI111791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek I., Axel R. Glucocorticoids enhance stability of human growth hormone mRNA. Mol Cell Biol. 1987 Apr;7(4):1496–1507. doi: 10.1128/mcb.7.4.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulweber B., Onasch M. A., Nagy B. P., Levy-Wilson B. Similarities and differences in the function of regulatory elements at the 5' end of the human apolipoprotein B gene in cultured hepatoma (HepG2) and colon carcinoma (CaCo-2) cells. J Biol Chem. 1991 Dec 15;266(35):24149–24160. [PubMed] [Google Scholar]
- Petersen D. D., Koch S. R., Granner D. K. 3' noncoding region of phosphoenolpyruvate carboxykinase mRNA contains a glucocorticoid-responsive mRNA-stabilizing element. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7800–7804. doi: 10.1073/pnas.86.20.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B., Vicart P., Delouis C., Paulin D. Mammalian cell lines can be efficiently established in vitro upon expression of the SV40 large T antigen driven by a promoter sequence derived from the human vimentin gene. Biol Cell. 1991;73(1):7–14. doi: 10.1016/0248-4900(91)90003-6. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Segal R., Berk A. J. Promoter activity and distance constraints of one versus two Sp1 binding sites. J Biol Chem. 1991 Oct 25;266(30):20406–20411. [PubMed] [Google Scholar]
- Shai S. Y., Langford K. G., Martin B. M., Bernstein K. E. Genomic DNA 5' to the mouse and human angiotensin-converting enzyme genes contains two distinct regions of conserved sequence. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1128–1133. doi: 10.1016/0006-291x(90)90640-9. [DOI] [PubMed] [Google Scholar]
- Somma M. P., Gambino I., Lavia P. Transcription factors binding to the mouse HTF9 housekeeping promoter differ between cell types. Nucleic Acids Res. 1991 Aug 25;19(16):4451–4458. doi: 10.1093/nar/19.16.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubrier F., Alhenc-Gelas F., Hubert C., Allegrini J., John M., Tregear G., Corvol P. Two putative active centers in human angiotensin I-converting enzyme revealed by molecular cloning. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9386–9390. doi: 10.1073/pnas.85.24.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. E., Sheridan M. A., Hammon K. J., Erdös E. G. Angiotensin I converting enzyme (kininase II) of the brush border of human and swine intestine. Biochem Pharmacol. 1980 Jun 1;29(11):1525–1529. doi: 10.1016/0006-2952(80)90603-6. [DOI] [PubMed] [Google Scholar]
- Wei L., Alhenc-Gelas F., Soubrier F., Michaud A., Corvol P., Clauser E. Expression and characterization of recombinant human angiotensin I-converting enzyme. Evidence for a C-terminal transmembrane anchor and for a proteolytic processing of the secreted recombinant and plasma enzymes. J Biol Chem. 1991 Mar 25;266(9):5540–5546. [PubMed] [Google Scholar]
- Yang H. Y., Erdös E. G., Levin Y. A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim Biophys Acta. 1970 Aug 21;214(2):374–376. doi: 10.1016/0005-2795(70)90017-6. [DOI] [PubMed] [Google Scholar]